Abstract
Objective
The posterior cricoarytenoid (PCA) muscle is the sole abductor of the glottis and serves important functions during respiration, phonation, cough, and sniff. The present study examines vocal fold abduction dynamics during PCA muscle activation.
Study Design
Basic science study using an in vivo canine model and human subjects.
Methods
In four canines and five healthy humans vocal fold abduction time was measured using high speed video recording. In the canines, PCA muscle activation was achieved using graded stimulation of the PCA nerve branch. The human subjects performed coughing and sniffing tasks. High speed video and audio signals were concurrently recorded.
Results
In the canines the vocal fold moved posteriorly, laterally, and superiorly during abduction. Average time to reach 10%, 50% and 90% abduction was 23, 50, and 100 ms with low stimulation, 24, 58, and 129 ms with medium stimulation, and 21, 49, and 117 ms with high level stimulation. In the humans, 100% abduction times for coughing and sniffing tasks were 79 and 193 ms, respectively.
Conclusion
The PCA abduction times in canines are within the range in humans. The results also further support the notion that PCA muscles are fully active during cough.
Level of Evidence
N/A (Animal studies and basic research)
Keywords: Posterior cricoarytenoid muscle, cough, sniff, vocal fold abduction, high-speed video-endoscopy, voice production
INTRODUCTION
The varied and complex roles of the larynx in airway maintenance, deglutition, and phonation are primarily accomplished through purposeful activation of the intrinsic laryngeal muscles (ILMs). The ILMs include the vocal fold adductors [thyroarytenoid (TA), lateral - cricoarytenoid (LCA) and interarytenoid (IA) muscles], as well as the only paired abductor, the posterior cricoarytenoid (PCA) muscle. While the role of the laryngeal adductors in phonation and airway protection is studied extensively the PCA muscle has received less attention in investigations of neuromuscular control of the larynx.1–2
The PCA muscle originates in the posterior medial cricoid lamina and inserts medially and superiorly onto the muscular process of the arytenoid. Contraction of this muscle causes lateral movement of the vocal fold thus widening the glottis.3 In a detailed electromyographic evaluation of ILM activity during speech, swallowing, sniff, and cough tasks Hillel found set roles for the PCA in nearly all human subjects.2 The PCA was never active during simple /i/ phonation, and demonstrated some activity during increased pitch task in four of nine subjects. In contrast, the PCA was markedly active during production of voiceless sounds /p/ and during cough. During cough the adductor muscles activated first to close the larynx then the PCA muscles activated approximately 150 ms before the adductor activity abruptly stopped, glottis opened, and cough occurred. During deglutition the PCA was quiet but activated at the end of the swallow. On inspiration, PCA was active in all subjects during fast breathing and sniff tasks. Hirose found a role for the PCA muscle in voiceless sounds using LEMG where the PCA consistently activated for production of voiceless sounds during connected speech.4 Furthermore, the time course of PCA activity and glottal width were comparable, indicating that glottal abduction during speech is an active event mediated by PCA activation.
Poor cough is also observed in patients with neurodegenerative disease, and this is thought to be due in part, due to decreased ILM activation. The PCA muscle has important roles in airway protection, phonation, and deep inspiration. However, vocal fold abduction dynamics has not been previously studied in detail. In this report we investigated PCA muscle abduction dynamics in canines and humans by measuring vocal fold abduction time from five canines and three healthy humans using high speed video (HSV) recording.
MATERIALS AND METHODS
In vivo canine larynx preparation
The canine larynx is a close match to the human larynx in terms of its gross, microscopic, and histologic anatomy, and the utility of the in vivo canine model in voice research is well established.1, 5–6 This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals and was approved by the Institutional Animal Research Committee (ARC) of the University of California, Los Angeles.
Four mongrel canines (weight 20–25 kilograms) were used for this report. The PCA experiments were performed as part of a study of neuromuscular control of the larynx. Surgical exposure of the larynx and the laryngeal nerves was as described previously.1,6 In brief, after induction of general endotracheal anesthesia, a low tracheotomy was performed for intraoperative ventilation. A suprahyoid pharyngotomy was performed to expose the larynx in the neck and a supraglottic laryngectomy was performed for improved laryngeal exposure for high-speed video recording. In one of the canines the hemilarynx was exposed and video recording was performed of the medial surface. The recurrent laryngeal nerves (RLNs) were identified on the tracheoesophageal grooves bilaterally and followed distally towards the larynx. The posterior cricoarytenoid (PCA) nerve branch was identified and a suture tie was placed at the branch take-off. Cuff electrodes were placed on the PCA nerve branch distally. Eleven levels of graded nerve stimulation (from threshold to maximal stimulation) were applied to the nerve. Neuromuscular stimulation parameters were 1000 ms pulse trains, 0.1 ms long rectangular unipolar cathodic pulses at 100 Hz. To allow muscle recovery, each stimulation pulse train was followed by a pause of 4 seconds prior to the next pulse train.
India ink was used to mark several locations on the surface of the vocal fold such as the anterior commissure, mid membranous vocal fold, and vocal processes (see figures 1–2). A high-speed digital video camera (Phantom v210, Vision Research Inc., Wayne, New Jersey, USA) was used for imaging laryngeal deformation at 3000 frames per second (fps) at a resolution of 512 X 512 pixels. The camera was triggered concurrently to the nerve stimulation pulse train and recorded laryngeal deformations for 1500 ms. The distance from the high speed camera to the vocal folds did not vary during the experiment. The movement of the India Ink landmark at the vocal process was used to assess the onset and end of all visible vocal fold position changes from PCA activation. Measurement of postural change was assessed manually using the Phantom Camera Control Application software (Pcc 1.3, Vision Research Inc., Wayne, New Jersey, USA) which allows frame by frame analysis of landmark trajectory and measurement of distance travelled. The position of the landmark at onset and end of movement was marked with a ruler and the entire distance travelled was divided into 10 equal parts (10th percentiles). The time required for displacement was calculated based on the number of video frames needed to travel the distance (3 video frames per ms). To ensure accuracy, measurements of time to achieve 10%, 50%, and 90% displacement is presented. The angle of displacement of the vocal process was also measured relative to the glottal midline for the superior view and relative to a horizontal parallel line for the medial view.
Figure 1.
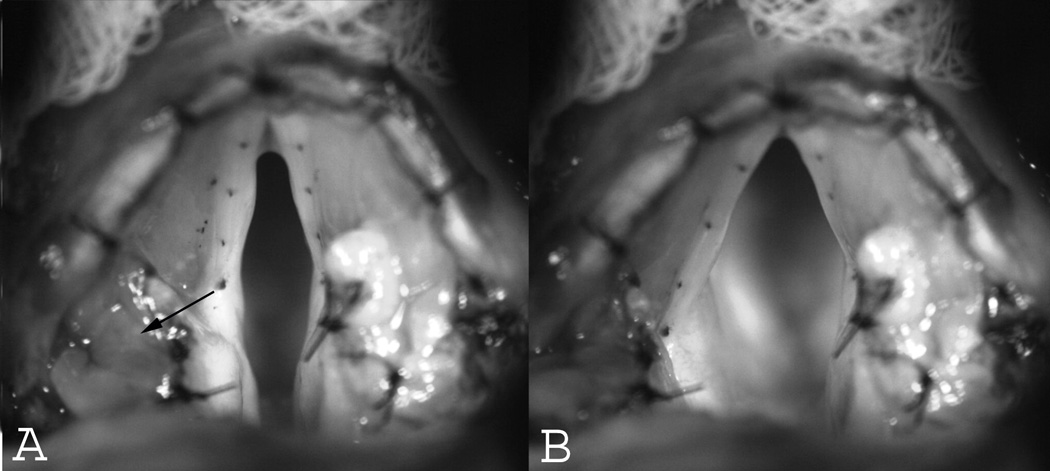
Superior views of the canine larynx. (A) Baseline resting position. The arrow points towards the direction of travel of the vocal process upon stimulation of the nerve branch to the PCA muscle. (B) Glottal posture at full abduction. The vocal process moved posteriorly and laterally.
Figure 2.
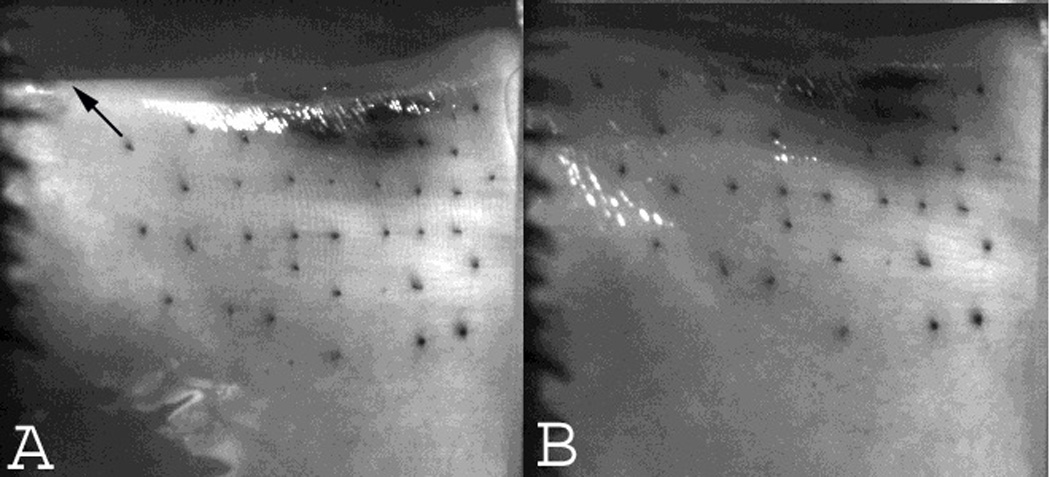
Medial views of the canine larynx. A) Baseline resting position. The arrow points towards the direction of travel of the vocal process upon stimulation of the nerve branch to the PCA muscle. (B) Glottal posture at full abduction. The vocal process moved posteriorly and superiorly.
Measurements of Human PCA Activity
PCA activity was observed from five humans subjects that participated in an IRB approved study evaluating synchronous audio and video recording of various glottal speech tasks. Among these, two subjects performed coughing tasks and three subjects performed sniffing tasks. A rigid 70 degree laryngoscope was used to visualize the larynx during tasks. For the cough task, subjects were asked to cough with effort but without shifting position of the head. HSV was performed at 20,000 fps. For the sniff task subjects were asked to phonate “eee” and then immediately perform a sniff maneuver (“eee_sniff”). HSV was performed at 10,000 fps. Acoustic signal was synchronized to the video signal and concurrently recorded using a high fidelity microphone. Total abduction time was calculated for both tasks by measuring video frames from onset of vocal fold movement at the vocal process until no more movement was perceived. Cases of cough and sniff tasks where the epiglottis blocked endoscopic view for adequate analysis of vocal fold motion were excluded from analysis.
RESULTS
In vivo canine larynx
Upon activation of the PCA nerve branch the vocal process moved posteriorly, laterally, and superiorly (Figures 1–2). From a superior view the vocal process moved laterally and posteriorly at an average angle of 57 degrees (Range 45 to 63 degrees), and from a medial view the vocal process moved posteriorly and superiorly at an angle of 62 degrees. The vocal folds traversed a greater distance with increasing stimulation level. At the lowest activation levels (levels 1–2) very small displacement occurred and prevented accurate measurements of displacement time. For simplicity, stimulation levels 3–11 were categorized into low (levels 3–5), medium (levels 6–8) and high (levels 9–11) activation levels and the displacement times averaged (Table 1). At low stimulation levels, average time to 10%, 50%, and 90% excursion was 23, 50, and 100 ms respectively. With medium stimulation, average time to 10%, 50%, and 90% excursion was 24, 58, and 129 ms, respectively. At the highest stimulation levels average time to 10%, 50%, and 90% excursion was 21, 49, and 117 ms, respectively. Since excursion times for the three stimulation conditions were similar but total displacement increased with increasing stimulation level, abduction velocity was greater with greater stimulation but we did not quantify the speed of abduction.
Table I.
Abduction Time (ms) by % Displacement in Canines
| Displacement | ||||
|---|---|---|---|---|
| 10% | 50% | 90% | ||
| Stimulation Level | Canine Vocal Fold | |||
| Low | 1 | 23.67 | 55.56 | 110.33 |
| 2 | 14.89 | 40.00 | 89.22 | |
| 3 | 27.44 | 59.89 | 112.11 | |
| 4 | 24.11 | 46.00 | 89.67 | |
| Average (ms) | 22.53 | 50.36 | 100.33 | |
| Medium | 1 | 23.89 | 53.78 | 114.89 |
| 2 | 23.33 | 75.00 | 165.11 | |
| 3 | 26.44 | 56.44 | 115.11 | |
| 4 | 21.33 | 47.22 | 120.11 | |
| Average (ms) | 23.75 | 58.11 | 128.81 | |
| High | 1 | 19.78 | 40.56 | 81.22 |
| 2 | 21.67 | 58.78 | 146.89 | |
| 3 | 21.56 | 51.89 | 122.67 | |
| 4 | 22.56 | 45.89 | 115.67 | |
| Average (ms) | 21.39 | 49.28 | 116.61 | |
Humans
Cough maneuvers were assessed from two human subjects (Table 2). The first subject produced two coughs in sequence and the second produced 4 coughs in one sequence and 2 coughs in a second sequence. A typical endoscopic cough cycle pattern was present in both subjects (Figure 3). The cough cycle began with glottic closure, followed by a period of laryngeal compression in the adducted position prior to sudden glottal opening, then sudden glottal opening and acoustic output of cough. It was possible to visualize the glottal closing phase prior to the compression phase in three coughs. The glottal closing time averaged 100 ms (range 60–127 ms). The compression phase in these three coughs lasted an average of 280 ms (range 154 o 417 ms). In subject two the faster closing time and longer compression time was perceptually associated with a stronger cough compared to the cough with longer closing time and shorter compression time. The cough phase lasted an average of 79 ms (range 56 to 93 ms) (Table 2). Analysis of the acoustic signal and laryngeal vibration by digital videokymography revealed that the first burst of acoustic activity during cough was associated with the first glottal opening cycle during the cough phase. Glottal vibration during cough phase lasted for approximately half the glottal opening time, whereas the latter half of the glottal opening was associated with cessation of glottal vibration (Figure 3). In all cough samples, the sudden glottal opening and acoustic output during cough was followed rapidly by glottal closing and a second acoustic output as the vocal folds adducted and vibrated, which we labeled the “clearing” phase of the cough (Figure 3).
Table II.
Cough Task in Humans
| Subject # | Cough # | Closing Time (ms) | Compression Time (ms) | Cough Time (ms) |
|---|---|---|---|---|
| 1 | 1 | - | - | 82 |
| 112 | 417 | 78 | ||
| 2 | 1 | - | - | 56 |
| 2 | - | - | 83 | |
| 3 | 127 | 154 | 78 | |
| 4 | - | - | 73 | |
| 1 | 60 | 268 | 93 | |
| 2 | - | - | 86 | |
| Average | 100 ms | 280 ms | 79 ms |
Figure 3.
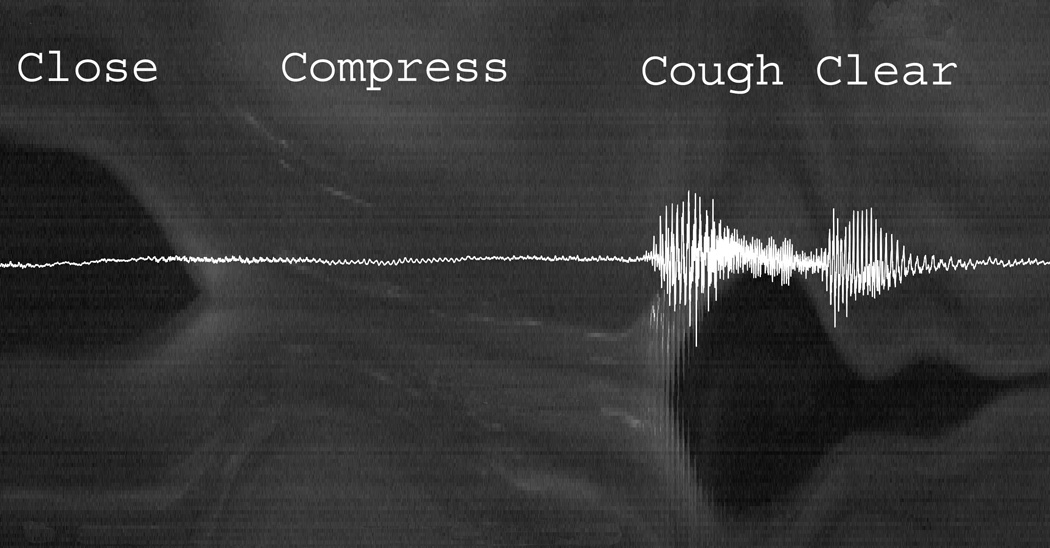
Digital videokymographic representation of the phases of the cough cycle superimposed with its acoustic signal (see text). (Video frame rate 20,000 fps)
Sniff maneuvers were elicited from three separate subjects and a total of 7 sniff cycles were assessed (Table 3). The average abduction time during sniff was 193 ms (range 179 – 211 ms). The glottal opening and closure for the “”eee-sniff” cycle was slower compared to the cough cycle (Figure 4).
Table III.
Sniff Task in Humans
| Subject # | Sniff # | 100% Abduction Time (ms) |
|---|---|---|
| 3 | 1 | 179 |
| 2 | 190 | |
| 3 | 197 | |
| 4 | 1 | 211 |
| 2 | 206 | |
| 5 | 1 | 187 |
| 2 | 180 | |
| Average | 193 ms |
Figure 4.
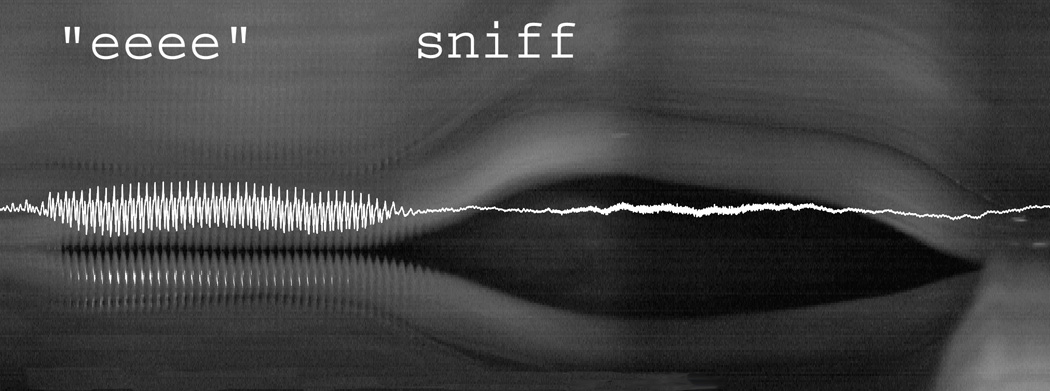
Digital videokymographic representation of the “eee-sniff” cycle superimposed with its acoustic signal (see text). (Video frame rate 10,000 fps)
DISCUSSION
This study, to our knowledge, provides the first descriptions of glottal abduction dynamics during direct stimulation of the PCA nerve in an animal model, and of cough and sniff maneuvers in humans using high speed video recording. Britton et al. reported endoscopic assessment of vocal fold movements during cough using videoendoscopic recordings (30 fps).7 That study was limited by the low video frame rate and measurements of the change in anterior commissure angle during cough and sniff as a surrogate abduction time (as opposed to the direct measurements of vocal process movement measured in this study) was used. However, despite the limitations, their mean measurement of the compression time (330 ms) is consistent with our measurements (280 ms). In addition, they measured a mean cough phase duration of 240 ms but this measurement included the post-cough adduction prior to the clear phase. At the post cough-adduction time prior for the clearing phase was typically about the same or slightly longer than the opening time during cough phase, their cough times are also consistent with our data (mean glottal opening time during cough of 79 ms). This study measured cough at 20,000 fps and sniff at 10,000 fps and provides high resolution assessment of the glottal posture during cough and sniff, and also is unique in demonstrating the relationship of the acoustic signal associated with the glottal posture changes during cough and sniff (Figures 3–4).
The abduction time in human larynges during cough (79 ms) was less than the abduction time in canines at full stimulation (116 ms). Speed of muscle contraction is dependent upon muscle fiber types and the degree of neuromuscular stimulation. There is sparse information on fiber types in PCA muscles. Few studied that exist have reported 65–67% type 1 (slow) fibers in human and 35–40% type 1 fibers in canine PCA muscles.8 In that case the canine PCA muscles would be somewhat faster than human PCA muscles. However, the fast abduction time in human subjects during cough is undoubtedly also assisted by aerodynamic energy from increased subglottal pressure during the compression phase and subsequent burst of airflow during the cough phase. Hillel has suggested that the 150 ms overlap of activity of both the abductors and the adductors just prior to the glottal opening for cough “spring loads” the glottis for abduction. Thus, during the cough phase adductors suddenly cease activity, PCAs are activate fully, and the buildup of subglottic pressure just prior to glottal opening enhances the abduction speed during cough. These results are consistent with the notion that PCA muscles are fully active during cough. We also noted that faster closure and longer compression time in one subject was associated with perceptually louder cough but no generalizations can be made from such a small sample.
Abduction time for sniff in humans (193 ms) was slower. It is possible that PCAs and not fully activated during sniff tasks. However, Hillel noted that during sniff the laryngeal adductors are also active “to provide counter tension” and to prevent turbulent flow through the glottis and likely accounts for the slower abduction dynamics of the vocal fold compared to cough.2
CONCLUSION
Vocal fold abduction serves important functions in speech tasks such as voiceless sounds and airway protective tasks such as cough. This study shows the utility of HSV in assessing laryngeal muscle dynamics. While both cough and sniff require increased PCA activity, the former event is about twice as fast as the latter. Neurodegenerative diseases result in decreased cough and airway clearance. Future studies should be focused on development of normative data of ILM dynamics in normal subjects, and the study of ILM dynamics in patients with neurodegenerative conditions.
Acknowledgments
This study was supported by Grant No. RO1 DC011300 from the National Institutes of Health
Footnotes
Financial Disclosure:
The authors have no other financial disclosures to make.
Conflict of Interest: None
This research will be presented as an oral presentation at Annual Meeting of the American Laryngological Association, Las Vegas, Nevada, May14–15, 2014
REFERENCES
- 1.Chhetri DK, Neubauer J, Berry DA. Neuromuscular control of fundamental frequency and glottal posture at phonation onset. J Acoust Soc Am. 2012;131(2):1401–1412. doi: 10.1121/1.3672686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillel AD. The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. Laryngoscope. 2001;111(4 Pt 2 Suppl 97):1–47. doi: 10.1097/00005537-200104001-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bryant BJ, Woodson GE, Kaufman K, Rosen C, Henqesteq A, Chen N, Yeung D. Human posterior cricoarytenoid muscle compartments. Anatomy and mechanics. Arch Otolaryngol Head Neck Surg. 1996;122:1331–1336. doi: 10.1001/archotol.1996.01890240039009. [DOI] [PubMed] [Google Scholar]
- 4.Hirose H. Posterior cricoarytenoid as a speech muscle. Ann Otol Rhinol Laryngol. 1976;85(3 pt 1):335–342. [PubMed] [Google Scholar]
- 5.Garrett CG, Coleman JR, Reinisch L. Comparative histology and vibration of the vocal folds: implications for experimental studies in microlaryngeal surgery. Laryngoscope. 2000;110:814–824. doi: 10.1097/00005537-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Chhetri DK, Neubauer J, Bergeron JL, Sofer E, Peng KA, Jamal N. Effects of asymmetric superior laryngeal nerve stimulation on glottic posture, acoustics, vibration. Laryngoscope. 2013;123:3110–3116. doi: 10.1002/lary.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britton D, Yorkston KM, Eadie T, Stepp CE, Ciol MA, Baylor C, Merati AL. Endoscopic assessment of vocal fold movements during cough. Ann Otol Rhinol Laryngol. 2012;121:21–27. doi: 10.1177/000348941212100105. [DOI] [PubMed] [Google Scholar]
- 8.Hoh JF. Laryngeal muscle fibre types. Acta Physiol Scand. 2005;183:133–149. doi: 10.1111/j.1365-201X.2004.01402.x. [DOI] [PubMed] [Google Scholar]


