Abstract
The dysregulation of glycogen synthase kinase-3 (GSK3) has been implicated in Alzheimer disease (AD) pathogenesis and in Aβ-induced neurotoxicity, leading us to investigate it as a therapeutic target in an intracerebroventricular Aβ infusion model. Infusion of a specific GSK3 inhibitor SB216763 (SB) reduced a downstream target, phospho-glycogen synthase 39%, and increased glycogen levels 44%, suggesting effective inhibition of enzyme activity. Compared to vehicle, Aβ increased GSK3 activity, and was associated with elevations in levels of ptau, caspase-3, the tau kinase phospho-c-jun N-terminal kinase (pJNK), neuronal DNA fragmentation, and gliosis. Co-infusion of SB corrected all responses to Aβ infusion except the induction of gliosis and behavioral deficits in the Morris water maze. Nevertheless, SB alone was associated with induction of neurodegenerative markers and behavioral deficits. These data support a role for GSK3 hyperactivation in AD pathogenesis, but emphasize the importance of developing inhibitors that do not suppress constitutive activity.
Keywords: GSK3, c-jun N-terminal kinase, Beta-amyloid, Tau, Neuron damage, Alzheimer's
Introduction
Glycogen synthase kinase-3 (GSK3α/β) is a constitutively active, ubiquitous serine/threonine kinase abundant in the central nervous system (CNS, (Woodgett, 1990)). It phosphorylates a broad range of substrates including β-catenin (Rubinfeld et al, 1996), the immediate early gene c-Jun (Boyle et al., 1991), the adaptor protein for trophic factor signalling, IRS-1 (Eldar-Finkelman and Krebs, 1997), the cytoskeletal protein tau (Hong et al., 1997; Takashima et al., 1993) and glycogen synthase (Beurel and Jope, 2006). Its regulation is similarly complex, being activated, by several factors, including intracellular calcium (Hartigan and Johnson, 1999), the tyrosine kinases ZAK1 (Kim et al., 1999) and Fyn (Lesort et al., 1999) (as reviewed by Forde and Dale (2007)). Its inhibition is mediated primarily by PI3K-Akt via serine phosphorylation (Ueki et al., 1998) or by Wnt signaling (Siegfried et al., 1992).
Although the general consensus is that aberrant over-expression of GSK3 promotes cell death (Carmichael et al., 2002; Jin et al., 2005; Maggirwar et al., 1999; Pap and Cooper, 1998; Tong et al., 2001) and inhibits cell proliferation (Sato et al., 2004; Tseng et al., 2006), emerging studies indicate that its relatively high constitutive activity is critical for neuron function (Hoeflich et al., 2000; Ougolkov et al., 2007; Takada et al., 2004). Two recent studies in transgenic mice supported this hypothesis that constitutive GSK3 is important for neuron viability (Gomez-Sintes et al., 2007) and for long-term depression (LTD), an electrophysiological measurement related to memory consolidation (Peineau et al., 2007).
GSK3 dysregulation is thought to contribute to pathogenesis not only in inflammatory diseases, mood disorders, diabetes and some cancers, but also in neurodegenerative diseases, notably AD (reviewed by Jope et al. (2007)). AD, a global cognitive disorder, is associated with neuron and synaptic loss, the accumulation of amyloid β (Aβ) and phosphorylated tau and neuroinflammation. GSK3 hyperactivation is implicated in AD because it can increase Aβ production and toxicity (Akiyama et al., 2005; Bayatti et al., 2003; Ryder et al., 2003; Su et al., 2004), neuroinflammation (Jope et al., 2007; Lipton, 2007) and tau phosphorylation (Hong et al., 1997; Takashima et al., 1993). Disruptions in insulin signaling, which commonly occur in AD and in insulin-resistant diabetes (Craft, 2007) and in AD models (Ho et al., 2004) can lead to GSK3 hyper-activation. Similarly increases in cyclooxygenase-2, another enzyme implicated in AD, are implicated in GSK3 hyperactivation in AD (Qin et al., 2006). Since GSK3 may have potential as a therapeutic target for AD, clinical trials with the Food and Drug Administration-(FDA) approved drug valproate, which is a non-specific inhibitor of GSK3, are underway (Loy and Tariot, 2002).
Since the FDA-approved GSK3 inhibitors lithium and valproate are non-specific, the effects in trials would not reveal target-specificity. The anti-convulsant valproate also increases GABA sensitivity while lithium is also a non-competitive inositol monophosphatase inhibitor. Because of the limited in vivo data on specific GSK3 inhibitors in AD models, we chose to investigate the impact of a highly specific GSK3 inhibitor on amyloid oligomer-induced neurodegenerative markers and behavior in aged rats.
Materials and methods
Animals, surgery and sacrifice
Surgical and animal procedures were carried out with adherence to the current guidelines set out in the NIH Guide for the Care and Use of Laboratory Animals and by the Association for Assessment and Accreditation of Laboratory Animal Care International (AALAC)-accredited VA-Greater Los Angeles Healthcare System. Animal procedures were approved by the VA Institutional Animal Care and Use Committee (IACUC), Institutional Biosafety (IBC) and Research and Development (R&D) Committees.
Sprague–Dawley rats (19 months, n=7–11 per group) were used for bilateral intracerebroventricular (icv) infusion of Aβ42 oligomer (Frautschy et al., 2001) and/or SB (SB216763 (3-[2,4-Dichlorophenyl]- 4-[1-methyl-1H-indol-3-yl]-1H-pyrrole-2,5-dione), Tocris Cookson Inc. Ellisville, MO), a highly selective and cell permeable GSK3 inhibitor. Following anesthesia with 3% isoflurane (oxygen 2 L/min), the double stainless steel cannula unit (Plastic One, 3280PD, Roanoke, VA, 3 mm spacing) was stereotaxically implanted into the left and right ventricle to allow bilateral delivery (0.26 mm posterior to Bregma, 4mm depth ventral to cranium). Each cannula was connected to its own mini-osmotic Alzet pump (model 1004); Durect Corporation, CA), one for each side. The pumps were implanted subcutaneously on the dorsal back. Pumps contained Aβ42 oligomer solution (releasing 50 ng/hour) and human HDL (releasing 37 µg/h, Calbiochem, CA) to stabilize oligomers during duration of study as previously described (Begum et al., 2008b; Craft et al., 2004; Frautschy et al., 2001). The infusion duration was for one month. Control animals were infused with HDL alone. GSK3 inhibitor SB solution (11 µM, releasing 78 pmol per day to maintain approximately 40 nM in the CSF in vivo) was infused either alone (with HDL) or co-infused with Aβ42/HDL. SB is a highly selective and cell permeable GSK3 inhibitor acting by ATP-binding competition, and the choice of dose for icv infusion was based on in vitro studies to prevent toxicity and without non-specific inhibition of other kinases such as CDk1, PKA, PKC, MAPK (Coghlan et al., 2000; Meijer et al., 2004). Since in vitro IC50 and in vivo IC50 may be different, we also assessed impact of SB infusion on GSK3 activity in vivo by measuring phospho-glycogen synthase and glycogen levels.
After one-month of water maze testing, rats were subsequently anesthetized with pentobarbital (100 mg/kg) and perfused with non-fixative HEPES-saline buffer containing protease inhibitors and phosphatase inhibitors as described (Lim et al., 2000). Half the brain was immersion-fixed in 4% paraformaldehyde and paraffin-embedded, and the remaining half dissected and snap-frozen, using liquid nitrogen, for biochemical analysis.
Aβ oligomer preparation and characterization
For in vivo pump infusion and in vitro experiments, Aβ oligomers were prepared according to Kayed et al. (2003). This procedure briefly utilizes double-lyophilized synthetic Aβ (1–42) peptide, which is then monomerized by solubilization in (1,1,1,3,3,3-hexafluoro-2-propanol (HFIP). Fibril formation was minimized by adding sterilized water (to achieve 315 µg/ml), prior to subsequent evaporation of HFIP. The aqueous Aβ solution is then continuously stirred (600 rpm) for 48 h at room temperature (25 °C). Two minor modifications were made to adapt the protocol for in vivo infusions. First, HDL is added after HFIP evaporation during the 48 h of stirring, since adding HDL after oligomers have formed prevents Aβ toxicity (Begum et al., 2008b). The second modification for pump oligomer preparation was that 1 mM NaHCO3 buffer was added after aggregation (stirring) step at a basic pH (pH 10) to maintain pH and oligomeric stability during infusion.
Aβ42 oligomers (C.G. Glabe, UC Irvine) were assessed by standard immunoblotting techniques using a 10–20% gradient tris-tricine gel (VWR Int.). Major oligomeric bands observed in the soluble fraction included dimer, trimer, 12-mer. Minor bands were 24-mer and 56-mer, and there was a high molecular weight band >250 kDa in the stacking gel (Fig. 1a). The pump preparation was also evaluated by dot blot, using polyclonal rabbit antibody A11 to confirm the presence of conformationally-specific high molecular weight oligomers as previously described (Yang et al., 2005). Pump contents were run on a Western for Aβ42 oligomers to evaluate the impact of SB on Aβ42 aggregation during infusion period, since this non-quantitative method was effective in predicting impact of drugs on aggregation using quantitative methods (Begum et al., 2008a; Yang et al., 2005). No noticeable impact on distribution of oligomers was observed (Supplementary Fig. 1).
Figure 1.
Viability and morphology of primary hippocampal neurons after GSK3 inhibitor and/or Aβ42. (a) Western blot of Aβ oligomers preparation using monoclonal antibody 6E10. (b–e) Lactodehydrogenase (LDH) in media was measured and used to assess toxicity. Values are shown as percent control (vehicle). (b) LDH values after two doses of Aβ at 24 h (c) Protective impact of SB on Aβ toxicity at 24 h if SB was applied during (Co, p=0.03) or 30 min prior to (Pre, p=0.003) addition of Aβ42 oligomer, but not if added 30 min after Aβ42 oligomer (Post). (d) Dose-dependent effect of SB216763 (SB) on LDH levels at 48 h. (e) Effects of two GSK3 inhibitors on LDH levels at 48 h. GSK3 inhibitors cause no toxicity at 24 h incubation at these doses (not shown). Each value represents the mean±SEM (n=3 wells), and is representative of 3 different experiments. (f) Representative micrographs of treatment effects on triple fluorescence staining of primary hippocampal neurons and their processes by anti-Tau5 as an axonal label (green), anti-MAP-2 as dendritic label (red) and nuclear dye (DAPI, blue) after 24 h treatment. White arrowheads show neurite fragmentation. Bar=15 µm.
Morris water maze
Water maze studies were as previously described (Frautschy et al., 2001) with the following modifications. Briefly, rats were first trained to find a randomly positioned visible platform in a rat pool (183 cm diameter swimming pool, maintained at 22–24 °C) for 4 blocks (4 consecutive swim trials per block, 1 block per day). Then rats were trained to find a hidden platform for 4 blocks at one position. A second acquisition study was performed at a new platform position for six blocks to examine working memory. Retention was assessed after both acquisition studies by a probe (removal of platform, and allowing rat to swim for 60 s). Time and path in zones and quadrants, swimming paths, speed, and latencies were recorded by video tracking and digital images were analyzed by water maze software (HVS image, United Kingdom).
Quantitative western blotting
Brain tissue homogenates were sonicated in PBS lysis buffer with a protease inhibitor cocktail as we previously described (Ma et al., 2006). The samples were centrifuged at 55,000 rpm for 30 min, and the protein lysate supernatants were collected and stored at −80 °C for further analysis. The protein concentration of the supernatant was measured using the Bio-Rad (Hercules, CA) DC protein assay, and equal amounts of protein were loaded per lane (25 µg). The electrophoresed proteins were then transferred to Immobilon-P PVDF membrane. For GSK3 activity, the proteins were immunoblotted with rabbit polyclonal pGSK3βser9 (Cell Signaling, Danvers, MA), or pGSK3α/βtyr279/216, or total GSK3α/β (Biosource International, Camarillo, CA), both at dilutions 1:1000. All values were normalized to total GSK3α/β. To detect changes in glial proteins, the supernatant was also analyzed with a cocktail of antibodies as previously described (Calon et al., 2004) using mouse anti-GFAP (1:1000, Sigma, St. Louis MO) and normalizing to mouse anti-actin (1:2000, Chemicon International, Temecula, CA). Secondary antibodies (peroxidase labeled anti-rabbit or anti-mouse, Pierce, Rockford, IL) were incubated for 1 h at 1:50,000 dilutions. Immunoreactive bands were visualized by enhanced chemiluminescent detection (ECL, Pierce, Rockford, IL) by exposure to X-ray film (Fuji Medical X-ray film) and then quantified by densitometric software (Molecular Analyst II, Bio-rad, Hercules, CA) as we have described previously (Calon et al., 2004; Frautschy et al., 2001; Ma et al., 2006).
Measurement of GSK3 inhibition by SB in vivo
Since SB is an ATP competitive GSK3 inhibitor, its impact on GSK3 inhibition is not effectively represented by changes in phospho-GSK3. Therefore we examined a downstream target phospho-glycogen synthase (Cell Signaling, Danvers MA, 1:1000 dilution) by Western analysis in the hippocampus. Besides examining glycogen histologically, we also quantified glycogen levels in dissected brain (thalamus), using methods previously described (Chun and Yin, 1998). Briefly, tissue was dissolved in 30% potassium hydroxide (KOH) and heated at 100 °C for 10 min. Anhydrous ethanol was added, and samples were centrifuged at 5700 rpm for 15 min. The pellet was re-suspended in 0.5 ml of H2O. Freshly made anthrone reagent (1 ml 0.2% in H2SO4) was added and mixed. The mixture was incubated at room temperature for 30 min, and measured at 650 nm with a microplate reader (Precision microplate reader, Emax). A standard curve was setup by dissolving glucose from 1.6 to 100 µg/ml (1.6 µg of glucose is equivalent to 1.44 µg of glycogen).
Histology and immunochemistry
Immunohistochemistry and its quantitation were performed as we have previously described (Calon et al., 2004; Frautschy et al., 2001; Lim et al., 2000; Ma et al., 2007; Zhao et al., 2006). In this study, three continuous 8 µM thick paraffin embedded sections mounted onto gelatin and polylysine coated slides were chosen at three distances from cannulas (adjacent, 1 mm posterior and 2 mm posterior). After removing paraffin, sections were steamed in citrate buffer antigen unmasking solution for 1 h, and endogenous peroxidase activity quenched with 0.3% hydrogen peroxide for 30 min. Sections were first blocked with 5% normal serum and then incubated with primary antibodies in 3% bovine serum albumin at 4 °C for 24 h. Primary antibodies used were: mouse anti-tau-1 (1:200, Cedarlane labs, Ontario, Canada), rabbit anti-pGSK3α/βtyr279/216 (1:100, Biosource International, Camarillo, CA), rabbit anti-active caspase-3 (1:600, Sigma), rabbit anti-MAP-2 (1:800, Sigma), mouse anti-GFAP (1:10,000, Sigma, St. Louis, MO), pJNK (1:200, Cell Signaling) mouse anti-ptauSer202 (CP13, Peter Davies, Albert Einstein College, (Ishizawa et al., 2003) or microglial antigen rabbit anti-Iba1 (1:200, Wako, Richmond, VA). After washing in PBS, sections were then incubated with secondary antibodies against appropriate species, conjugated with horseradish peroxidase (1:1200, HRP, Vector Laboratories, Burlingame, CA), which was detected with metal-enhanced 3,3′-diaminobenzidine tetrahydrochloride (Pierce Chemicals, Rockford, IL). Adjacent slides, which were treated with the same immunohistochemical methods except in the absence of primary antibody, were evaluated as negative controls.
Neuronal damage was also detected with Cresyl Violet. The fluorochrome FluoroJade (Histo-Chem Inc. Jefferson, AR) was used as another method of detecting neuron damage, and performed according to the manufacturer's instructions, except that sections were steamed for 1 h and 20 min, which was required to eliminate background staining in controls.
DNA fragmentation was assessed using TUNEL assay (Calbiochem, San Diego, CA) according to the manufacturer's instructions with the following modifications. We used Pierce DAB instead of the manufacturer's DAB, steamed tissue for 60 min with antigen unmasking citrate buffer (Vector), incubated with proteinase K at 37 °C for 3 min (20 µg/ ml), and quenched endogenous peroxidase for 35 min with 0.6% H2O2.
Quantitative image analysis was performed using routine procedures in our laboratory as previously described (Calon et al., 2004; Frautschy et al., 2001; Lim et al., 2000; Ma et al., 2007; Zhao et al., 2006). Briefly, microscopic images were captured and analyzed with NIH-Image public domain software (http://rsb.info.nih.gov/nih-image) to assess particle size, density (particles/0.1 mm2) and intensity (rel OD, compared to background). Multiple images at high magnification were combined for quantification of staining in hippocampal layers for each Bregma analyzed. Microscopic lighting and density slice thresholding were maintained constant for each immunostaining analyzed. Immunohistochemistry on all sections for all treatments was performed together, and sequence of image analysis of slides was randomized to ensure that treatment effects were not due to unforeseen variations in room lighting. After analysis of coded treatments, treatments were unblinded.
Primary hippocampal neuron culture
The methods for primary culture of rat hippocampal neurons studies were as we previously described (Zhao et al., 2004). Briefly, cells were diluted in Neurobasal medium (NBM) supplemented with B27 components (GibcoBRL, Grand Island, NY) and plated onto poly-d-lysine (Sigma, St. Louis, MO, 50 µg/ml) and laminin (1 µg/ml, GibcoBRL, Grand Island, NY) coated 24 well plates at a density of 1.1 × 105 cells per well. Neurons were maintained at 37 °C in a 5% CO2 atmosphere for 10 to 12 days prior to treatment. SB (0.5 µM) and a GSK3β inhibitor (Inhibitor 1, Inh 1, also known as 4-Benzyl-2-methyl-1, 2,4-thiadiazolidine-3,5-dione or TDZD-8, 10 µM, Calbiochem, San Diego, CA) were dissolved in dimethyl sulfoxide (DMSO, final concentration 0.1%) and added into neuron cultures. Pre-made Aβ42 oligomer (0.5 or 500 nM) or GSK3 inhibitors were dissolved into NBM without B27 and added to neuron cultures.
Neurodegeneration (neuron damage) was assessed by morphological changes including loss of neurites in MAP-2 (1:800, Sigma, St Louis, MO) or tau5 (1:500, Chemicon, Temecula, CA), stained cells, and condensed chromatin by DAPI staining. Neurotoxicity was also quantified by lactate dehydrogenase (LDH) assay of media using CytoTox96 Non-radioactive cytotoxicity assay kit (Promega, Madison, WI).
Statistical analysis
Statistical analysis was performed using JMP software (SAS Institute). Immunocytochemical analysis was performed using 2 × 2 ANOVA (treatment × region), and differences between treatments were determined by Fisher's post hoc analysis. Behavior analysis was performed using linear regression analysis (Block × Path) and 2 × 2 ANOVA (Block × Treatment). When Bartlett's tests showed lack of homogeneity, log transformation was used to analyze data (distance acquisition). Zone behavioral data, biochemistry and LDH toxicity assays were all analyzed using 1 × 1 ANOVA.
Results
GSK3 inhibition protected against acute Aβ toxicity in primary hippocampal neuronal culture
In our primary hippocampal neuronal cultures, Aβ oligomers (Fig. 1a) were acutely toxic to primary hippocampal neurons at 24 h at two different doses (Fig. 1b). Using this model we show that GSK3 inhibition, using the highly specific GSK3 inhibitor SB, ameliorated Aβ toxicity if co-administered throughout or administered 30 min prior to Aβ, but was not protective if given 30 min after application of Aβ to the neurons (Fig. 1c). Since over-inhibition of GSK3 may impair viability based on several studies (Hoeflich et al., 2000; Ougolkov et al., 2007; Takada et al., 2004), we then investigated the dose–response impact on viability. We saw no impact on viability at 24 h (not shown), but with prolonged incubation (48 h), a dose–response curve showed that levels at 0.1 µM or above increased LDH levels by 60% (Fig. 1d). These results suggested that below the reported in vitro IC50 for GSK3α (34 nM), SB was not toxic, but near the IC50 for GSK3β (75 nM), it was exerting some toxicity after prolonged incubation. To ensure that the toxicity was dependent on specificity of the drug for GSK3, we also examined a non-ATP site GSK3-specific inhibitor I (Inh 1, TDZD, IC502 µM). Results revealed that this drug caused similar toxicity at the 48 h timepoint (Fig. 1e). Toxicity of 500 nM SB was confirmed by immunohistochemical staining (Fig 1f). SB treatment alone caused reduction in the axonal network (Tau5) but did not modify MAP-2-ir dendrites, while the impact of Aβ was more severe, causing elimination of both axons and most dendrites (Fig. 1f). Fields showed that many DAPI stained neuronal nuclei were present in all fields, so the apparent elimination of axons and dendrites was not just occurring because of cell loss.
Aβ increased GSK3 activity in vivo
In order to demonstrate that the Aβ infusion model was one of GSK3 hyperactivation, we first measured levels of the phosphoepitopes GSK3αtyr279/βtyr216 (pGSK3α/β, active form) and GSK3βser9 (pGSK3β/ser9, inactive from) in the detergent-extracted hippocampal homogenates by Western analysis and by immunohistochemical (IHC) staining as previously described (Ma et al., 2006) (Fig. 2). By Western, data showed that Aβ reduced pGSK3βser9 (inactive form, Fig. 2a, top 47 kDa) about 55% (p=0.04) (Fig. 2a), with non significant trend for increase in the pGSK3α/β subunits (active form, Fig. 2a, lower 51/47 kDa). For quantitation, pGSK3 data are normalized to total GSK3 and ratios multiplied by 10. By immunocytochemistry Aβ infusion caused a selective 50% increase in immunoreactivity of the active pGSK (pGSK3α/β) in the CA1 perikarya (Fig. 2b). There was no staining in the absence of primary antibodies (not shown).
Figure 2.
GSK3 phosphoepitopes (pGSK) in hippocampus after Aβ infusion. (a) Representative lanes are shown of the Western immunoblot of the inactive (pGSK3βser9), and active (pGSK3α/βtyr279/216) phosphoepitopes which were normalized to total GSK3α/β in the histograms (10× ratio on ordinate axes). Quantitation shows that Aβ infusion reduced levels of the inactive form (pGSK3βser9) with a trend for an increase in the active form (pGSK3α/βtyr279/216), suggesting increased activity. (b) Representative immunohistochemical (IHC) micrographs of the active form pGSK3α/β in hippocampal CA1 pyramidal neurons, showing that Aβ appeared to increase punctate staining prominent in nuclei. Both treatments show diffuse staining of non-pyramidal layers, suggesting possible process staining. Dark punctate staining is quantified using relative optical density thresholding. Data are expressed as mean±SEM, n=7–11 per group. Scale bar=100 µM.
SB attenuated GSK3 activity in vivo
GSK3 activity in vivo was measured in response to the GSK3 inhibitor SB (at ~40 nM) by assessing the downstream target phospho-glycogen synthase, which was reduced by 39% (9.991±1.026 versus 6.018±1.079, p<0.05), suggesting that the inhibitor effectively reduced but did not abolish GSK3 activity. We also measured a downstream intermediate, glycogen, showing that compared to vehicle glycogen levels (1.208±0.164 mM, SB significantly increased glycogen levels (1.742±0.125 mM) by 44% (p<0.01), further supporting that the inhibitor was effectively reducing but not abolishing GSK3 activity.
Together these data would indicate that the dose chosen was adequate to reduce activity, but not above an EC50. Although SB interacts directly with the kinase, it does not directly impact the phosphoepitopes. Nevertheless, it can impact phosphoepitopes indirectly. We did observe an impact of SB on one GSK3 phosphoepitope. SB reduced levels of the pGSK3α/β by 25% (0.922±0.208 compared to 1.225±0.286, p=0.03). This indirect inhibition of the active pGSK3 would be expected since pGSK3α/βtyr279/216 autophosphorylates (Hughes et al., 1993). In fact, it has been described that both SB and another structurally unrelated and highly specific GSK3 inhibitor (AO14418, AR) but not lithium reduce pGSK3α/βtyr279/ 216 in vitro (Simon et al., 2008). Lochhead et al. (2006) have shown that the intramolecular tyrosine kinase activity of GSK3 on tyr 279/216 occurs during a transition intermediate conformation and that some specific inhibitors, like SB can block this tyrosine kinase activity. Further, pharmacological inhibition of GSK3 with two additional structurally unrelated inhibitors, kenpaullone and indirubin and even specific genetic inhibition with dominant-negatives GSK-3β (K85R or K85A) blocked tyrosine 279/216 phosphorylation (Cole et al., 2004). Thus, reduced levels of pGSK3α/βtyr279/216 found with 4 specific inhibitors (including SB, AR, kenpaullone and indirubin) or with dominant negative mutations argue that for SB, inhibition of pGSK3α/βtyr279/216 is a useful index of specific drug activity.
Aβ increased neurodegenerative markers: prevention by GSK3 inhibition
We then examined the impact Aβ on neurodegeneration, whether GSK3 inhibition could impart neuroprotection from Aβ. By Cresyl violet (CV) staining, we found that compared to vehicle treatment, Aβ was associated with an increase in pyknotic nuclei distributed in the CA1, CA3 and particularly the dentate gyrus (DG; Fig. 3, p<0.0001). Although the interaction between treatment and neuronal layer region (DG, CA1, CA3) did not reach significance (p=0.111), the impact of Aβ on pyknosis of pyramidal neurons was more evident in the DG than in the CA1 and CA3 (p<0.01). The impact of Aβ on CV staining in neurons was fully prevented by SB treatment, but SB treatment given to control animals induced severe pyknosis (p<0.001). Similar effects were also seen in the subiculum (not shown).
Figure 3.
Aβ and/or GSK3 inhibitor (SB) impact on pyknosis of hippocampal neurons. (a) Cresyl Violet staining of hippocampal neurons in the CA1, CA3, or dentate gyrus (DG). Neurons of vehicle and SB groups typically showed intact nuclei and clearly visible Nissl granules, while neurons in the SB or Aβ group more typically exhibited an increased number of darkened and pyknotic neurons, or loss of Nissl (black arrows). Neurons from rats co-infused with SB and Aβ were similar to vehicle. Scale bar=50 µM. (b) Quantitation of dark pyknotic neurons revealed a significant treatment effect (p<0.01). There was no treatment×region interaction. Post hoc LSD Fisher analysis showed differences between groups as indicated. Asterisks indicate difference between Veh. ****p<0.0001; ***p<0.001. Data were expressed as mean±SEM, n=7–11 per group.
Paralleling effects observed with CV, immunohistochemical examination of the hippocampal regions revealed that, compared to vehicle, Aβ increased three neurodegenerative markers, which were fully prevented by co-administration of SB. For example, in the CA1, Aβ increased staining for active caspase-3 (Figs. 4a–d, m, p<0.0001), the DNA fragmentation marker TUNEL (Figs. 4e–h, n, p<0.0001), and the anionic fluorochrome, FluoroJade B, but SB treatment also increased these neurodegenerative markers (Fig. 4, and Supplementary Fig. 2, p<0.0001). Similar results were observed with antibodies to the active (phosphorylated) c-jun terminal kinase (pJNK) as shown by representative micrographs (Figs. 4i–l) and the quantification of the pJNK response (Fig. 4o).
Figure 4.
Neurodegenerative markers after infusion of Aβ oligomer and/or GSK3 inhibitor (SB). Staining of the CA1 of the hippocampus for active caspase-3 (a–d), TUNEL (e–h) and active c-jun N-terminal kinase (pJNK) (i–l). (m–o) Quantitative image analysis within the CA1 for the three neurodegenerative markers. Arrows indicate examples of affected neurons. Increased staining for all three markers compared to vehicle (a, e, i) or to Aβ plus SB infusion (d, h, l) was seen in SB (b, f, j) or Aβ treatment (c, g, k). Asterisks indicate significant difference from Veh. *p<0.05, **p<0.01, ***p<0.001. Data are expressed as mean±SEM, n=7–11 per group. Scale bar=100 µm.
Prevention of Aβ-induced aberrant dendritic morphology and ptau by GSK3 inhibition
To explore the impact of treatments on dendrites and axons, we performed immunohistochemistry for microtubule-associated protein-2 (MAP-2) (Fig. 5a), tau-1 (non-phosphorylated tau), and phosphorylated tau (ptau, CP13) (Figs. 5b and c). In the stratum radiatum the apical dendrites of CA1 neurons stained for MAP-2 were generally straight and non-vacuolated in the vehicle treatment group. In contrast, Aβ commonly induced curly and vacuolated dendrites and a thinning of dendrites, best seen at high magnification (Fig. 5a, right panels). Although SB treatment prevented this Aβ-associated dendritic abnormality, SB treatment alone also caused aberrant dendritic morphology.
Figure 5.
Cytoskeletal proteins in the hippocampus after infusion of Aβ oligomers and/or GSK3 inhibitor (a) MAP-2-ir in representative sections shows dendritic staining in the stratum radiatum (SR) in response to intracerebroventricular infusion of vehicle (Veh), GSK3 inhibitor (SB), Aβ, or Aβ+SB. Treatment effects on induction of aberrant curly dendrites (black arrows) and MAP-2-ir accumulation in soma (black arrowheads) are shown. The right panel is a high magnification of the adjacent panel's black-boxed area to demonstrate the treatment impact on dendritic blebbing (white arrowheads). (b) In the CA1, Tau-1 (non-phosphorylated tau epitope 191–225) staining is present in the non-pyramidal layers. Phospho-Tau (CP13, phosphorylated serTau) staining is induced in soma and dendrites in responses to some treatments. (c) In the CA3, treatment-dependent induction of Tau-1 (non-phosphorylated) and CP13 (phosphorylated) tau in neuritic processes (black arrows) and soma (arrowheads). (d) Image analysis of CP13-ir in CA1 and CA3, demonstrating increased CP13-ir in SB and Aβ infusion groups (p<0.0001). Data are expressed as mean±SEM, n=7–11 per group. Scale bar=100 µm.
We also explored how treatments influenced tau-1, which binds only non-phosphorylated tau residues 191–225, and the reciprocal aberrantly phosphorylated (CP13, ser202) tau in the stratum radiatum (CA1 apical dendrites and CA3 mossy fiber axons, Figs. 5b and c, respectively). Although perikarya of CA1 pyramidal neurons in all treatments showed no staining for tau-1 as expected, the limited diffuse staining of the apical dendrite layer appeared slightly reduced by either Aβ or SB treatments alone, compared to vehicle or to combined Aβ and SB (Fig. 5b). In contrast, Aβ treatment alone induced strong ptau immunoreactivity (CP13) in the apical dendrites, and the associated degenerating perikarya, which was prevented by co administration of SB (Figs. 5b, d, p<0.0001). The SB treatment alone given to control animals also induced tau pathology, relative to the vehicle.
Tau-1-ir was mainly observed in the mossy fibers in vehicle-treated rats consistent with normal, primarily axonal tau distribution in vivo, but Aβ-treated rats showed loss of tau-1, which was prevented by SB (Fig. 5c). Compared to vehicle treatment, Aβ enhanced ptau (CP13) immunostaining in the CA3 pyramidal neurons and in neurites, and coinfusion with SB prevented this effect (arrows, Figs. 5c, d, p<0.0001). But relative to vehicle, infusion of SB increased pTau staining in CA3 neurons and neurites.
Impact of infusion of Aβ and/or SB on gliosis
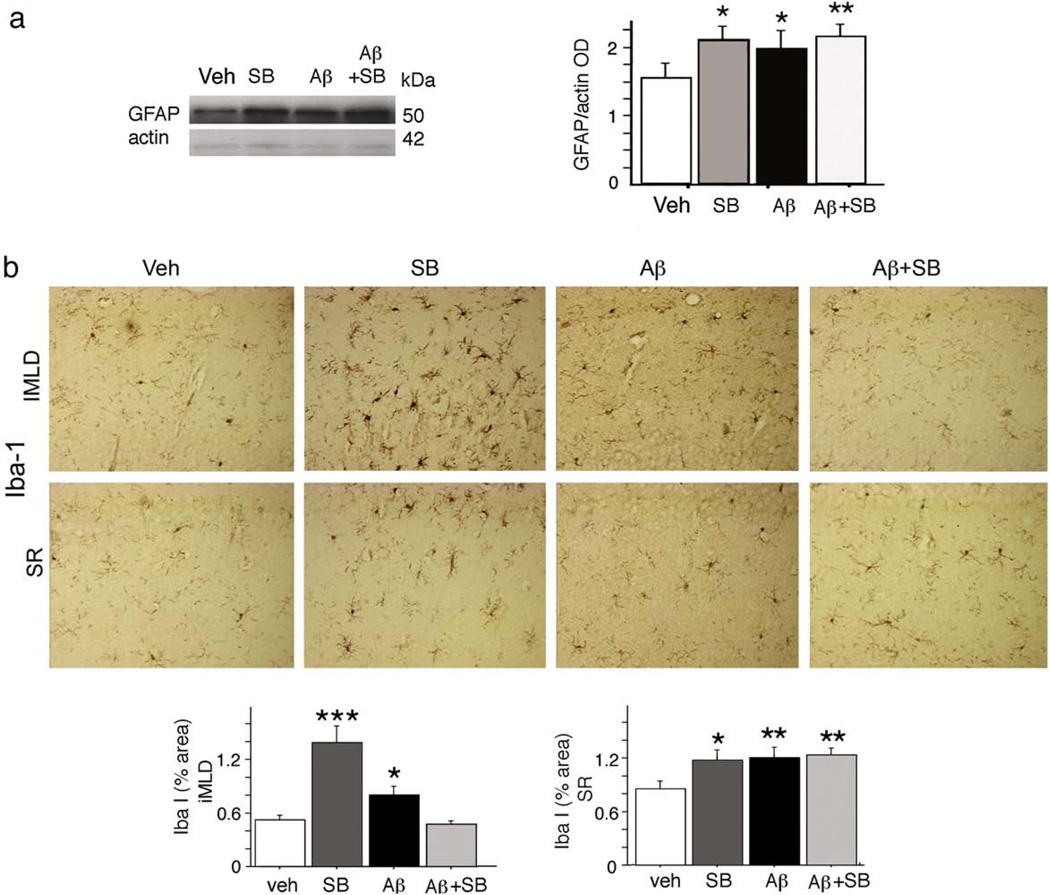
We measured the astrocyte response to treatment by Western and immunocytochemistry. We also quantified the microglial response by immunocytochemistry, using the microglial antigens Iba-1 and phosphotyrosine (Frautschy et al., 2001; Frautschy et al., 1998). Western analysis of GFAP-ir showed that, compared to vehicle, all other treatments (SB alone, SB+Aβ, Aβ) increased levels of GFAP (Fig. 6a, p<0.05). Parallel responses confirming the results of Western were observed by immunocytochemical analysis of hippocampal and cortical layers (now shown).
Figure 6.
Gliosis in the hippocampus after Aβ and/or GSK3 inhibitor (SB) infusion. (a) Astrocyte marker GFAP was measured in the hippocampal lysate by Western analysis. Values were quantified by densitometry and normalized to β-actin. Representative lanes and results of statistical analysis are shown. (b) Iba-1 staining of brain sections, used to assess the microglial response to treatment. Representative micrographs of the inner molecular layer of the dentate gyrus (iMLD) and stratum radiatum (SR) are shown and quantified by ANOVA of % area. Data are expressed as mean±SEM, n=7–11 per group. Scale bar=100 µm; asterisks indicate significance of Fisher's LSD post hoc analysis of planned comparisons, indicating difference between groups without asterisks: *p<0.05, **p<0.01, ***p<0.0001.
Compared to vehicle, Aβ induced microgliosis; and even more robustly, SB induced microgliosis. SB resolved Aβ-dependent microgliosis in some (inner molecular layer of the dentate, IMLD Fig. 6b, p<0.01) but not all (stratum radiatum, Fig. 6b) layers of the hippocampus. Most notable and specific for SB treatment was the impact on microglial cell body size, which was increased by 84.9% compared to other groups (25.2±5.9 µm2 versus 46.575±7.3 µm2, respectively, p<0.05). This is evident in the micrograph, particularly in the IMLD region. Similar microglial responses were observed using a different microglial antigen (phosphotyrosine, not shown).
Effect of Aβ and GSK3 inhibition on performance in Morris water maze
Animals were first trained to find a visible platform (Figs. 7a and b). There were no differences in the swim speed to find the visible platform (not shown), suggesting no differences in motor skills and/or visual skills.
Figure 7.
Morris water maze performance after infusion of Aβ and/or GSK3 inhibitor (SB) (n=7–11 per group). (a) Schematic diagram showing quadrant, platform, zone (A, B, C) and thigmotaxis locations. (b) Paths (meters) to alternating visible platforms are similar between groups (significant regression, r=0.473, p<0.0001). (c–f) Performance to find hidden platforms at two locations. Line graphs of acquisition data for path to hidden platform are shown for blocks 1–4 (platform # 3) and blocks 5–10 (platform #1), according to treatment comparisons: Aβ vs. vehicle (c), SB vs. vehicle (d) and Aβ+SB versus Veh (e). (f) These data are summarized in a histogram of treatment means±SEM for acquisition paths to hidden platform. (g)We also examined thigmotaxis (circling near wall of tank) to investigate alterations in search strategy. Line graphs show thigmotaxis data (% area), according to block for both platform positions. (h) A summary histogram of treatment means±SEM for thigmotaxis (% area, p<0.05) for blocks 5–10 is shown. (i) Search strategies were further examined by assessing swimming in different zones, in particular, the central zone, A (% seconds in center of pool). Line graph values represent average±SEM of four trials per block (b–e, g). (f) Histogram shows p value differences between planned comparisons, using post hoc Fishers LSD. (h and i) Asterisks indicate treatments that are significantly different than all other treatments. *p<0.05; ***p<0.0001. (j) Representative swim paths in the trial without the platform (Probe) in each group.
Rats were then trained to find a submerged platform first located at position #3 for 4 blocks of training (4 swim trials per block) (Figs. 7c–f). There were no treatment-dependent deficits in this test since with repeated trials the animals' performance uniformly improved (significant regression, r=0.163, p<0.001), and there were no treatment differences in path lengths during acquisition (Figs. 7c–f) or difference in percent path in target quadrant during probe retention (not shown). In contrast, when the platform position was moved to a new position (#1), there were treatment differences. The Aβ infusions showed a mild learning deficit to finding new platform #3, compared to vehicle infusion (Figs. 7c and f, blocks 5–10, p=0.07 and block 7, p=0.05), and SB exacerbated deficits, showing more significant deficits (Figs. 7d–f, p=0.0002).
Although there was no difference in retention between the groups, there were differences in search strategies during probe trials and during acquisition. Vehicle infused rats quickly crossed over the correct platform position, and when they did not find it (probe test), they immediately started searching for a new location by crossing across quadrants. Interestingly, swim paths of Aβ infused rats had a different search strategy compared to vehicle, staying away from side walls (Figs. 7g and h, p=0.015) and swimming or circling mostly in the center (zone A) (Fig. 7i, p=0.05), which was corrected by SB (Figs. 7h and i, p=0.0004 and p=0.0003, respectively). SB given to control animals showed no impact on thigmotaxis when training for first platform position, but when training for second platform position, SB increased thigmotaxis dramatically (Figs. 7g and h, p<0.0001). Representative search paths during the probe trial after training for new platform position illustrate the opposing impacts of Aβ compared with SB on search strategies (Fig 7j).
Discussion
Our data showed that chronic infusion of Aβ oligomers was sufficient to induce hyper-activation of GSK3 activity, paralleling large changes in neurodegenerative markers including ptau, caspase-3, TUNEL, as well as stimulating neuroinflammation and activating tau kinases. A highly specific GSK3 inhibitor protected against Aβ induced neuronal damage, but only partially ameliorated neuroinflammatory and behavioral deficits. Interestingly, in control animals, modest GSK3 inhibition itself induced neurodegenerative markers, inflammation and behavioral deficits. These data corroborated a role for GSK3 in AD pathogenesis but pointed to the importance of normal constitutive activity of GSK3 in neuron function.
Role of GSK3 in Aβ induced phospho-tau and neuronal damage
GSK3, also called tau protein kinase-1 (Imahori and Uchida, 1997) phosphorylates tau, and its active phospho-epitope is elevated in neurofibrillary tangles, granulovacuolar degenerative vacuoles and dystrophic neurites (Ferrer et al., 2002; Ishiguro, 1998; Leroy et al., 2007; Pei et al., 1999; Shiurba et al., 1996; Yamaguchi et al., 1996). Unlike active GSK3, total GSK3 does not correlate (Harr et al., 1996) or is reduced (Baum et al., 1996) in AD. The Aβ infusion model enables one to examine the impact of oligomers independent of APP overexpression and was found to induce aberrant somatodendritic ptau staining and loss of normal axonal tau in hippocampal neurons, which were prevented by GSK3 inhibition. Pre-formed Aβ oligomer infusion also induced neuron damage indexed by pyknotic changes (using Cresyl violet), increased caspase 3 and JNK activation, TUNEL and FluoroJadeB, all which were prevented by GSK3 inhibition, strongly implicating GSK3 hyperactivation in Aβ-dependent neuron damage.
Aβ induced GSK3 activation in vivo
In vivo exogenous Aβ infusion increased GSK3 activity by reducing the inactive phospho-GSK3 and stimulating the active phospho-GSK3. Although GSK3 hyperactivation can be induced by Aβ in vitro and by APP or PS1 overexpression in transgenic models that may derive from intraneuronal Aβ (Leroy et al., 2007; Qin et al., 2006; Takashima et al., 1993), this is the first report to our knowledge for direct effects of exogenous Aβ oligomers on GSK3 activity in vivo, consistent with our report that anti-Aβ antibody infusion into Tg2576 mice increased inhibitory GSK3ser9 phosphorylation (Ma et al., 2006). Thus our new data is the first to demonstrate that Aβ oligomers are sufficient to activate GSK3 activity not only by decreasing inactive GSK3 phosphoepitope but also by increasing active GSK3 phosphoepitopes.
Role of GSK3 in neuron survival
Despite protection against infused Aβ, our data demonstrated that the GSK3 inhibitor alone also induced neurodegenerative markers and ptau. This was surprising, considering that the dosage chosen for GSK3 inhibitor only mildly reduced activity as evidenced by reduction in downstream targets pGS and increased glycogen. Caspase-3 induction by GSK3 inhibition, may activate other tau kinases such as phospho c-jun activated kinase (pJNK) (Kumagae et al., 1999), which we found elevated in a similar pattern as the dying neurons in the CA1 (Figs. 4i–l). Baseline constitutive activity may be critical for neuron function as suggested by a recent study, showing that deletion of GSK3β can induce apoptosis of adult motor neurons in transgenic mice with conditional (tetracycline system) expression of dominant-negative-GSK-3β (Gomez-Sintes et al., 2007).
Our data with primary hippocampal neurons, with two GSK3 inhibitors offer some support to the need for constitutive activity. It has been reported that under certain conditions, GSK3 inhibition can facilitate apoptosis by stimulation of death domain-containing receptors (Song et al., 2004), suppress NFκB transcriptional activity and decreases expression of anti-apoptotic proteins XIAP and Bcl-2 (Ougolkov et al., 2007). In fact, GSK3 activity is required for TNF induced-NFkB function, which induces anti-apoptotic transcription factors such as Bcl-xL, TRAF1 (Takada et al., 2004). These findings could explain our observations of increased caspase 3 and TUNEL staining.
Impact of Aβ and/or GSK3 inhibition on microglia and astrocytes
Consistent with role of Aβ induced neuroinflammation in AD pathogenesis (Akiyama et al., 2000; Frautschy et al., 2001), Aβ infusion increased astrogliosis and microgliosis. Although the impact of Aβ on many neurodegenerative parameters was corrected by GSK3 inhibition, astrocytosis remained robust with the combined group (GSK3 inhibition plus Aβ), compared to vehicle. In addition, Aβ-induced neuroinflammation (microgliosis) was attenuated by co-infusion with GSK3 inhibitor in some, but not all, hippocampal layers. One possibility is that GSK3 inhibitors can directly stimulate glia, an idea supported by our work in primary astrocytes (Hu et al., 2006). Our data seem at odds with a general anti-inflammatory role for GSK3 that has been postulated (De Sarno et al., 2008). Together these data support the idea that GSK3 can attenuate neuroinflammation arising under conditions of GSK3 hyperactivation, but may aggravate neuroinflammation in conditions where constitutive GSK3 activity is impaired.
Role of GSK3 in mediating effects of Aβ on learning and search strategy
Aβ oligomer caused mild impairment in working memory in addition to altering the search strategy, causing circling in the center, which is possibly indicative of disinhibition. The data may suggest that some Aβ-dependent effects may arise only in relation to working memory. Dis-inhibitory tendencies have been described in transgenic models using other behavior paradigms in Tg2576 mice (Ognibene et al., 2005) or APPswe + PS1/DeltaE9 mice (Lalonde et al., 2005). In addition to cognitive deficits, behavioral and psychiatric disturbances are common in AD patients (Shin et al., 2005; Srikanth et al., 2005). SB completely corrected the Aβ-induced change in search strategy, suggesting that GSK3 hyperactivation was involved in some Aβ-dependent behavioral changes.
Noteworthy is that acquisition deficits in learning second platform position were not corrected by SB. Incomplete resolution of behavioral deficits with GSK3 inhibition is consistent with a report using a triple transgenic AD model, where a GSK3 inhibitor failed to ameliorate working memory deficits (Caccamo et al., 2007). Inhibiting GSK3 may be insufficient on its own, since evidence suggests that Aβ activates multiple signal transduction pathways (not just GSK3), for example Aβ-activation of calpain and cdk5. Nevertheless, unlike Aβ-dependent effects on neurodegenerative markers, effects on acquisition were mild, so it is difficult to make major conclusions about cognition.
GSK3 inhibition attenuated Aβ-dependent neuroinflammation in layers adjacent to the dentate gyrus, but not in layers adjacent to the CA1. Thus GSK3 inhibition may have region-specific effects on synaptic or glial elements influencing behavior, particularly since CA1 and dentate gyrus synapses play different functional roles in spatial learning (Okada et al., 2003).
Role of GSK3 in learning and behavior
GSK3 inhibition interfered with the ability of rats to learn a new platform position, which may reflect a role for GSK3 in working memory. This is consistent with GSK3′s normal role in mediating long-term depression, an important mechanism for memory consolidation (Peineau et al., 2007). This is consistent with findings in healthy patients, showing that two non-specific GSK3 inhibitors, valproate and lithium, interfered with verbal tasks; lithium interfered more with working memory, while valproate interfered more with spatial memory (Bell et al., 2005). Valproate has been known to interfere with working memory, but it was not clear whether this was mediate by valproates impact on GABA or GSK3 (Willner and Birbeck, 1986).
GSK3 inhibitors induced severe thigmotaxis after changing the platform's location. This may be primarily an anxiety effect of GSK3 inhibition. One report in a congenitally learned helpless rat model, found a deficiency in GSK3 (Kohen et al., 2003). Thus it is possible that constitutive GSK3 may impact non-cognitive behaviors as well. Again these findings suggest that more work needs to be done to not only understand the normal role of GSK3 in behavior, but to develop approaches that limit hyper-activation without reducing constitutive activity.
Potential for SB–Aβ interactions
One might argue that protective effects of SB are due to Aβ–SB interactions and not due to effects on GSK3 activity. However, coincubating SB with Aβ did not influence oligomer aggregation and co-infusion of SB with Aβ antagonized some (neurodegeneration) but not all (gliosis) Aβ-dependent effects. There were also differential effects on behavior since co-infusion of SB with Aβ corrected Aβ-induced circling in the center of the tank, but did not correct the mild acquisition deficits associated with finding a new platform position. Finally, in vitro, SB and Aβ were not co-incubated, but added separately and still SB and other structurally different GSK3 inhibitors protected from Aβ toxicity. Collectively, these results argue that SB's protective effects were not mediated by direct interactions on oligomer formation or stability.
Conclusion
In conclusion, our results support the hypothesis that GSK3 hyperactivation is involved in Aβ-induced neurodegeneration in vivo, but argue that therapeutic intervention must take into account the critical role of constitutive GSK3 in neuron function, memory and perhaps non-cognitive behaviors. Robust induction of 6 of 8 markers of neurodegeneration by Aβ was prevented by GSK3 inhibition, but GSK3 inhibition did not completely block Aβ-induced neuroinflammation. Clinical development of function-specific GSK3 inhibitors that disrupt some actions of GSK3 but leave others intact may help to minimize adverse events (Harwood, 2001).
Supplementary Material
Acknowledgments
Funding was provided by NIH RO1 AG021975 and a VA Merit. We would like to thank Dr. Atul Deshpande (UCLA, Dept Medicine) for reviewing and editing the manuscript.
Abbreviations
- AD
Alzheimer's disease
- CNS
central nervous system
- FDA
Food and Drug Administration
- GSK3
glycogen synthase kinase-3
- HDL
High density lipoprotein
- ir
immunoreactivity
- Inh 1
Inhibitor 1, also known as 4-Benzyl-2-methyl-1, 2,4-thiadiazolidine-3,5-dione or TDZD-8
- IRS-1
Insulin Receptor Substrate-1
- icv
intracerebroventricular
- LDH
Lactodehydrogenase
- LTD
Long Term Depression
- MAP-2
microtubule associated protein-2
- icv
Morris water maze
- pJNK
phospho-c-jun N-terminal kinase
- pGS
phospho-glycogen synthase
- ptau
phosphorylated tau
- SB
SB216763
- GABA
Gamma-amino butyric acid
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.nbd.2008.10.007.
Footnotes
Disclosure/conflicts of interest
The authors declare no conflict of interest.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Shin RW, Uchida C, Kitamoto T, Uchida T. Pin1 promotes production of Alzheimer's amyloid beta from beta-cleaved amyloid precursor protein. Biochem. Biophys. Res. Commun. 2005;336:521–529. doi: 10.1016/j.bbrc.2005.08.130. [DOI] [PubMed] [Google Scholar]
- Baum L, Hansen L, Masliah E, Saitoh T. Glycogen synthase kinase 3 alteration in Alzheimer disease is related to neurofibrillary tangle formation. Mol. Chem. Neuropathol. 1996;29:253–261. doi: 10.1007/BF02815006. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Zschocke J, Behl C. Brain region-specific neuroprotective action and signaling of corticotropin-releasing hormone in primary neurons. Endocrinology. 2003;144:4051–4060. doi: 10.1210/en.2003-0168. [DOI] [PubMed] [Google Scholar]
- Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA. Curcumin structure–function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J. Pharmacol. Exp. Ther. 2008a;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum AN, Yang F, Teng E, Hu S, Jones MR, Rosario ER, Beech W, Hudspeth B, Ubeda O, Cole GM, Frautschy SA. Use of copper and insulin-resistance to accelerate cognitive deficits and synaptic protein loss in a rat Aβ-infusion Alzheimer's disease (AD) model. Journal Alzheimer's Disease. 2008b;15:1–16. doi: 10.3233/jad-2008-15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Differential effects of chronic lithium and valproate on brain activation in healthy volunteers. Hum. Psychopharmacol. 2005;20:415–424. doi: 10.1002/hup.710. [DOI] [PubMed] [Google Scholar]
- Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog. Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, Smeal T, Defize LH, Angel P, Woodgett JR, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Oddo S, Tran LX, LaFerla FM. Lithium reduces tau phosphorylation but not A beta or working memory deficits in a transgenic model with both plaques and tangles. Am. J. Pathol. 2007;170:1669–1675. doi: 10.2353/ajpath.2007.061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J, Sugars KL, Bao YP, Rubinsztein DC. Glycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington's disease mutation. J. Biol. Chem. 2002;277:33791–33798. doi: 10.1074/jbc.M204861200. [DOI] [PubMed] [Google Scholar]
- Chun Y, Yin ZD. Glycogen assay for diagnosis of female genital Chlamydia trachomatis infection. J. Clin. Microbiol. 1998;36:1081–1082. doi: 10.1128/jcm.36.4.1081-1082.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- Cole A, Frame S, Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem. J. 2004;377:249–255. doi: 10.1042/BJ20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft JM, Watterson DM, Frautschy SA, Van Eldik LJ. Aminopyridazines inhibit beta-amyloid-induced glial activation and neuronal damage in vivo. Neurobiol. Aging. 2004;25:1283–1292. doi: 10.1016/j.neurobiolaging.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr. Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, Jope RS. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 2008;181:338–345. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9660–9664. doi: 10.1073/pnas.94.18.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Barrachina M, Puig B. Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol. 2002;104:583–591. doi: 10.1007/s00401-002-0587-8. [DOI] [PubMed] [Google Scholar]
- Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell. Mol. Life Sci. 2007;64:1930–1944. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am. J. Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging. 2001;22:993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Gomez-Sintes R, Hernandez F, Bortolozzi A, Artigas F, Avila J, Zaratin P, Gotteland JP, Lucas JJ. Neuronal apoptosis and reversible motor deficit in dominantnegative GSK-3 conditional transgenic mice. EMBO J. 2007;26:2743–2754. doi: 10.1038/sj.emboj.7601725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr SD, Hollister RD, Hyman BT. Glycogen synthase kinase 3 alpha and 3 beta do not colocalize with neurofibrillary tangles. Neurobiol. Aging. 1996;17:343–348. doi: 10.1016/0197-4580(96)00025-5. [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Johnson GV. Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J. Biol. Chem. 1999;274:21395–21401. doi: 10.1074/jbc.274.30.21395. [DOI] [PubMed] [Google Scholar]
- Harwood AJ. Regulation of GSK-3: a cellular multiprocessor. Cell. 2001;105:821–824. doi: 10.1016/s0092-8674(01)00412-3. [DOI] [PubMed] [Google Scholar]
- Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Hong M, Chen DC, Klein PS, Lee VM. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J. Biol. Chem. 1997;272:25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- Hu S, Simmons M, Beech WA, Begum A, Baba O, Frautschy SA. GSK3 inhibition prevents amyloid beta toxicity to hippocampal neurons, but on its own when reducing baseline GSK3 can increase neuron damage and gliosis. Soc. Neurosci. 2006;33 [Google Scholar]
- Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imahori K, Uchida T. Physiology and pathology of tau protein kinases in relation to Alzheimer's disease. J. Biochem. (Tokyo) 1997;121:179–188. [PubMed] [Google Scholar]
- Ishiguro K. Involvement of tau protein kinase in amyloid-beta-induced neurodegeneration. Rinsho Byori. 1998;46:1003–1007. [PubMed] [Google Scholar]
- Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J. Neuropathol. Exp. Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- Jin N, Kovacs AD, Sui Z, Dewhurst S, Maggirwar SB. Opposite effects of lithium and valproic acid on trophic factor deprivation-induced glycogen synthase kinase-3 activation, c-Jun expression and neuronal cell death. Neuropharmacology. 2005;48:576–583. doi: 10.1016/j.neuropharm.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem. Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kim L, Liu J, Kimmel AR. The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell. 1999;99:399–408. doi: 10.1016/s0092-8674(00)81526-3. [DOI] [PubMed] [Google Scholar]
- Kohen R, Neumaier JF, Hamblin MW, Edwards E. Congenitally learned helpless rats show abnormalities in intracellular signaling. Biol. Psychiatry. 2003;53:520–529. doi: 10.1016/s0006-3223(02)01503-2. [DOI] [PubMed] [Google Scholar]
- Kumagae Y, Zhang Y, Kim OJ, Miller CA. Human c-Jun N-terminal kinase expression and activation in the nervous system. Brain Res. Mol. Brain Res. 1999;67:10–17. doi: 10.1016/s0169-328x(99)00013-3. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Kim HD, Maxwell JA, Fukuchi K. Exploratory activity and spatial learning in 12-month-old APP(695)SWE/co+PS1/DeltaE9 mice with amyloid plaques. Neurosci. Lett. 2005;390:87–92. doi: 10.1016/j.neulet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Leroy K, Yilmaz Z, Brion JP. Increased level of active GSK-3beta in Alzheimer's disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 2007;33:43–55. doi: 10.1111/j.1365-2990.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- Lesort M, Jope RS, Johnson GV. Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3beta and Fyn tyrosine kinase. J. Neurochem. 1999;72:576–584. doi: 10.1046/j.1471-4159.1999.0720576.x. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J. Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat. Rev. Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- Lochhead PA, Kinstrie R, Sibbet G, Rawjee T, Morrice N, Cleghon V. A chaperone-dependent GSK3beta transitional intermediate mediates activationloop autophosphorylation. Mol. Cell. 2006;24:627–633. doi: 10.1016/j.molcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Loy R, Tariot PN. Neuroprotective properties of valproate: potential benefit for AD and tauopathies. J. Mol. Neurosci. 2002;19:303–307. doi: 10.1385/jmn:19:3:301. [DOI] [PubMed] [Google Scholar]
- Ma QL, Lim GP, Harris-White ME, Yang F, Ambegaokar SS, Ubeda OJ, Glabe CG, Teter B, Frautschy SA, Cole GM. Antibodies against beta-amyloid reduce Abeta oligomers, glycogen synthase kinase-3beta activation and tau phosphorylation in vivo and in vitro. J. Neurosci. Res. 2006;83:374–384. doi: 10.1002/jnr.20734. [DOI] [PubMed] [Google Scholar]
- Ma QL, Harris-White ME, Ubeda OJ, Simmons M, Beech W, Lim GP, Teter B, Frautschy SA, Cole GM. Evidence of Abeta- and transgene-dependent defects in ERK-CREB signaling in Alzheimer's models. J. Neurochem. 2007;103:1594–1607. doi: 10.1111/j.1471-4159.2007.04869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tatmediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J. Neurochem. 1999;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol. Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Middei S, Daniele S, Adriani W, Ghirardi O, Caprioli A, Laviola G. Aspects of spatial memory and behavioral disinhibition in Tg2576 transgenic mice as a model of Alzheimer's disease. Behav. Brain Res. 2005;156:225–232. doi: 10.1016/j.bbr.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Okada T, Yamada N, Tsuzuki K, Horikawa HP, Tanaka K, Ozawa S. Long-term potentiation in the hippocampal CA1 area and dentate gyrus plays different roles in spatial learning. Eur. J. Neurosci. 2003;17:341–349. doi: 10.1046/j.1460-9568.2003.02458.x. [DOI] [PubMed] [Google Scholar]
- Ougolkov AV, Bone ND, Fernandez-Zapico ME, Kay NE, Billadeau DD. Inhibition of glycogen synthase kinase-3 activity leads to epigenetic silencing of nuclear factor {kappa}B target genes and induction of apoptosis in chronic lymphocytic leukemia B cells. Blood. 2007;110:735–742. doi: 10.1182/blood-2006-12-060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J. Biol. Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J. Neuropathol. Exp. Neurol. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Qin W, Peng Y, Ksiezak-Reding H, Ho L, Stetka B, Lovati E, Pasinetti GM. Inhibition of cyclooxygenase as potential novel therapeutic strategy in N141I presenilin-2 familial Alzheimer's disease. Mol. Psychiatry. 2006;11:172–181. doi: 10.1038/sj.mp.4001773. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Ryder J, Su Y, Liu F, Li B, Zhou Y, Ni B. Divergent roles of GSK3 and CDK5 in APP processing. Biochem. Biophys. Res. Commun. 2003;312:922–929. doi: 10.1016/j.bbrc.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Shin IS, Carter M, Masterman D, Fairbanks L, Cummings JL. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am. J. Geriatr. Psychiatry. 2005;13:469–474. doi: 10.1176/appi.ajgp.13.6.469. [DOI] [PubMed] [Google Scholar]
- Shiurba RA, Ishiguro K, Takahashi M, Sato K, Spooner ET, Mercken M, Yoshida R, Wheelock TR, Yanagawa H, Imahori K, Nixon RA. Immunocytochemistry of tau phosphoserine 413 and tau protein kinase I in Alzheimer pathology. Brain Res. 1996;737:119–132. doi: 10.1016/0006-8993(96)00717-2. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Chou TB, Perrimon N. Wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- Simon D, Benitez MJ, Gimenez-Cassina A, Garrido JJ, Bhat RV, Diaz-Nido J, Wandosell F. Pharmacological inhibition of GSK-3 is not strictly correlated with a decrease in tyrosine phosphorylation of residues 216/279. J. Neurosci. Res. 2008;86:668–674. doi: 10.1002/jnr.21523. [DOI] [PubMed] [Google Scholar]
- Song L, Zhou T, Jope RS. Lithium facilitates apoptotic signaling induced by activation of the Fas death domain-containing receptor. BMC Neurosci. 2004;5:20. doi: 10.1186/1471-2202-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Nagaraja AV, Ratnavalli E. Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer's disease, vascular dementia and frontotemporal dementia. J. Neurol. Sci. 2005;236:43–48. doi: 10.1016/j.jns.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Su Y, Ryder J, Li B, Wu X, Fox N, Solenberg P, Brune K, Paul S, Zhou Y, Liu F, Ni B. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-beta precursor protein processing. Biochemistry. 2004;43:6899–6908. doi: 10.1021/bi035627j. [DOI] [PubMed] [Google Scholar]
- Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J. Biol. Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- Takashima A, Noguchi K, Sato K, Hoshino T, Imahori K. Tau protein kinase I is essential foramyloid beta-protein-induced neurotoxicity. Proc.Natl. Acad. Sci. U.S.A. 1993;90:7789–7793. doi: 10.1073/pnas.90.16.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong N, Sanchez JF, Maggirwar SB, Ramirez SH, Guo H, Dewhurst S, Gelbard HA. Activation of glycogen synthase kinase 3 beta (GSK-3beta) by platelet activating factor mediates migration and cell death in cerebellar granule neurons. Eur. J. Neurosci. 2001;13:1913–1922. doi: 10.1046/j.0953-816x.2001.01572.x. [DOI] [PubMed] [Google Scholar]
- Tseng AS, Engel FB, Keating MT. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem. Biol. 2006;13:957–963. doi: 10.1016/j.chembiol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Ueki K, Yamamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering BM, Coffer PJ, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J. Biol. Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- Willner P, Birbeck KA. Effects of chlordiazepoxide and sodium valproate in two tests of spatial behaviour. Pharmacol. Biochem. Behav. 1986;25:747–751. doi: 10.1016/0091-3057(86)90381-3. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Ishiguro K, Uchida T, Takashima A, Lemere CA, Imahori K. Preferential labeling of Alzheimer neurofibrillary tangles with antisera for tau protein kinase (TPK) I/glycogen synthase kinase-3 beta and cyclin-dependent kinase 5, a component of TPK II. Acta Neuropathol. 1996;92:232–241. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Zhao L, Teter B, Morihara T, Lim GP, Ambegaokar SS, Ubeda OJ, Frautschy SA, Cole GM. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer's disease intervention. J. Neurosci. 2004;24:11120–11126. doi: 10.1523/JNEUROSCI.2860-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat. Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.