Abstract
Background
Adherence is key to antiretroviral therapy (ART) success. Enhanced partner support may benefit patients with prior treatment failure.
Methods
We conducted a 1:1 randomized trial of a partner-based modified directly observed therapy (mDOT) compared with standard of care (SOC) at 9 sites in 8 countries. Participants had failed a first-line regimen with HIV RNA >1000 copies/mL and a willing partner. Randomization was computer generated and balanced by site. Participants and site investigators were not masked to group assignment. ART included lopinavir/ritonavir (400/100 mg) twice daily and emtricitabine/tenofovir disoproxil fumarate (200/300 mg) once daily. Trained partners observed one ART dose daily ≥5 days/week for 24 weeks. Primary outcome was HIV RNA >400 copies/mL before or at week 48 and adherence measured with microelectronic monitors was a secondary outcome.
Findings
We randomized 129 participants to mDOT and 128 to SOC, 130 (51%) males, 204 (79%) of African origin, 52 (20%) Latino, with median age 38 years. Partners were parents, 57 (22%), spouses 55 (21%), siblings 50 (19%), friends 41 (16%), and others 54 (21%). Primary outcome occurred in 26% (34/129) of mDOT and 18% (23/128) of SOC participants at week 48 (p=0.13). Median adherence was similar [Q1: 95% vs. 96% p=0.38, Q2: 91% vs. 94% p=0.40, Q3: 90% vs. 93% p=0.17, Q4: 90% vs. 93% p=0.36] in mDOT and SOC, respectively.
Interpretation
This intervention had no effect on outcomes. Potential reasons include study visits maximizing adherence in both groups and control partners already providing sufficient support. Partner-based training with mDOT does not appear promising to enhance adherence. Intensive follow-up with clinic staff may be a viable strategy in this setting.
Introduction
The roll-out of antiretroviral therapy (ART) in resource-limited settings has resulted in remarkable increases in the life expectancy of HIV infected individuals1. Adherence rates have generally surpassed those observed in resource rich settings2, yet, virologic failure due to suboptimal adherence is an ongoing problem 3,4. Because patients in these settings often present with low CD4 counts5, virologic failure is associated with high rates of morbidity and mortality 6. Further, options for alternate treatment are limited. Patients failing first-line regimens containing two nucleoside analog reverse transcriptase inhibitors (NRTIs) and a non-nucleoside analog reverse transcriptase inhibitor (NNRTI) commonly develop resistance to both of these drug classes7–9 making these regimens much less effective even if patients subsequently achieve optimal adherence. Second-line regimens are more complex and expensive when available10 and there is often no availability of third-line regimens in many settings. Thus, preventing treatment failure due to non-adherence is a high priority for optimal patient outcomes.
Interventions to improve adherence have been developed over the past decade; the most promising combine modalities such as problem solving, motivational interviewing, skill enhancement, and technologies such as electronic reminders11–13. Directly observed therapy (DOT) is a complex series of steps14 that has been effective as an antiretroviral adherence intervention in special populations such as incarcerated patients and those receiving concomitant methadone maintenance therapy15. The mechanisms by which DOT is purported to improve adherence include facilitating medication access, providing encouragement by the observer, and, when lack of adherence observed, activating clinical and social services needed for enhanced support for an individual at high risk for stopping ART.
Unfortunately, as a sustainable option in community settings, the implementation of DOT can be costly, and may require resources that are unavailable, such as community healthcare workers with the skills to manage medication delivery to multiple clients. We hypothesized that the roles of a DOT worker including medication reminders, encouragement, and early alert of non-adherence might, alternatively, be carried out by members of a patient’s social circle16. If so, the personnel costs of DOT would be substantially reduced, limited to only the expenses needed for training the partner. Therefore, we designed an enhanced partner-based support intervention including modified directly observed therapy (mDOT) to improve adherence to second line therapy in HIV patients who had failed first-line.
Methods
We conducted a multi-site, international randomized clinical trial to test whether a partner-based mDOT intervention would result in higher virologic suppression and adherence rates than standard of care (SOC) adherence counseling in HIV-infected individuals who had experienced first-line ART failure. We believed that for the intervention to be maximally clinically meaningful, the effect should be measurable at 48 weeks. Yet, to decrease the burden and costs on sites and partners, we stopped requiring partners to implement mDOT and stopped site support for intervention activities after 24 weeks, while expecting any effect to be sustained for the subsequent 24 weeks.
All participants received a regimen consisting of lopinavir/ritonavir (400/100 mg) twice daily and emtricitabine/tenofovir disoproxil fumarate (200/300 mg) once daily. Partners were drawn from participants’ friend and family networks to serve in the DOT roles of monitoring medication taking, assisting in reminding the participant to take their medication, providing positive social support, and serving as a liaison to the clinical site if the participant was unwilling or unable to ask for help with adherence barriers. The study was approved by the ethics committees at each site and at the University of Pennsylvania. Written informed consent was obtained from all participants and partners.
Study Sites and Participants
The study was conducted at nine AIDS Clinical Trials Group (ACTG) sites in Botswana, Brazil, Haiti, Peru, South Africa, Uganda, Zambia, and Zimbabwe. We enrolled HIV-infected adults 18 years of age or older who had confirmed virologic failure with plasma HIV RNA >1000 copies/mL after having been treated for at least 16 weeks with a standard first-line ART regimen containing two NRTIs (lamivudine with either zidovudine or stavudine) and an NNRTI (efavirenz or nevirapine). Patients who changed ARVs within these classes due to toxicity that occurred prior to their virologic failure remained eligible.
Virologic failure was confirmed with two viral load tests at least one week apart. Participants were required to identify a potential mDOT partner at the time of enrollment. Partners had to be willing and able to observe at least one dose per day for five days per week and not expect to be away for longer than two weeks during the 24-week intervention period. Exclusion criteria for participants were having more than one episode of ART failure, having been treated with NRTIs other than those listed above or prior ART other than NRTIs and NNRTIs. Exclusion criteria for partners were inability to comprehend the goals of the training or unable or unwilling to carry out the mDOT roles due to substance abuse or other limitations. Recruitment strategies were not systematic and followed local practices.
Intervention Development
The intervention manual for partner training was developed by the core team with input from each of the site investigators to ensure cultural appropriateness. The manual consisted of training in basic HIV and antiretroviral treatment details, including the rationale for strict adherence, potential side effects and their management. It also focused on strengthening positive social support messages and decreasing negative ones (e.g., nagging) and psychosocial barriers to adherence and how to help manage them. The training also covered how to handle late or missed doses and how and when to contact the site for more help. A visual education aid was developed specifically for this study to allow for both verbal and visual learning to accommodate different learning styles and/or low literacy.
All site investigators and intervention staff attended an in-person meeting to review the details of the intervention manual. Mock training sessions were conducted in English with site staff role playing as interventionists and partners. Mock training sessions were attended by the co-investigators who critiqued the performance and adapted the intervention when problematic issues arose.
Intervention Implementation
The intervention included one ninety-minute educational and behavioral skills-building session with the partner. The session was conducted one-on-one by site staff trained by the staff who attended the in-person mock training sessions. Partners were provided a prepaid telephone card (value ~$5) for calling the site for additional advice and support and how and when to do so. Of note, the training emphasized that the ultimate responsibility for adherence to the medication remained with the participant.
The mDOT partners were instructed to document the doses they observed on a preprinted form. They were to contact the site by telephone for assistance if the participant missed 3 doses in any 14 day period, if the participant was experiencing adverse effects that they were unable to manage, or if they were concerned with the participant’s behavior with respect to future adherence.
Fidelity to the intervention was assessed by audio taping the first 3 sessions of each partner educator, which were graded by trained site staff. If fidelity was suboptimal, the partner educators were retrained and fidelity reassessed until it met the study standards. The partners of control participants only received basic HIV and health education information which included information about the definition of adherence and its importance 17; intervention staff were explicitly instructed not to implement mDOT training with SOC partners to avoid contamination of the control arm.
mDOT partners could be replaced at the request of the participant or the partner at any time for the first 24 weeks; the newly identified partner received the same training. After 24 weeks, documentation of mDOT was no longer required and telephone cards were no longer dispensed. If requested, mDOT logs were provided beyond 24 weeks.
Randomization and Masking
Participants and partners were assigned 1:1 to the experimental or control group, stratified by screening HIV RNA≥10,000 copies/mL. Study arm was allotted by computer algorithm at the Statistical and Data Analysis Center of the ACTG. The algorithm randomly assigned participants to treatment arm. However, it also tracked the prior assignments to allow for monitoring of the numbers assigned to the two groups at each site. If a pre-set threshold for imbalance was reached at a site, the computer assigned the next participant to the less represented group. Notably, the investigator team and sites were blinded to this algorithm.
Due to the nature of the intervention, it was not possible to mask participants, partners, or site personnel to group assignment. However, no other investigators except the Biostatisticians, had access to group assignment information.
Study Visits
Study staff evaluated participants in both arms at weeks 4, 8, 12, 24, 36, 48 and 52. These visits consisted of clinical and laboratory (complete blood counts, electrolytes, liver and kidney function tests, CD4 counts, and plasma HIV RNA) assessments for disease complications or adverse drug effects, self-reports of adverse experiences, and queries regarding adherence (using the ACTG adherence instrument 18. If non-adherence was reported by participants in either arm, the importance of adherence was reinforced per site standard of care.
Outcomes
The primary efficacy endpoint was confirmed virologic failure at or prior to week 48 based on two successive HIV-1 RNA measurements at least seven days apart that were either: (a) <1 log10 copies/mL below the baseline level and >400 copies/mL at the week 12 HIV-1 RNA evaluation (obtained at least 11 weeks after the date of the randomization) or (b) >400 copies/mL at or after the week 24 HIV-1 RNA evaluation. Participants who died or were lost to follow-up were also considered as failing at the first missing scheduled measurement time at or after week 12.
Secondary endpoints included confirmed virologic failure by week 24, and adherence, assessed using microelectronic monitors (MEMS, Aardex, Zug, Switzerland) on the lopinavir/ritonavir bottle for all doses of both groups. Microelectronic monitors were chosen to measure adherence given their higher sensitivity than other options 19. Adherence was summarized as percent of prescribed doses taken per quarter.
Statistical Considerations and Sample Size
Virologic failure rates were compared between groups using Fisher’s exact test. Comparisons taking into account stratification were carried out using Cochran-Mantel-Haenszel test and stratified log-rank test. Cumulative probability of the primary endpoint was estimated using Kaplan-Meier method. Adherence was categorized as >95%, 90–95%, 80–89.9%, 70–79.9%, and ≤70%13,20,21. Wilcoxon rank sum tests and ordinal regression with generalized estimating equations were used to compare the study groups in terms of magnitude of adherence, averaging across quarters.
We targeted enrollment of 248 participants (and partners) to achieve ~90% power to detect a 20% difference in virologic suppression rates between groups assuming successful suppression in 60% of controls.
Study Monitoring
The study was monitored by a Data and Safety Monitoring Board convened by the National Institute of Allergy and Infectious Diseases. The board met three times over the course of the study. The study was registered on ClinicalTrials.gov (NCT00608569).
Role of the Funding Source
The ACTG sponsored the study and provided advice regarding the study design, data collection, data analysis, data interpretation, and writing of the report. The study was also supported by Abbott Laboratories and Gilead Pharmaceuticals, which provided the medications. The pharmaceutical supporters monitored the development of the protocol and provided input into the design. They also reviewed earlier drafts of the manuscript prior to submission and suggested modifications. The decision to incorporate industry supporters’ suggestions was exclusively the purview of the study team. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Participants
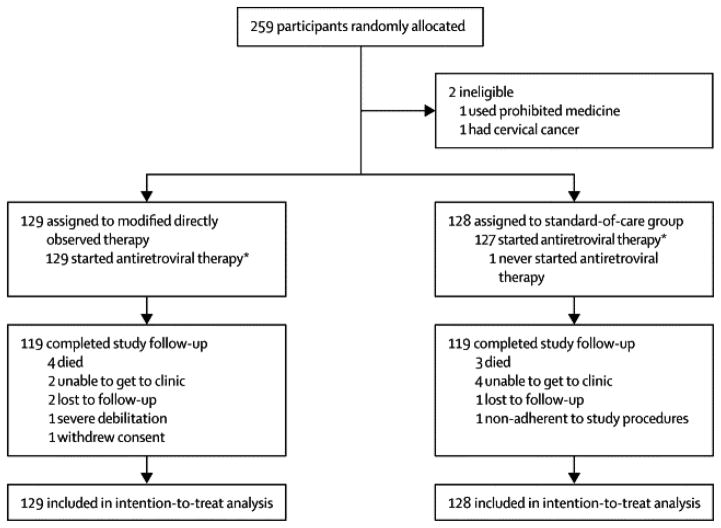
We enrolled 259 participants between April 2009 and September 2011 with 129 randomized to the mDOT group and 130 to the SOC group (see Figure 1). The site with the highest enrollment was Haiti with 73 participants, followed by Uganda (50), Zimbabwe (34) and South Africa (32). Two eligibility violations were reported after randomization with both in the SOC group. They were excluded from the analyses.
Figure 1.
Enrollment was nearly equal between males (51%) and females (49%) with a majority of black race (79%), 20% Latino ethnicity and median age of 38 years (interquartile range, IQR, 33, 45). Baseline characteristics were well balanced between the groups (see Table 1), except for baseline CD4+ cell count. Participants assigned to the SOC group had higher baseline CD4+ cell count with a median 201 cells/mm3 in the SOC group and 164 cells/mm3 in mDOT group although they had slightly lower median nadir CD4+ cell counts and durations of antiretroviral therapy. The failing NNRTI-containing regimen prior to randomization was zidovudine + lamivudine + efavirenz or neviripine for 62% of the participants with 63% in mDOT group and 61% in SOC group and stavudine + lamivudine + efavirenz or nevirapine for 38% with 37% in the mDOT group and 39% in the SOC group.
Table 1.
| Modified directly observed therapy group (n=129) | Standard-of-care group (n=128) | |
|---|---|---|
| Women | 62(48%) | 65(51%) |
| Black race | 101(78%) | 103(80%) |
| Hispanic ethnic origin | 27(21%) | 25(20%) |
| Age (years) | 38(34–44) | 37(33–45) |
| Body-mass index (kg/m2) | 22·1(20·7–24·7) | 22·6(20·1–26·2) |
| Duration of previous regimen (weeks) | 153(82–230) | 144(89–245) |
| Nadir CD4 cell count (cells per mm3) | 122(37–187) | 109(45–202) |
| Baseline CD4 cell count (cells per mm3) | 164(91–250) | 201(97–292) |
| Baseline plasma HIV RNA (log10 copies per mL) | 4·2(3·8–4·9) | 4·3(3·8–4·9) |
Partner Participants
A total of 260 potential partners were enrolled of whom 257 were paired with eligible participants who had been randomized. The majority of partners were male (163, 63%) with a median age of 38 years. The partner relationships to participants at randomization are included in Table 2. Seventy percent of the partners reported living with the participant and 28% reported being HIV-positive.
Table 2.
| Modified directly observed therapy group (n=129) | Standard-of-care group (n=128) | |
|---|---|---|
| Parent | 24(19%) | 33(26%) |
| Spouse | 28(22%) | 27(21%) |
| Sibling | 27(21%) | 23(18%) |
| Friend | 23(18%) | 18(14%) |
| Sexual partner | 7(5%) | 9(7%) |
| Other | 20(16%) | 18(14%) |
Study Status and Compliance with Procedures
Two hundred and thirty-eight participants (93%) completed the planned 52 weeks of study follow-up. A total of seven deaths occurred during study follow-up with four in mDOT group and three in SOC group. The causes of death were: HIV infection or HIV-1 related (n=2), non-HIV diagnosis (n=2), toxicity (n=2) and no information available (n=1). Twelve participants (5%) discontinued study follow-up prior to visit week 52: 6 each from the mDOT and SOC groups. Among the 256 participants who initiated LPV/r, 235 (91%, 117 on the mDOT group and 118 on the SOC group) completed protocol-defined treatment duration of 52 weeks on LPV/r and 21 (8%) (11 on the mDOT group and 10 on the SOC group) discontinued LPV/r prematurely. Among participants randomized to the mDOT group, 98% of participants indicated that their doses were being observed at week 4 and 87% at the week 24 planned end of mDOT. Thirty-one percent of partners continued to provide mDOT through week 52.
Few calls were made to report problems either with partners or with participant adherence to study drugs. Sites were contacted for 12 (10 on mDOT group) out of 252 participants evaluated at week 4, for 4 (3 on mDOT group) out of 249 at week 8, 1 (none on mDOT group) out of 250 at week 12, and 3 (all on mDOT group) out of 248 at week 24. For post week 24 visits, only four participants contacted the sites; two were from each group with three of the contacts at week 48 and one at week 52. In total, the reason for eight of the contacts were for participants to notify sites of a change in partner with seven of those in the mDOT group and one in the SOC group. Six of the seven mDOT partners were changed prior to week 24 and new partners were trained. The one SOC partner that was changed was at enrollment.
Primary Endpoint
Fifty-seven (22%) participants reached the primary endpoint by week 48 (34/129 (26%) in the mDOT group and 23/128 (18%) in the SOC group), among which, there were 47 virologic failures (27 in the mDOT group and 20 in the SOC group), five premature discontinuations (four in the mDOT group and one in the SOC group) and five deaths (three in the mDOT group and two in the SOC group). There was no significant difference in the primary endpoint rate by week 48 between the two strategy groups (p=0.13). Although more failures occurred in the higher screening RNA stratum (44/164 [26.8%] vs. 13/93 [14.0%]), there was no difference between the treatment strategies after adjusting for the screening HIV-1 RNA (p=0.11). The Kaplan-Meier estimated cumulative probability of the primary endpoint by week 48 was 25.1% in the mDOT group and 17.3% in the SOC group, for a weighted difference in SOC versus mDOT of −6.6% with a 95% confidence interval (CI) of (− 16.5%, 3.2%), p=0.19.
Secondary Endpoints
Virologic Failure by Week 24
By week 24, a total of 41 participants (16%), (24 in the mDOT group and 17 in the SOC group) experienced a primary endpoint, with 34 virologic failures (19 in the mDOT group and 15 in the SOC group), four premature study discontinuations (three in the mDOT group and one in the SOC group) and three deaths (two in the mDOT group and one in the SOC group). There was no significant difference observed between the two strategy groups in primary endpoint rate at or prior to week 24 (p=0.31).
Virologic Suppression
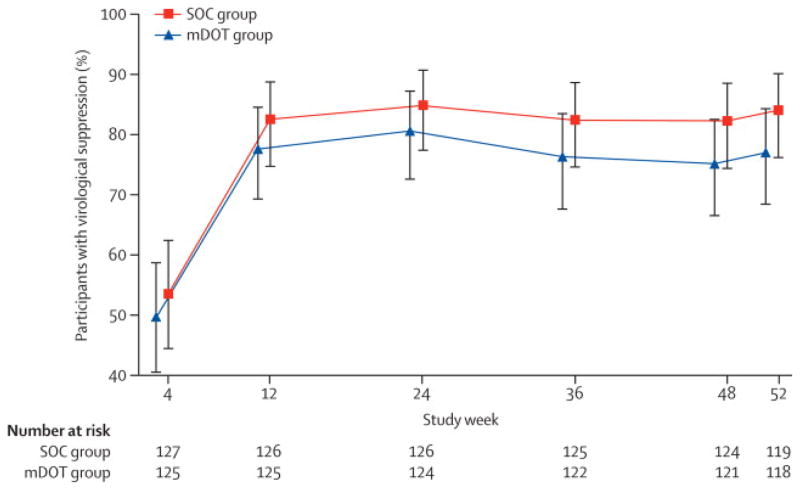
Figure 2 displays the proportion of individuals in each arm with plasma HIV RNA ≤400 copies/mL over time using intent-to-treat approach (missing data treated as failure). At all time points after week 12, the proportion with undetectable HIV RNA in the SOC group exceeded that of the mDOT group, but at all of the time points, the 95% confidence intervals overlapped. At week 48, 75% (95% CI: 67%, 83%) of mDOT and 82% (74%, 89%) of SOC subjects had HIV RNA ≤ 400 copies/mL. There were no significant differences between the groups at week 24 (p=0.37) or week 48 (p=0.18).
Figure 2.
Adherence
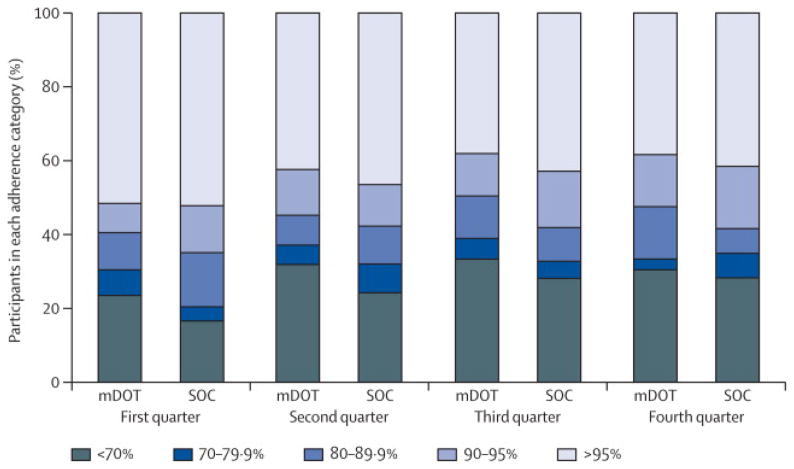
Adherence was similarly high [Q1: 95% vs. 96% p=0.38, Q2: 91% vs. 94% p=0.40, Q3: 90% vs. 93% p=0.17, Q4: 90% vs. 93% p=0.36] in the mDOT and SOC groups, respectively. Figure 3 displays the adherence categories by study group per quarter. In the ordinal regression models, the association between the intervention and adherence category (estimated odds ratio) was 1.21 (95% CI: 0.80 to 1.83) indicating a non-significant difference in adherence between study groups.
Figure 3.
Safety
A total of 36 participants (14%, 21 on mDOT and 15 on SOC) reported at least one grade 3 or higher sign or symptom event or laboratory abnormality. The most commonly reported sign/symptom was ache/pain/discomfort (n=8), followed by fever (n=5). One participant (mDOT group) had grade 4 cachexia/wasting/weight loss and one participant (SOC group) reported a grade 4 difficulty breathing/dyspnea/shortness of breath. The most commonly reported laboratory abnormality was low neutrophil count (n=5), followed by low hemoglobin (n=3).
One hundred and five participants (41%) reported a total of 177 new diagnoses after randomization (48 reported 84 new diagnoses on the mDOT group and 57 reported 93 new diagnoses on the SOC group). The most commonly reported diagnoses were renal system disease/disorder (18 cases, seven on mDOT and 11 on SOC), diarrhea - (17 cases, nine on mDOT and eight on SOC) and eye, ear, nose disease (12 cases, eight on mDOT and four on SOC). A total of nine pregnancies from eight participants were reported on study (two on mDOT and seven from six participants on SOC).
Discussion
Both the partner-based intervention group and SOC group had high rates of treatment success and high rates of medication adherence. We found no evidence of a difference in virologic suppression at week 48, or evidence to suggest difference in other virologic or adherence endpoints between the intervention and SOC. The lack of effect of this intervention is similar to that seen for other attempts to improve ART adherence using different approaches to DOT 22, including in a resource constrained setting23.
There are several possible explanations for the lack of significant differences in the intervention versus SOC group. The participants, irrespective of group, had sufficient social supports to include a partner in their care, were more engaged with their study site, had more clinic visits than usual patients, and met with skilled study nurses trained to counsel against non-adherence which may have helped prevent adherence issues equally in both groups. Or, the lack of readily available third-line therapy in these settings in the event of second-line failure might have been motivating. It is possible that the single session of adherence education was insufficient to provide partners with additional skills to help the participants or to build sufficient rapport between the partner and site staff as problems arose. We did not assess whether the partners understood how to implement our intervention to see if the training was sufficient. Another potential explanation for our findings includes self-selection for participation by participants likely to be able and willing to adhere to procedures, thereby reducing the likelihood of non-adherence overall and making it impossible to demonstrate an effect of any intervention. Although most of the partners carried out the mDOT procedures, few of them contacted the site for help. This may have been due to a lack of a perceived need for extra services or a distrust of the healthcare providers to help rather than scold the participants for non-adherence.24
This study had both limitations and strengths. Although it was conducted in resource-constrained settings, the sites had more services available than many clinical care facilities in their respective countries and had high levels of adherence in the control group, potentially limiting generalizability. However, if the partner-based intervention were effective over and above this extra attention, we would have expected to observe some magnitude of benefit. Another limitation is that the sample size did not permit formal evaluation of the effects of the intervention on subgroups (e.g., by partner type or whether the partner was HIV infected). Additionally, we cannot comment on the potential effect of this intervention were it rolled out outside of a trial to patients who chose not to participate because it was a trial. Yet, we think it is unlikely that patients who were not interested in participating would have had a better outcome with the intervention than the already high success rate observed. One key strength was the measurement of adherence using microelectronic monitors. While these devices may have some effect in improving adherence by themselves (i.e., a Hawthorne effect), prior studies have demonstrated that even if present, the effect is likely to be small and short-lived25. In addition, we monitored fidelity to the training provided to study participants, thus maximizing the likelihood that the study provided a standardized intervention.
Although the intervention was not successful, the findings are encouraging for the potential for achieving high rates of virologic success in patients with a history of virologic failure in a resource-constrained setting who bring a partner to their clinic visit for initiation of second line therapy. Since the rates of response exceed those expected for second line therapy, we believe the structure of the clinical trial itself may have benefitted these patients. Potentially beneficial components include the frequent study visits, reminders of the importance of adherence, and supportive counseling and advice by the study staff. However, we are unable to determine which components were most impactful.
In conclusion, a single 90-minute session of training with partner-based direct observation and enhanced linkage to the study site did not result in improved HIV treatment outcomes in patients with prior treatment failure over and above study participation and the clinic’s standard of care. Caution is warranted against implementing interventions that are superficially appealing without the type of rigorous testing we have undertaken. Further development of targeted scalable adherence interventions and access to second-line and later therapies are needed for patients failing ART worldwide. Future trials should further attempt to avoid selecting populations destined to be adherent without additional assistance and to minimize potential adherence improving effects of the study structure itself while maintaining rigor in evaluating the intervention.
Acknowledgments
Funding: AIDS Clinical Trials Group, NIAID, NIH
The authors wish to thank the study participants for their contributions. We would also like to acknowledge the following individual sites’ grant support, study team members, and site personnel:
Drs. Valerie Francois and Samuel Pierre - Les Centres GHESKIO CRS (Site 30022) ACTG CTU Grant
Dr. Michael Ssemmanda and Haspha Nassolo – Joint Clinical Research Centre CRS (Site 12401) ACTG CTU Grant U01- A1069501
Drs. Wadzanai Samaneka and James G Hakim - Parirenyatwa CRS (Site 30313) ACTG CTU Grant UM 1AI069436
Drs. Mohammed Rassool and Pauline Vunandlala - Wits HIV CRS (Site 11101) ACTG CTU Grant AI069463
Jorge Sanchez, MD, MPH and Fanny Rosas, RN - Barranco CRS (Site 11301) ACTG CTU Grant 2UM1AI069438-08
Rosa Infante, MD and Fanny Garcia, RN - San Miguel CRS (Site 11302) ACTG CTU Grant AI069438
Drs. Elizabeth Stringer and Margaret Kasaro - Kalingalinga Clinic CRS (Site 12801) ACTG CTU Grant 7UMIA1069455
Mpho Shakes and Lesedi Tirelo - Gaborone Prevention/Treatment Trials CRS (Site 12701) ACTG CTU Grant 2UMIAI069456-08; CFDA Grant 93.865
Drs. Brenda Hoagland and Isabel Tavares -Instituto de Pesquisa Clinica Evandro Chagas (IPEC) CRS (Site 12101) ACTG CTU Grant AI069476
Footnotes
Trial Registration: ClinicalTrials.gov NCT00608569
Declaration of Interests
We declare that we have no conflicts of interest related to this work.
Contributors Statements
Robert Gross: conception and design of the work, analysis and interpretation of data, drafting manuscript
Lu Zheng: analysis and interpretation of data, revising manuscript for important intellectual content
Alberto La Rosa: conception and design of the work, interpretation of data, revising manuscript for important intellectual content
Xin Sun: analysis and interpretation of data, revising manuscript for important intellectual content
Susan L. Rosenkranz: analysis and interpretation of data, revising manuscript for important intellectual content
Sandra Wagner Cardoso: conception and design of the work, interpretation of data, revising manuscript for important intellectual content
Francis Ssali: conception and design of the work, acquisition and interpretation of data, revising manuscript for important intellectual content
Rob Camp: conception and design of the work, interpretation of data, revising manuscript for important intellectual content
Catherine Godfrey: conception and design of the work, interpretation of data, revising manuscript for important intellectual content
Susan E. Cohn: conception and design of the work, interpretation of data, revising manuscript for important intellectual content
Gregory K. Robbins: conception and design of the work, interpretation of data, revising manuscript for important intellectual content
Anthony Chisada: acquisition and interpretation of data, revising manuscript for important intellectual content
Carole L. Wallis: interpretation of data, revising manuscript for important intellectual content
Nancy R. Reynolds: conception and design of the work, interpretation of data, revising manuscript for important intellectual content
Darlene Lu: analysis and interpretation of data, revising manuscript for important intellectual content
Steven Safren: conception and design of the work, revising manuscript for important intellectual content
Lara Hosey: design of the work, interpretation of data, revising manuscript for important intellectual content
Patrice Severe: conception and design of the work, interpretation of data, revising manuscript for important intellectual content
Ann C. Collier: conception and design of the work, interpretation of data, revising manuscript for important intellectual content
All authors gave final approval of this version and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial Disclosure:
The project described was supported by Award Numbers UM1AI068636, UM1AI069434, UM1AI069481, and UM1AI068634 from the National Institute of Allergy and Infectious Diseases and supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson LF, Mossong J, Dorrington RE, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10(4):e1001418. doi: 10.1371/journal.pmed.1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006 Aug 9;296(6):679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 3.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Int Med. 2007 Apr 17;146(8):564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bisson GP, Rowh A, Weinstein R, Gaolathe T, Frank I, Gross R. Antiretroviral failure despite high levels of adherence: discordant adherence-response relationship in Botswana. J Acquir Immune Defic Syndr. 2008 Sep 1;49(1):107–110. doi: 10.1097/QAI.0b013e3181820141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002 Jul 13;360(9327):119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 6.Keiser O, Tweya H, Braitstein P, et al. Mortality after failure of antiretroviral therapy in sub-Saharan Africa. TM & IH. 2010 Feb;15(2):251–258. doi: 10.1111/j.1365-3156.2009.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockman S, Hughes M, Sawe F, et al. Nevirapine- versus lopinavir/ritonavir-based initial therapy for HIV-1 infection among women in Africa: a randomized trial. PLoS Med. 2012;9(6):e1001236. doi: 10.1371/journal.pmed.1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009 Jun 1;23(9):1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R, Hill A, Sawyer AW, Pillay D. Emergence of drug resistance in HIV type 1-infected patients after receipt of first-line highly active antiretroviral therapy: a systematic review of clinical trials. Clin Infect Dis. 2008 Sep 1;47(5):712–722. doi: 10.1086/590943. [DOI] [PubMed] [Google Scholar]
- 10.Boyd MA, Cooper DA. Second-line combination antiretroviral therapy in resource-limited settings: facing the challenges through clinical research. AIDS. 2007 Jul;21( Suppl 4):S55–63. doi: 10.1097/01.aids.0000279707.01557.b2. [DOI] [PubMed] [Google Scholar]
- 11.Simoni JM, Amico KR, Pearson CR, Malow R. Strategies for promoting adherence to antiretroviral therapy: a review of the literature. Curr Inf Dis Rep. 2008 Nov;10(6):515–521. doi: 10.1007/s11908-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010 Nov 27;376(9755):1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 13.Gross R, Bellamy SL, Chapman J, et al. Managed problem solving for antiretroviral therapy adherence: a randomized trial. JAMA IM. 2013 Feb 25;173(4):300–306. doi: 10.1001/jamainternmed.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2007;(4):CD003343. doi: 10.1002/14651858.CD003343.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007 Sep 15;45(6):770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware NC, Idoko J, Kaaya S, et al. Explaining adherence success in sub-Saharan Africa: an ethnographic study. PLoS Med. 2009 Jan 27;6(1):e11. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Center F-XB. About HIV: Rutgers School of Nursing. 2006 [Google Scholar]
- 18.Reynolds NR, Sun J, Nagaraja HN, Gifford AL, Wu AW, Chesney MA. Optimizing measurement of self-reported adherence with the ACTG Adherence Questionnaire: a cross-protocol analysis. J Acquir Immune Defic Syndr. 2007 Dec 1;46(4):402–409. doi: 10.1097/qai.0b013e318158a44f. [DOI] [PubMed] [Google Scholar]
- 19.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999 Jun;21(6):1074–1090. doi: 10.1016/S0149-2918(99)80026-5. discussion 1073. [DOI] [PubMed] [Google Scholar]
- 20.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Int Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 21.Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001 Nov 9;15(16):2109–2117. doi: 10.1097/00002030-200111090-00006. [DOI] [PubMed] [Google Scholar]
- 22.Ford N, Nachega JB, Engel ME, Mills EJ. Directly observed antiretroviral therapy: a systematic review and meta-analysis of randomised clinical trials. Lancet. 2009 Dec 19;374(9707):2064–2071. doi: 10.1016/S0140-6736(09)61671-8. [DOI] [PubMed] [Google Scholar]
- 23.Nachega JB, Chaisson RE, Goliath R, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 2010 Jun 1;24(9):1273–1280. doi: 10.1097/QAD.0b013e328339e20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laws MB, Beach MC, Lee Y, et al. Provider-patient adherence dialogue in HIV care: results of a multisite study. AIDS Behav. 2013 Jan;17(1):148–159. doi: 10.1007/s10461-012-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deschamps AE, Van Wijngaerden E, Denhaerynck K, De Geest S, Vandamme AM. Use of electronic monitoring induces a 40-day intervention effect in HIV patients. J Acquir Immune Defic Syndr. 2006 Oct 1;43(2):247–248. doi: 10.1097/01.qai.0000246034.86135.89. [DOI] [PubMed] [Google Scholar]