Abstract
Background
Few studies have been conducted that make comparisons between traditional measures of cholesterol and cholesterol subfractions, and only one study has compared low-density lipoprotein cholesterol (LDL-C) particle number, LDL-C particle size and LDL-C among end-stage renal disease (ESRD) patients. The purpose of this study was to examine the relationships between cholesterol measures and differences in risk stratification when using ATP-III guidelines compared with cholesterol particle number and size in ESRD patients.
Methods
ESRD patients (n=1,092) from clinics associated with the Central Texas Nephrology Associates were recruited to participate in this study.
Results
LDL particle size categorized more patients at-risk when compared with LDL-C, non-HDL-C and triglycerides. Pearson correlation coefficients revealed a strong significant correlation between LDL-C and LDL particle number (r2=0.908, p=0.0001) and a significant correlation between LDL particle number and LDL particle size (r2=−0.290, p=0.0001). A significant but weak correlation existed between LDL-C and LDL particle size (r2=0.107, p=0.0001). A significant correlation existed between LDL particle number and triglycerides (r2=0.335, p=0.0001) and a significant inverse relationship between LDL particle size and triglycerides (r2=−0.500, p=0.0001).
Conclusions
Our study seems to suggest that using LDL particle size may help to identify those who would not be considered at-risk using LDL-C, non-HDL-C or triglycerides alone, and can be used as a further screening measure that may be more predictive of coronary heart disease outcomes.
Keywords: Cholesterol, End-stage renal disease, LDL-C, LDL particle number, LDL size
Introduction
Current National Cholesterol Education Program (NCEP) guidelines suggest standard lipid measurements are to be used to assess risk for coronary heart disease (CHD) in healthy and diseased populations (1). Low-density lipoprotein cholesterol (LDL-C) levels as a causal mechanism of coronary artery disease (CAD) are well established (2), with LDL-C lowering therapy being an accepted practice in reducing the risk for CAD. Though some studies (3–10) have suggested subfractions of cholesterol might be better predictors of CHD, the NCEP has yet to endorse these measures of risk prediction, although they have labeled them as emerging risk factors (1). The NCEP suggests there is uncertainty regarding how much additional risk is associated when subfractions of lipids are greater than triglycerides and/or high-density lipoprotein cholesterol (HDL-C) (11). Additionally, there is no definite answer as to how much additional risk can be assigned, or attenuated, by measuring and controlling subfractions of cholesterol (12, 13). Recently, the American Diabetes Association and the American College of Cardiology have endorsed the use of 2 cholesterol subfractions in clinical practice: LDL particle number (LDL-P) and apolipoprotein B (6). Moreover, some studies suggest that LDL-P has a stronger association with disease than LDL-C or HDL-C (14, 15).
One technology that researchers use to measure LDL-P and LDL particle size is nuclear magnetic resonance (NMR) spectroscopy (16). Additionally, some less-readily used measures are large HDL-C (5) and large very-low-density lipoprotein (VLDL) cholesterol. Traditional measures of cholesterol, such as LDL-C and HDL-C, quantify the cholesterol and triglyceride content of lipoproteins in milligrams per deciliter, and then use the amount measured to assess risk. However, an issue that arises in using such measures in predicting risk is that the measures and models do not account for individual differences. For example, individuals can vary in their LDL-P and LDL particle sizes, meaning that even though they may have equivalent LDL-C levels, they can vary in their risk for CHD (12, 14, 17).
To date, few studies have compared traditional measures of cholesterol and cholesterol subfractions (3–10), and only one study has specifically compared LDL-P, LDL particle size and LDL-C among end-stage renal disease (ESRD) patients (17). The few studies that have been conducted have demonstrated differing risk profiles among participants, when assessing risk using traditional measures of cholesterol and particle number and size with many suggesting an increased risk associated with LDL-P and particle size that is independent of LDL-C (12, 16). Some study authors (2, 3) have reported that differences in traditional measures and subfractions are significant, as these measures are clinically different and may measure different aspects of risk. For example, Jayarajah et al (3) found LDL-P to be more predictive than LDL-C levels, and thus better able to predict morbidity. Many of the participants in this particular study had normal LDL-C levels, but abnormal levels of particle concentration and size. However, El Harchaoui et al (2) found no increased risk associated with LDL-P and LDL particle size when compared to LDL-C cholesterol in the EPIC-Norfolk study.
Therefore, as some of the few studies that have investigated cholesterol subfractions have found contradictory results, we designed the current study to examine the relationships between cholesterol and cholesterol subfraction measures in stratifying risk for CHD in ESRD patients.
Subjects and methods
ESRD patients (n=1,092; 519 of whom were female) from clinics associated with the Central Texas Nephrology Associates were recruited to participate in this study after signing informed consent statements in compliance with the human subjects guidelines of the university and clinics for the protection of human subjects in research. Institutional Review Board (IRB)/Ethics Committee approval was obtained. This study adhered to the principles of the Declaration of Helsinki. Patients were chronic hemodialysis patients who sought treatment 3 times per week. Exclusion criteria included: (a) a life-expectancy of less than 6 months based on physician prognosis, (b) a diagnosis of cancer or HIV infection, (c) no desire to participate in the study, (d) previous cardiovascular events, (e) hospitalizations or surgery in the last 12 weeks, (f) age less than 18 years, (g) pregnancy or (h) malignant hypertension (altered mental status clinically related to hypertension or blood pressure greater than 200/120 mm Hg). Patients were recruited through clinic medical records and their treating physician.
Using the risk categories identified by the National Cholesterol Education Program Adult Treatment Panel-III (ATP-III), we classified each patient separately, based on their LDL-C, HDL-C, total cholesterol, VLDL and triglycerides. For LDL particle size, we classified patients from 18.0 to 21.2 as phenotype B and patients from 21.3 to 23.0 as phenotype A (4). For LDL-P, we categorized patients as optimal (<1,100), near optimal (1,101–1,399), borderline-high (1,400–1,799), high (1,800–2,100) or very high (>2,100). We used the risk categorization from the Multi-Ethnic Study of Atherosclerosis (MESA) study for LDL particle size and LDL-P (4).
Dialysis protocol
Polysulfone membranes were used in all associated dialysis clinics with a dialysate flow rate of 800 ml/min with a mean flow rate of 376.49 ml/min. Dialysis dose was Kt/V range of 1.1–2.0 with a mean of 1.35. Ninety-five percent of patients were dialyzed using a Fresenius-160 dialyzer, and 5% used a Fresenius-180 dialyzer. All patients followed the standard dialysis unit protocol of 4-hour dialysis duration.
Lipid analysis
Approximately 20 mL of blood was collected from each patient after fasting for 12 hours and immediately prior to dialysis, using standardized venipuncture techniques in the antecubital vein. Venous samples were centrifuged and immediately placed in a cold storage unit and sent for assay. For each patient, we obtained a standard clinical lipid profile (triglycerides, total cholesterol, HDL-C, LDL-C, non-HDL-C and VLDL) in milligrams per deciliter (mg/dL). Lipids were measured by standardized automated methods (LipoScience Inc., Raleigh, NC, USA), and calculated LDL-C by the Friedewald equation (18). LDL-P and LDL particle size were measured using NMR spectroscopy (LipoScience Inc., Raleigh, NC, USA).
Statistical analysis
The analysis consisted of 3 steps. First, we assessed the relationship between the variables LDL-C, LDL-P, LDL size, triglycerides, total cholesterol, VLDL, HDL-C and non-HDL-C. Initially, we examined pairwise scatterplots of these same variables to assess for any curvilinear relationships. None of the relationships appeared to have nonlinear relationships with each other, therefore we then examined the Pearson product moment correlations between the same variables. Second, we categorized each patient for risk of CHD by their levels for the following variables using ATPIII or MESA guidelines: LDL-C, non-HDL-C, triglycerides, LDL particle size and LDL-P. In addition, based on the initial classifications, we then classified the patients a second time into either normal (no elevated risk, based on ATP-III or MESA guidelines) or elevated risk (any patient who was categorized as borderline-high, high, very high or phenotype B, based on NCEP-III or MESA guidelines). Third, we then assessed for any disconnect between the variables by comparing the percentage of ESRD patients quantified as at-risk (using ATP-III guidelines) for LDL-C, triglycerides, total cholesterol, HDL-C and non-HDL-C, with the number of participants classified as at-risk using LDL-P and LDL particle size (MESA guidelines). We also acquired percentiles (10th, 25th, 50th, 75th, 90th, 95th and 99th) for comparisons between lipid values.
Results
Descriptive statistics of participants are given in Table I. Percentiles of cholesterol values are provided in Table II. Pearson correlation coefficients for the inter-lipid relationships are given in Table III. Of note, there was an almost collinear relationship between LDL-C and LDL-P, a strong inverse relationship between LDL particle size and triglycerides, a medium-sized correlation between LDL-P and triglycerides, a medium-sized inverse correlation between LDL-P and LDL particle size, and small-to-nonexistent correlations among the other measures. Using ATP-III and MESA cutpoints, we were able to discover what percentage of study participants exceeded the cutpoints for each variable. The cutpoint for LDL-P (≥1,100 LDL particles) occurred at the 62nd percentile, LDL size (<21.2 nm) at the 53rd percentile, triglycerides (≥150 mg/dL) at the 72nd percentile, total cholesterol (>200 mg/dL) at the 84th percentile, LDL (≥100 mg/dL) at the 53rd percentile and HDL-C (<40 mg/dL) at the 50th percentile.
TABLE I.
DEMOGRAPHIC INFORMATION FOR THE SAMPLE POPULATION OF END-STAGE RENAL DISEASE PATIENTS
| Sex, no. (%) | |
| Women | 519 (47.39) |
| Men | 576 (52.61) |
| Age, years (SD) | 63.25 (12.54) |
| Ethnicity, no. (%) | |
| African American | 496 (45.29) |
| White | 334 (30.50) |
| Hispanic | 256 (23.38) |
| Other | 9 (.008) |
| Diabetes diagnosis, no. (%) | 505 (46.12) |
| Months on dialysis, mean (SD) | 32.20 (9.29) |
| Hypertensive, no. (%) | 739 (67.49) |
| Medication usage, no. (%) | |
| Statins | 305 (28.77) |
| ACE-inhibitors | 277 (25.30) |
| ARB | 302 (27.58) |
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker.
TABLE II.
CHOLESTEROL PERCENTILES OF THE SAMPLE POPULATION OF END-STAGE RENAL DISEASE PATIENTS
| 10th | 25th | 50th | 75th | 90th | 95th | 99th | |
|---|---|---|---|---|---|---|---|
| Total cholesterol | 115.0 | 135.0 | 161.0 | 186.0 | 213.0 | 232.0 | 268.0 |
| LDL cholesterol | 62.0 | 77.0 | 96.0 | 120.0 | 139.0 | 157.0 | 189.0 |
| LDL-P | 630.2 | 792.0 | 1,004.0 | 1,235.0 | 1,537.2 | 1,695.0 | 2,100.0 |
| LDL size | 20.2 | 20.6 | 21.3 | 21.7 | 22.0 | 22.2 | 22.4 |
| HDL cholesterol | 26.0 | 32.0 | 40.0 | 49.0 | 58.0 | 64.0 | 79.0 |
| Triglycerides | 55.2 | 73.0 | 107.0 | 161.0 | 246.0 | 324.0 | 491.0 |
| VLDL cholesterol | 24.0 | 42.0 | 74.0 | 127.0 | 210.8 | 287.0 | 459.0 |
| Non-HDL | 77.0 | 93.0 | 118.0 | 144.0 | 171.0 | 190.0 | 226.0 |
HDL = high-density lipoprotein; LDL = low-density lipoprotein; LDL-P = LDL particle number; VLDL = very-low-density lipoprotein.
TABLE III.
PEARSON CORRELATIONS BETWEEN LIPID MEASURES
| LDL-P | LDL particle size | Triglycerides | VLDL | LDL cholesterol | HDL cholesterol | |
|---|---|---|---|---|---|---|
| LDL-P | 1.000 | |||||
| LDL particle size | −0.290 | 1.000 | ||||
| Triglycerides | 0.335 | −0.500 | 1.000 | |||
| VLDL | 0.274 | −0.519 | 0.994 | 1.000 | ||
| LDL cholesterol | 0.908 | 0.107 | 0.141 | 0.066 | 1.000 | |
| HDL cholesterol | −0.162 | 0.531 | −0.309 | −0.338 | 0.053 | 1.000 |
HDL = high-density lipoprotein; LDL = low-density lipoprotein; LDL-P = LDL particle number; VLDL = very-low-density lipoprotein.
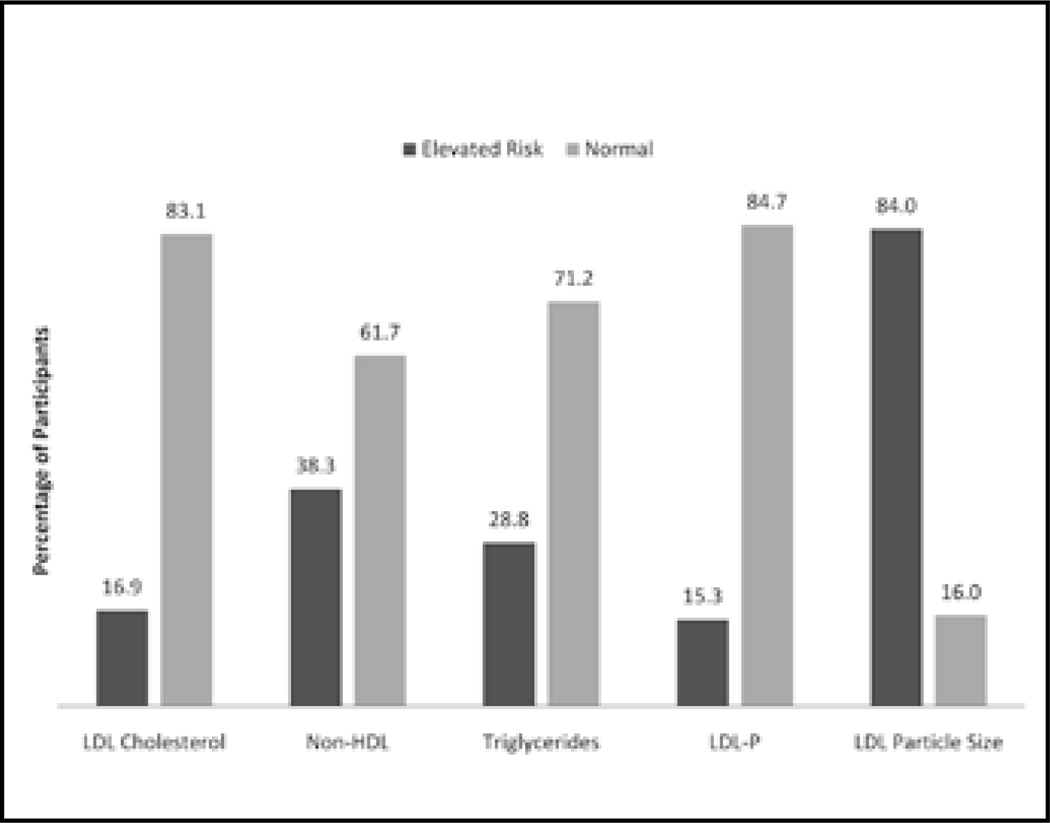
Using the ATP-III guidelines for LDL-C risk stratification, 587 patients (53.8%) fell into the optimal category (<100 mg/dL), 320 (29.3%) in the near-optimal/above-optimal (100–129 mg/dL), 138 (12.6%) in the borderline-high (130–159 mg/dL), 38 (3.5%) in the high (160–189 mg/dL) and 9 (0.8%) in the very high (>190 mg/dL) categories. Based on the ATP-III guidelines, 185 patients (16.9%) (borderline-high, high and very high combined) were considered to have elevated risk for CHD based on LDL-C levels.
Using categorization of non-HDL-C (total cholesterol-HDL-C) by Cromwell and Otvos (14) (which is based on ATP-III guidelines), 674 patients (61.4%) fell into an optimal (<130 mg/dL) category and 418 patients (38.3%) fell into an at-risk (≥130 mg/dL) category.
Using ATP-III guidelines for triglyceride risk stratification, 777 patients (71.2%) were classified as normal (<150 mg/dL), 145 (13.3%) were classified as borderline-high (150–199 mg/dL), 160 (14.7%) were classified as high (200–499 mg/dL) and 10 (0.9%) were classified as very high risk (≥500 mg/dL).
Using MESA (4) recommendations for LDL-P risk stratifications, 674 patients (61.7%) fell into the optimal category (<1,100 LDL particles), 251 (23.0%) fell into the near-optimal category (1,100–1,399 LDL particles), 134 (12.3%) fell into the borderline-high category (1,400–1,799 LDL particles), 23 (2.1%) fell into the high category (1,800–3,100 LDL particles) and 10 (0.9%) fell into the very high category (≥2,100 LDL particles). Based on MESA guidelines, 167 patients (15.3%) were considered at-risk.
Using MESA (4) for LDL particle size, the patients were grouped into categories according to LDL particle size definitions for phenotype A and phenotype B. Using these definitions, 175 patients (16.0%) were classified as phenotype A (21.3–23.0 nm) and 917 patients (84.0%) were classified as phenotype B (18.0–21.2 nm), which has been associated with increased risk for morbidity and mortality.
Disconnect
We examined the disconnect in risk categorization as proposed by Jayarajah et al (3) across all the variables, and the results are presented in Figure 1. Of note, using LDL-P risk stratifications, 18 fewer patients (1.6%) were classified as at-risk, than those classified using LDL-C, 253 fewer patients (23.2%) were classified than those classified using non-HDL-C, and 3 fewer patients (0.3%) were classified than those classified using triglycerides. Conversely, LDL particle size categorizes 732 more patients (67.0%) as at-risk, compared with LDL-C, 497 (45.5%) more compared with non-HDL-C and 747 (68.4%) more compared with triglycerides. It should also be noted that a very small correlation (0.107) existed between LDL-C and LDL particle size.
Figure 1.
Risk categorization by lipid measure. HDL = high-density lipoprotein; LDL = low-density lipoprotein; LDL-P = LDL particle number.
Discussion
LDL-C, non-HDL-C and triglycerides have been documented as a means to predict CHD, yet many ESRD patients continue to experience cardiovascular events, even when their LDL-C levels are normal (14). Some study authors (3, 19) report that differentiation between LDL-C and LDL-P and size is an important clinical distinction, as these measures are not equivalent. The NCEP does not include LDL size among clinical criteria used to identify people with metabolic syndrome, based on the uncertainty regarding how much risk can be predicted beyond LDL-C and triglycerides. Yet, lipoproteins such as LDL-C are lipid molecules that can vary widely and are a measure of cholesterol that does not quantify the number or size of LDL particles. Therefore any 2 patients may have the same LDL-C level, but 1 may have more LDL particles or smaller LDL particles and possibly more risk. In an effort to develop novel treatment strategies that can be effective in this population, it may be necessary to ascertain differences in risk attenuation using LDL-P and LDL particle size. Many ESRD patients have a higher number of LDL-P with smaller and denser particles that may increase risk and/or risk stratification. Our study discovered using LDL-P has less ESRD patients identified as at-risk when compared with using LDL-C, which was not reported in a previous study (17), but LDL particle size had a substantial increase (approximately 68% more) in the number of patients deemed at-risk when compared with LDL-C. Identifying an additional 68% is substantially higher than the approximately 11% of patients at increased risk with LDL particle size in one previous study (17) looking at the same variables. The previous study had a smaller sample size and used ATP-III guidelines but not MESA guidelines. This substantial increase in risk stratification in ESRD patients may suggest that LDL particle size may contribute more risk than previously reported in other studies. Additionally, the correlation between LDL-C and LDL particle number (r2=0.107) in the present study suggests that these 2 measures may not be equivalent. Therefore the results of our study suggest that a different measure of risk for ESRD patients may be LDL particle size, but not LDL-P, which disagrees with previous literature (9, 14, 19), and confirms the result of a previous study on the same topic (17). Cromwell and Otvos (9) suggest that LDL-P is more important than LDL particle size in predicting CHD. However, it should be noted that this is only the second study that compares LDL-C, LDL-P and LDL size in ESRD patients (17). This patient population has a number of comorbid conditions, whereas previous studies have used apparently healthy populations and other chronic disease states that may not have the same comorbid conditions.
El Harchaoui et al (2) report in a published study that LDL-P and LDL particle size have the prospect of improving treatment decisions, as these measures have been reported to be an independent predictor from LDL-C. Van der Graaf et al (20) report that small dense LDL particles have less affinity for the LDL-C receptor, spending more time in circulation with increased exposure to the arterial endothelium and have increased susceptibility to oxidative modification which has a direct effect on foam cell formation and CHD. It has been postulated that people with elevated triglyceride levels tend to have cholesterol depletion that enhances the enrichment of LDL particles and increases the formation of small dense LDL particle numbers. Elevated triglycerides are an important comorbidity in ESRD patients along with small dense LDL particles due to a VLDL defect (17), along with low HDL-C levels due to a transfer of cholesterol esters from HDL-C particles to apoB-containing lipoproteins. Kathiresan et al (11) report that small LDL particles are more atherogenic as they enter endothelium more readily with a greater affinity for oxidation. Our study supports these findings, as LDL particle size seemed to identify substantially more at-risk patients using a phenotype-B designation rather than LDL-C, non-HDL-C, triglycerides or LDL-P.
The Pearson correlation between LDL-C and LDL particle size (r2=0.107, p=0.0001) helps to support these findings. LDL-P and LDL-C are highly correlated (r2=0.908, p=0.0001), suggesting these 2 cholesterol variables are virtually the same with little unexplained variation. Conversely, the weak correlation between LDL-C and LDL particle size suggest 2 distinctly different measures and disagrees with many of the previous findings regarding LDL-P and LDL particle size (9, 14, 19). This may suggest that LDL size is measuring a different aspect of CHD risk that is not explained by LDL-C or LDL-P. LDL particle size may be a novel risk factor that when controlled may help to reduce CHD that occurs in patients that have normal LDL-C levels. Yet, until more studies have been conducted that look at outcomes such as cardiac events and mortality, this is highly speculative.
Some limitations existed in this study and should be noted. Our study was cross-sectional and limited to ESRD patients, making causation difficult to establish in both ESRD patient and apparently health populations. Additionally, our study did not track mortality, which should be included in future studies. More longitudinal studies in ESRD patients are warranted to help understand how LDL-P and LDL particle size may impact mortality and morbidly.
Finally, current understanding of CHD suggests that LDL particles are a significant part of cholesterol transport that has downstream effects of entering the artery wall, becoming modified through oxidation, and which are engulfed by macrophages to substantially increase plaque accumulation and calcification by the creation of foam cells. A greater question that must be answered through research with ESRD patients is whether LDL particle size is simply a marker for CHD that is reflective of increased risk that is no greater than LDL-C, non-HDL-C or triglycerides, or if LDL particle size has a direct causative effect that directly promotes atherosclerotic plaque accumulation that is independent (2, 16). Small LDL particles may simply be a reflection of increased triglyceride levels and low HDL-C, which are common in ESRD patients. Our study seems to suggest that at the very least using LDL particle size may help to identify those who would not be considered at-risk using LDL-C, non-HDL-C, LDL-P or triglycerides alone, and that it can possibly be used as a further screening measure. Small LDL particles may play a more important role as a means of primary prevention in those with ESRD, rather than in an apparently healthy population. Finally, as discussed in Mora et al (4), when considering important markers of CHD from both a clinical and public health perspective, a decision has to be made to use the appropriate or more appropriate approach to prevention and treatment. Reclassifying individuals using LDL particle size in this case may offer another means to identify at-risk patients and therefore modify therapeutic approaches.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Financial support: A.A.B. was supported by award number R03HD058464 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Footnotes
Conflict of interest statement: None declared.
References
- 1.National Institutes of Health. Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults - Third Report. Washington, DC: US Government Printing Office; 2002. [Google Scholar]
- 2.El Harchaoui K, van der Steeg WA, Stroes ES, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Jayarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Mora S, Szklo M, Otvos JD, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;192:211–217. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 5.El Harchaoui K, Arsenault BJ, Franssen R, et al. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 6.Mora S. Advanced lipoprotein testing and subfractionation are not (yet) ready for routine clinical practice. Circulation. 2009;119:2396–2404. doi: 10.1161/CIRCULATIONAHA.108.819359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivellese A, Patti L, Kaufman D, et al. Lipoprotein particle distribution and size, insulin resistance and metabolic syndrome in Alaska Eskimos: the GOCADAN study. Atherosclerosis. 2008;200:350–358. doi: 10.1016/j.atherosclerosis.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsia J, Otvos J, Rossouw JE, et al. Lipoprotein particle concentrations may explain the absence of coronary protection in the Women’s Health Initiative Hormone Trials. Arterioscler Thromb Vasc Biol. 2008;28:1666–1671. doi: 10.1161/ATVBAHA.108.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cromwell WC, Otvos JD. Heterogeneity of low-density lipoprotein particle number in patients with type 2 diabetes mellitus and low-density lipoprotein cholesterol <100 mg/dl. Am J Cardiol. 2006;98:1599–1602. doi: 10.1016/j.amjcard.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Chung CP, Oeser A, Raggi P, et al. Lipoprotein subclasses and particle size determined by nuclear magnetic resonance spectroscopy in systemic lupus erythematosus. Clin Rheumatol. 2008;27:1227–1233. doi: 10.1007/s10067-008-0890-4. [DOI] [PubMed] [Google Scholar]
- 11.Kathiresan S, Otvos JD, Sullivan LM, et al. Increased small low-density lipoprotein particle number a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20–29. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 12.Mora S, Otvos J, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119(7):931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toth PP. Low-density lipoprotein reduction in high-risk patients: how low do you go? Curr Atheroscler Rep. 2004;6:348–352. doi: 10.1007/s11883-004-0045-2. [DOI] [PubMed] [Google Scholar]
- 14.Cromwell WC, Otvos JD. Low-density lipoprotein particle number and risk for cardiovascular disease. Curr Atheroscler Rep. 2004;6:381–387. doi: 10.1007/s11883-004-0050-5. [DOI] [PubMed] [Google Scholar]
- 15.Soedamah-Muthu SS, Chang YF, Otvos J, Evans RW, Orchard TJ. Lipoprotein subclass measurements by nuclear magnetic resonance spectroscopy improve the prediction of coronary artery disease in Type 1 Diabetes: a prospective report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2003;46:674–682. doi: 10.1007/s00125-003-1094-8. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, Hirano T, Shiobara T, Suguro T, Adachi M. Small dense LDL concentration is closely associated with serum apolipoprotein B, comparisons of non-HDL cholesterol or cholesterol. Rinsho Byori. 2006;54:569–575. [PubMed] [Google Scholar]
- 17.Bowden RG, Hebert S, Wilson R, Gentile M, Lanning B. Comparison of lipid measures and risk stratification among endstage renal disease patients. J Nephrol. 2007;21:212–218. [PubMed] [Google Scholar]
- 18.Mora S, Rifai N, Buring JE, Ridker PM. Comparison of LDL cholesterol concentrations by Friedewald calculation and direct measurement in relation to cardiovascular events in 27,331 women. Clin Chem. 2009;55:888–894. doi: 10.1373/clinchem.2008.117929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Contois JH, McConnell JP, Sethi AA, et al. Apolipoprotein B and cardiovascular disease risk: position statement from the AACC Lipoprotein and Vascular Diseases Working Group on Best Practices. Clin Chem. 2009;55:407–419. doi: 10.1373/clinchem.2008.118356. [DOI] [PubMed] [Google Scholar]
- 20.van der Graaf A, Rodenburg J, Vissers MN, et al. Atherogenic lipoprotein particle size and concentrations and the effect of pravastatin in children with familial hypercholesterolemia. J Pediatr. 2008;152:873–878. doi: 10.1016/j.jpeds.2007.11.043. [DOI] [PubMed] [Google Scholar]