Abstract
Severe intraoperative hypotension has been reported in patients on angiotensin-converting enzyme inhibitors and angiotensin II receptor subtype 1 antagonists. We describe a patient on lisinopril who developed refractory intraoperative hypotension associated with increased serum tryptase level suggesting mast cell activation (allergic reaction). However, allergology workup ruled out an allergic etiology as well as mastocytosis, and hypotension recalcitrant to treatment was attributed to uninterrupted lisinopril therapy. Elevated serum tryptase was attributed to our patient's chronic renal insufficiency.
Keywords: Anaphylaxis; Renal insufficiency, Chronic; Hypotension; Lisinopril; Mastocytosis; Tryptase
INTRODUCTION
Preoperative use of angiotensin-converting enzyme inhibitors (ACEI) has been associated with intraoperative hypotension [1]. We describe a patient on the ACEI lisinopril who developed severe intraoperative hypotension, but because hypotension was associated with increased serum tryptase levels a comprehensive allergology work-up was conducted.
CASE REPORT
The Mayo Clinic Institutional Review Board gave written permission for the authors to publish this report. A 66-year-old male with chronic renal insufficiency (serum creatinine, 2.0 mg/dL) and hypertension was scheduled for cryoablation of recurrent renal carcinoma on a solitary kidney. Two hours before the procedure the patient took propranolol (120 mg) and lisinopril (10 mg). Anesthesia was induced with lidocaine, fentanyl, propofol, and succinylcholine, and he immediately developed hypotension recalcitrant to treatment with intravenous crystalloids, ephedrine, phenylephrine and vasopressin and bradycardia recalcitrant to ephedrine and glycopyrrolate. Only epinephrine, 20 µg every 3 minutes, would transiently increase blood pressure and heart rate. Epinephrine and phenylephrine infusions were initiated, both at 0.05 µg/kg/min. Though clinical features of an allergic reaction were absent (urticaria, bronchospasm, etc.), anaphylaxis was still considered as a part of differential diagnosis and hydrocortisone, diphenhydramine, and famotidine were administered. Transesophageal echocardiography showed good ventricular filling (therefore hypotension was unrelated to hypovolemia) and myocardial contractility (therefore unrelated to decreased myocardial contractility). The procedure was aborted and patient was transferred to the intensive care unit where cardiac troponins, and the adrenocorticotropic hormone stimulation test were normal. Three hours after the hypotensive event the total serum tryptase level was measured 16.2 µg/L (reference, <11.5 µg/L), raising the possibility of an allergic reaction. After 6 hours the patient was weaned from vasopressors. A baseline total tryptase level, measured 72 hours after the hypotension remained high (16.5 µg/L), but both 24-hour urinary N-methylhistamine (NMH), 67 µg/g creatinine, (reference, 30-200 µg/g creatinine) and 11-β prostaglandin F2α (11-β PGF2α), 874 ng per 24 hours (reference, <1,000 ng per 24 hours) were normal.
Allergology consult was obtained and patient interview revealed that 2 years earlier he underwent 2 uneventful anesthetics with the same agents as during the present anesthetic, but at that time he was not receiving lisinopril, which pointed to its potential role in encountered hypotension. Cryoablation was rescheduled one week later, and the lisinopril had been withheld for the entire week. In addition, preoperatively our patient received prednisone (50 mg) 13, 7 , and 1 hour prior to the procedure; montelukast (10 mg) and cetirizine (10 mg) both day before and on the morning of surgery; and diphenhydramine (50 mg) and famotidine (10 mg) both on the morning of surgery. The procedure was uneventful. Six weeks later the patient was scheduled for allergy testings. Surprisingly, the serum tryptase remained elevated (23.9 µg/L). Allergy testings for medications used perioperatively (midazolam, propofol, succinylcholine, lidocaine, dexamethasone, penicillin, benzylpenicilloyl moiety, alkaline hydrolysis product, cefazolin, povidone-iodine swab stick, and chlorhexidine) were all negative. Repeat NMH and 11-β PGF2α were normal along with negative c-kit D816V mutation analysis on peripheral blood.
DISCUSSION
We describe a patient on the ACEI, lisinopril, who developed refractory hypotension after anesthetic induction, with elevated serum tryptase which suggested a possibility for allergic etiology. However, although the acute serum tryptase level was elevated, so was the baseline level (level remote from event), making an acute mast cell activation event an unlikely culprit. This notion was further supported by normal urine NMH and 11-β PGF2α levels [2]. The fact that the patient underwent subsequent uneventful anesthetic using the same drugs but with lisinopril withheld, reiterated the likelihood that the ACEI therapy was the likely culprit. The persistently elevated tryptase was attributed to renal insufficiency.
The enzyme tryptase is secreted from mast cells in immature proforms (α- and β-protryptases). Protryptases undergo processing within the cell to become mature tryptase, which is stored in mast cell granules and released only during mast cell activation. Portions of the protryptases that do not mature are constitutively secreted from mast cells and are always present in 'normal' plasma. Total tryptase serum assay measures both pro- and mature (β)-forms of tryptase [3]. In the absence of mast cell activation, nearly all of the serum tryptase is the proform, and reflects the mast cell burden, which is typically elevated in systemic mastocytosis. During severe anaphylaxis mature β-tryptase is released from store granule, raising total tryptase levels above baseline. Furthermore, prostaglandin D2 and histamine are also released and their degradation products appear in the urine as 11-β PGF2α and NMH [4].
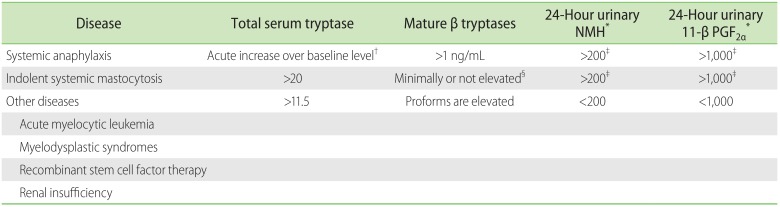
To diagnose the cause for increased serum tryptase one should ideally know the total and mature β-tryptase, unfortunately testing for mature β-tryptase is available in only one center in the United States [5]. When both total (>11.5 µg/L) and mature β (>1 µg/L) tryptases are elevated, anaphylaxis may be considered [3]. In patients with mastocytosis associated with mast cell activation, acute total tryptase levels are increased above baseline (Table 1). Comparison of acute (within 4 hours of event) to baseline levels (at least 24 hours after signs have resolved) of total tryptase are necessary to distinguish between an increased mast cell burden (mastocytosis with an cute mast cell degranulation) and mast cell degranulation [3]. Therefore, if the baseline total tryptase remains chronically elevated a nonanaphylactic causes, such as mastocytosis, may be considered. The Red Española de Mastocytosis or Spanish Network on Mastocytosis (REMA) score may be used to assess the probability of systemic mastocytosis [6]. The REMA score assigns positive or negative points: male (+1), female (-1), serum baseline tryptase levels <15 µg/L (-1) or >25 µg/L (+2), presence (-2) or absence (+1) of pruritus, hives or angioedema and presence (+3) of presyncope or syncope [7]. A REMA score of ≥2 indicates a high likelihood of bone marrow mast cell clonality. In our case, the REMA score was 2, but mastocytosis was excluded because of the atypical clinical history, negative NMH and PGF2α on two independent occasions, and a negative D816V c-kit mutation [8]. Finally, in our patient elevated serum tryptase was attributed to renal insufficiency [9]. The proposed mechanism of elevated serum tryptase in patients with renal insufficiency is based on decreased clearance of stem cell factor which causes mast cell hyperplasia resulting in serum tryptase elevation [10].
Table 1.
Tryptase, N-methylhistamine (NMH) and 11-β prostaglandin F2α (11-β PGF2α) as tests for differentiating the etiology of increased serum tryptase
Reference: total serum tryptase, <11.5 µg/L; Mature β tryptases, <1 µg/L; 24-Hour urinary NMH, 30-200 µg/g creatinine; 24-Hour urinary 11-β PGF2α, <1,000 ng.
*Measured from urine collected over 24 hours. †Elevations are transient; a clinically-significant acute tryptase elevation has been recommended as more than 2 + 1.2 (µg/L) × baseline tryptase level. ‡Elevations in urinary metabolites may be transient, either one or the other or be persistent. §Systemic mastocytosis is typically associated with normal levels of mature tryptase, except in patients who have recently experienced a mast-cell activation, in which case acute NMH and 11-β PGF2α should also be elevated.
In our patient severe hypotension refractory to treatment was likely associated with lisinopril therapy. Angiotensin system inhibitors (as well as angiotensin receptor blockers) interfere with regulation of blood pressure and have a vasodilatory action via multiple mechanisms. These drugs induce a direct sympathetic blockade and reduce the responsiveness to alpha-adrenergic agonists, inhibit the vasoconstrictor effects of angiotensin II by binding to its receptor, and impair the degradation of vasodilators such as bradykinin. Patients receiving chronic antihypertensive therapy with angiotensin system inhibitors are at higher risk for refractory hypotension during anesthesia [1, 11, 12, 13].
This case emphasizes that the presence of elevated serum tryptase cannot be taken as a definitive diagnostic proof of mast cell activation, and other conditions associated with increased tryptase production, in our case chronic renal insufficiency, should be considered.
References
- 1.Comfere T, Sprung J, Kumar MM, Draper M, Wilson DP, Williams BA, Danielson DR, Liedl L, Warner DO. Angiotensin system inhibitors in a general surgical population. Anesth Analg. 2005;100:636–644. doi: 10.1213/01.ANE.0000146521.68059.A1. [DOI] [PubMed] [Google Scholar]
- 2.Moreno F, Blanca M, Fernandez J, Ferrer A, Mayorga C, del Cano A, Aguilar F, Juarez C, Garcia J. Determination of inflammatory markers in allergic reactions to drugs. Allergy Proc. 1995;16:119–122. doi: 10.2500/108854195778690309. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;26:451–463. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Ono E, Taniguchi M, Mita H, Fukutomi Y, Higashi N, Miyazaki E, Kumamoto T, Akiyama K. Increased production of cysteinyl leukotrienes and prostaglandin D2 during human anaphylaxis. Clin Exp Allergy. 2009;39:72–80. doi: 10.1111/j.1365-2222.2008.03104.x. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz LB. Laboratory tests to support the clinical diagnosis of anaphylaxis. Waltham, MA: UpToDate; 2014. [Google Scholar]
- 6.van Doormaal JJ, van der Veer E, van Voorst Vader PC, Kluin PM, Mulder AB, van der Heide S, Arends S, Kluin-Nelemans JC, Oude Elberink JN, de Monchy JG. Tryptase and histamine metabolites as diagnostic indicators of indolent systemic mastocytosis without skin lesions. Allergy. 2012;67:683–690. doi: 10.1111/j.1398-9995.2012.02809.x. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Twose I, Gonzalez-de-Olano D, Sanchez-Munoz L, Matito A, Jara-Acevedo M, Teodosio C, Garcia-Montero A, Morgado JM, Orfao A, Escribano L. Validation of the REMA score for predicting mast cell clonality and systemic mastocytosis in patients with systemic mast cell activation symptoms. Int Arch Allergy Immunol. 2012;157:275–280. doi: 10.1159/000329856. [DOI] [PubMed] [Google Scholar]
- 8.Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, Castells M, Escribano L, Hartmann K, Lieberman P, Nedoszytko B, Orfao A, Schwartz LB, Sotlar K, Sperr WR, Triggiani M, Valenta R, Horny HP, Metcalfe DD. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–225. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirvent AE, Gonzalez C, Enríquez R, Fernandez J, Millan I, Barber X, Amoros F. Serum tryptase levels and markers of renal dysfunction in a population with chronic kidney disease. J Nephrol. 2010;23:282–290. [PubMed] [Google Scholar]
- 10.Kitoh T, Ishikawa H, Ishii T, Nakagawa S. Elevated SCF levels in the serum of patients with chronic renal failure. Br J Haematol. 1998;102:1151–1156. doi: 10.1046/j.1365-2141.1998.00902.x. [DOI] [PubMed] [Google Scholar]
- 11.Brabant SM, Eyraud D, Bertrand M, Coriat P. Refractory hypotension after induction of anesthesia in a patient chronically treated with angiotensin receptor antagonists. Anesth Analg. 1999;89:887–888. doi: 10.1097/00000539-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler AD, Turchiano J, Tobias JD. A case of refractory intraoperative hypotension treated with vasopressin infusion. J Clin Anesth. 2008;20:139–142. doi: 10.1016/j.jclinane.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Bjerregaard J, Jaffe RA. Intraoperative cardiac arrest: was it the ACE inhibitor? J Clin Anesth. 2014;26:62–64. doi: 10.1016/j.jclinane.2013.10.002. [DOI] [PubMed] [Google Scholar]