Abstract
The use of mutant mouse models of neurodevelopmental and neurodegenerative disease is essential in order to understand the pathogenesis of many genetic diseases such as fragile X syndrome and fragile X-associated tremor/ataxia syndrome (FXTAS). The choice of which animal model is most suitable to mimic a particular disease depends on a range of factors, including anatomical, physiological, and pathological similarities; presence of orthologs of genes of interest; and conservation of basic cell biological and metabolic processes. In this chapter, we will discuss two mouse models of the fragile X premutation which have been generated to study the pathogenesis of FXTAS and the effects of potential therapeutic interventions. Behavioral, molecular, neuropathological, and endocrine features of the mouse models and their relation to human FXTAS are discussed.
14.1 Introduction
The FMR1 gene is polymorphic for the length of a tandem CGG trinucleotide repeat in the 5′ untranslated region (UTR). In the general population there are fewer than 55 CGG repeats [mean 30 – Hagerman (2008)]. In some individuals there is a repeat expansion wherein the number of CGG repeats expands beyond 200 repeats in length (i.e., full mutation – FM), and this is associated with FMR1 promoter and CpG island hyper-methylation and subsequent gene silencing – leading to no measurable FMR1 transcription and no FMRP translation and Fragile X Syndrome (FXS; Hagerman and Hagerman 2004). This FM occurs in roughly 1:4,000 males and 1:6,000 females, and virtually all FM males will develop FXS and 60% of FM women will develop FXS. CGG repeat lengths between those found in the general population and the FM are called the Fragile X premutation (55–200 CGGs; PM) zone and occurs in ~1:130–200 females and 1:800 males (Hagerman 2008). CGG trinucleotide repeat lengths in the PM were historically considered to lack a clinical phenotype, so the PM was used as a descriptor to emphasize the high probability for the PM to maternally expand into the FM across subsequent generations (Hagerman 2008; Hagerman and Hagerman 2004; Jacquemont et al. 2004; Kraff et al. 2007; Leehey et al. 2007, 2008; Senturk et al. 2009).
In 2001, a late onset neurodegenerative disorder called Fragile-X associated tremor/ataxia (FXTAS) was described in a subset of elderly carriers of PM alleles (Hagerman et al. 2001). FXTAS patients exhibit gait ataxia, intention tremor, and Parkinsonism, as well as presence of eosinophillic, ubiquitin-positive intranuclear inclusions in neurons and astrocytes throughout the brain (Greco et al. 2002, 2006, 2007, 2008; Tassone et al. 2004a). This finding, along with the findings that elevated FMR1 mRNA levels and concomitant mild reductions in FMRP levels are associated with the PM (Tassone et al. 2000a,b,c, 2004b, 2007; Tassone and Hagerman 2003), has led to the proposal that FXTAS is the result of an RNA gain of function resulting in cellular toxicity, similar to myotonic dystrophy (Garcia and Hagerman 2010; Raske and Hagerman 2009; Sellier et al. 2010; Tassone et al. 2000a). What remains unclear in FXTAS is the cause of incomplete penetrance of FXTAS within PM carriers: in PM carriers from known fragile X probands, only 30% of the males and 10–15% of the females may develop FXTAS, a number that may be lower if samples were ascertained through non-fragile X probands (Jacquemont et al. 2003, 2004).
14.2 Mouse Models of the Fragile X Premutation and FXTAS
The first mouse models were initially developed to model repeat instability and potential expansion to FM across generations. However, these transgenic mouse models, both within and outside the context of the FMR1 gene, did not show instability in the trinucleotide repeat length (Bontekoe et al. 1997; Lavedan et al. 1997, 1998).
The first model to be reported as a putative model for the PM and potentially FXTAS was the CGG Knock-In mouse model (CGG KI), which was generated by a homologous recombination whereby the endogenous mouse CGG repeat (CGG8) was replaced with a PM length CGG repeat of human origin (CGG98) on the endogenous mouse Fmr1 promoter (Bontekoe et al. 2001; Willemsen et al. 2003). These CGG KI mice, with minimal changes to the endogenous mouse Fmr1 promoter, showed moderate instability upon paternal and maternal transmission, and both expansions and contractions have been observed (Brouwer et al. 2007). Later, another CGG-CCG knock-in mouse (CGG-CCG mouse) was developed wherein CGG-CCG repeats (CGG-CCG124) were serially ligated and expressed in the endogenous mouse CGG repeat on the endogenous promoter (Entezam et al. 2007). This model also shows a trend toward gradual increases in CGG (or CGG-CCG) repeat lengths. Furthermore, the CGG-CCG mice show the same general pattern of repeat instability as that reported in the PM, namely that the paternal mutation shows small repeat expansions, and this expansion occurs preferentially in mice lacking ATM, with a bias toward greater expansions in males (Entezam and Usdin 2008, 2009).
Maternally transmitted mutations show larger repeat expansions that occur preferentially in mice lacking ATR. These results support models proposed in the human PM research concerning the differential expansion of male–female PM alleles into FM alleles across generations.
It has recently been reported that there may be environmental contributions to the CGG repeat instability in humans, or at least a contribution of environmental factors in the time course of neurodegeneration (Paul et al. 2010). The CGG-CCG mouse has been used to determine the role of oxidizing agents on CGG-CCG repeat expansion. When a DNA oxidizing agent is introduced to CGG-CCG mice, there appears to be a higher frequency and size of repeat expansions (Entezam et al. 2010). The authors suggest that such oxidizing agents may play a role in CGG repeat expansion seen in the PM and FXTAS.
Recently, another model of FXTAS has been developed in mice (Hashem et al. 2009). These mice used constructs and promoters either independent of the Fmr1 gene or used non-Fmr1 promoters. These mice specifically express CGG90 RNA in Purkinje cells with either Fmr1 or eGFP. Therefore these models target the implications of CGG90 mRNA overexpression for FXTAS. These models expressing an expanded CGG RNA without the context of the Fmr1 gene are very promising for the study of the RNA gain of function hypothesis.
There is another transgenic mouse model, into which a 1,057 bp fragment of genomic DNA from FMR1 including the translation initiation site and a repeat of 26 CGG repeats was cloned (Baskaran et al. 2002). These mice show intergenerational instability during both male and female transmission. Baskaran et al. (2002) find methylation in lines lacking repeat expansion and absence of methylation in lines that do show expansion, indicating that methylation and expansion are potentially independent events. This mouse model will not be covered in this chapter, as this mouse serves as a better model for Fmr1 CGG repeat expansion and gene methylation and thus is a better model for FXS than for FXTAS.
14.3 Utility of CGG KI and CGG-CCG Mice for the Study of FXTAS
As FXTAS is a late onset neurodegenerative disorder, it is difficult to determine precisely the factors that may contribute to the cellular dysfunctions thought to underlie the disease progression across the lifespan of any individual. In FXTAS patients we can only study the end-stage of the disease progression in brain tissue. The benefit of evaluating mouse models of neurodegenerative disorders is the relative shortness of the mouse lifespan. If a researcher wished to determine the natural history of the disease process in FXTAS, both the CGG KI and CGG-CCG mouse models will serve to provide invaluable insight (see Table 14.1).
Table 14.1.
Comparison of FXTAS with CGG KI and CGG-CCG FXTAS mouse models
| FXTAS | CGG KI mouse | CGG-CCG mouse | |
|---|---|---|---|
| Molecular measures | |||
| CGG Repeat | 55–200 repeats | 70–350 repeats | 120 to >200 repeats |
| FMR1 mRNA | 2–8-fold increase (blood) | 3–5-fold increase (brain) | 2–6-fold increase (brain) |
| FMRP Level | Slightly reduced | Slightly reduced | Markedly reduced |
| Neuropathology | |||
| Inclusions | Neurons and astrocytes | Neurons and astrocytes | In cells of brain |
| Gross Pathology | Purkinje cell dropout | No gross pathology | Purkinje cell dropout |
| Motor Function | Tremor and ataxia | Motor deficit with age | Normal motor function |
| Cognition | |||
| Social | Social anxiety | – | Reduced sociability |
| Anxiety | Anxiety disorders | Elevated anxiety | Reduced anxiety |
| Memory | Poor memory | Memory impairments | Memory impairments |
The CGG KI mouse has been used to evaluate the hypothesis that FXTAS, a late onset neurodegenerative disorder, may be the end stage of earlier, perhaps even neurodevelopmental, effects accumulated across the lifespan (Hagerman and Hagerman 2004; Bourgeois et al. 2011; Cornish et al. 2008a, 2009; Garcia-Arocena and Hagerman 2010). Recently, it has been shown that the CGG KI mouse shows abnormal cortical neuron differentiation and migration patterns in utero (Cunningham et al. 2011). Furthermore, it has been demonstrated in vitro, using primary neuronal cultures from the CGG KI mice, that immature neuronal morphologies predominate (thinner, filapodial dendrites), and reduce cellular viability (Chen et al. 2010). It has also been shown in vivo that CGG KI mice as young as 12 weeks of age show ubiquitin-positive intranuclear inclusions in neurons and astrocytes in the hippocampus and only later similar pathological features appear to develop in the parietal neocortex (Hunsaker et al. 2009). Similarly, intranuclear inclusions are present in the internal granule cell layer in the cerebellum at 12 weeks of age (MR Hunsaker, unpublished observations). These data suggest that there are developmental influences that may contribute to later neurodegenerative processes, or at least that the progressive neuropathology begins to form relatively earlier in life than previously thought.
14.3.1 Modeling Molecular Correlates of FXTAS in CGG KI and CGG-CCG Mice
Both CGG KI and CGG-CCG mice have been used to evaluate the molecular cascades associated with the PM that potentially underlie FXTAS pathophysiology. The brains of the CGG KI mouse show elevated Fmr1 mRNA levels and reduced Fmrp levels, similar to those observed in the PM and FXTAS (Tassone et al. 2000a, b, 2004a, 2007; Tassone and Hagerman 2003; Brouwer et al. 2007, 2008a,b, 2009a, b; Entezam et al. 2007). An average of twofold elevation in Fmr1 mRNA levels was detected as early as 1 week of age in CGG KI mice that persisted throughout development (Willemsen et al. 2003). In contrast to what was reported for the linear correlation between FMR1 mRNA levels and the repeat size in human FXTAS patients (Kenneson et al. 2001), the increase in Fmr1 mRNA levels was not correlated with the length of the repeat (Brouwer et al. 2008a). However, the data from the human patients were not from brain samples, but from blood samples or lymphoblasts. Entezam et al. (2007) were able to show a direct relationship between CGG-CCG repeat size and Fmr1 mRNA levels in the brains of the CGG KI mice, although the number of mice studied for the different repeat sizes was limited. Despite the increase in mRNA levels, both the CGG KI and the CGG-CCG mouse strain show an inverse correlation between CGG repeat length and Fmrp expression in the brain (Entezam et al. 2007; Brouwer et al. 2008c). One explanation is that the CGG repeat hampers the initiation of translation at the ribosome, possibly due to secondary structures formed.
14.3.2 Modeling Cellular Dysfunction Associated with FXTAS in CGG KI and CGG-CCG Mice
The CGG KI mouse has been used (in concert with engineered human cell lines) to demonstrate potential interacting partners of the CGG-expanded Fmr1 mRNA to directly test a model that suggest the CGG repeat itself acts to sequester proteins from the cell and by that mechanism causes cellular dysfunction (Raske and Hagerman 2009; Garcia-Arocena and Hagerman 2010). For example, it was demonstrated that Sam68, a splicing factor, is sequestered by the CGG repeat expansion and thus subsequently titrated out from the rest of the cell. This results in reduced Sam68-dependent splicing events, which may be involved in the events leading up to inclusion formation as increasing Sam68 expression can prevent aggregate formation in mouse and cell lines (Sellier et al. 2010).
The CGG KI mouse has also been used to evaluate more systems level disruptions that may be present in the PM and FXTAS. In addition, the CGG KI mouse has been used to demonstrate altered expression of GABA-B receptors in the cerebellum but not neocortex (D’Hulst et al. 2009), as well as to demonstrate abnormalities along the HPA axis and amygdala similar to those proposed in PM and FXTAS that might explain the molecular mechanisms underlying the psychopathology in PM carriers and FXTAS patients (Brouwer et al. 2008b).
14.3.3 Modeling Pathological Features of FXTAS in CGG KI and CGG-CCG Mice
Pathologic neuroanatomical features have been demonstrated in the CGG KI mice that appear to phenocopy human FXTAS. Greco et al. (2006) evaluated gray and white matter of brain in a number of cases of FXTAS and found a relatively large percentage (1–5%) of neurons and astrocytes in the brain contained eosinophillic intranuclear inclusions. White matter pallor and apparent thinning of the gray matter were also reported, as well as Purkinje cell dropout and axonal pathology such as torpedo axons in the cerebellum. Both the CGG KI and the CGG-CCG mouse have intranuclear inclusions in neurons throughout the brain (Willemsen et al. 2003; Entezam et al. 2007; Hunsaker et al. 2009; Brouwer et al. 2008a,b; Wenzel et al. 2010) and the CGG KI mouse has further been shown to have intranuclear inclusions in astrocytes, as well as neurons (Wenzel et al. 2010; Fig. 14.1). In addition to the presence of intranuclear inclusions in neurons inclusion presence or absence in astrocytes has not been reported, the CGG-CCG mouse shows reduced numbers of Purkinje cells and evidence for torpedo axonal morphology similar to that reported in FXTAS (Entezam et al. 2007).
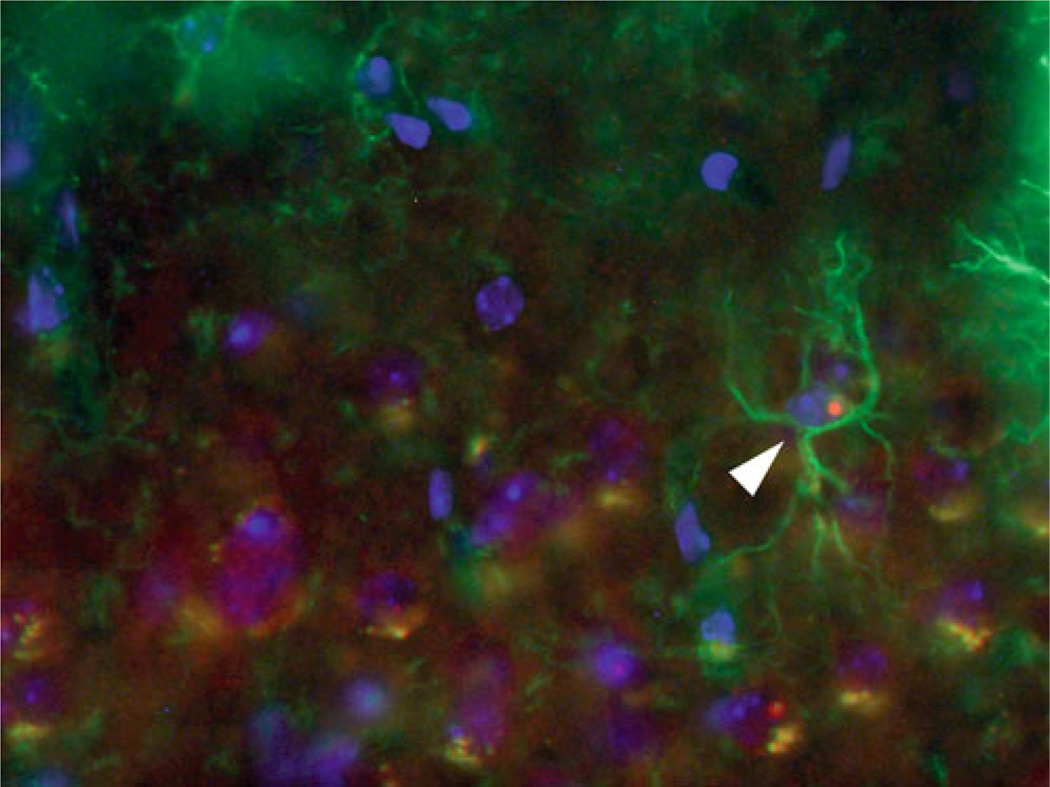
Fig. 14.1.
Astroglial cell containing an ubiquitin positive intranuclear inclusion (white arrow head) in the motor cortex of a 70 week old female CGG KI mouse with 9, 128 CGG repeats. Green = GFAP, red = ubiquitin, blue = DAPI
In the CGG KI mouse, the distribution of intranuclear inclusions has been carried out in mice ranging from 20 to 72 weeks of age (Willemsen et al. 2003). The analysis suggested that CGG KI mouse displays progressive neuropathological features (i.e., inclusions) that are most prominent in the rostral cortices, hypothalamus, olfactory nucleus, parafasicular nucleus of the thalamus, the inferior colliculus, pontine nuclei, vestibular nucleus, superficial dorsal horn of the spinal cord, and 10th cerebellar lobule. A later study further quantified intranuclear inclusion presence in the pituitary gland and amygdala (Brouwer et al. 2008b). Further analysis of CGG KI mice replicated these findings in a limited sample, but saw a much greater quantity of intranuclear inclusions in the hippocampus, particularly in the dentate gyrus (Brouwer et al. 2008c; Wenzel et al. 2010). The CGG-CCG mouse showed similar inclusions, but no regional quantifications were presented (Entezam et al. 2007).
An intriguing pattern can be seen in the distribution of the relatively early presence of intranuclear inclusions in the more primitive cortical structures, and later presence in more evolutionarily recent cortices (cf., Willemsen et al. 2003). A follow-up analysis of the distribution of intranuclear inclusions undertaken by Wenzel et al. (2010) and to a lesser extent Hunsaker et al. (2009) demonstrated that granular cells within the olfactory bulb, cerebellum, and dentate gyrus show the highest quantity of intranuclear inclusions (roughly 50% of neurons), followed by subcortical structures including the hypothalamus, thalamus, inferior colliculus, septal nuclei, various brainstem nuclei, and the cerebellum. In the cortex, the paleocortex associated with the amygdala and hippocampus and the entorhinal cortex (transitional cortex) show the greatest quantity of inclusions, followed by the limbic cortex and finally the rostral (i.e., sensory and motor cortices) and caudal (i.e. parietal and visual cortices) neocortex. This pattern suggests the potential for a primarily subcortical and limbic involvement in the neuropathology that spreads to the neocortex later in life.
Although the CGG KI and CGG-CCG mouse models appear to provide very good models for the primary neuropathological features present in FXTAS, there are a number of very important differences between the species that needs to be discussed. In FXTAS, a higher percentage of astrocytes in both the grey and white matter contain intranuclear inclusions compared to the local neuron populations (Greco et al. 2002, 2006; Wenzel et al. 2010). Furthermore, in FXTAS the intranuclear inclusions stain easily for eosin in a hematoxylin and eosin (H&E) stain, whereas the inclusions in mice are more difficult to stain – requiring the use of immunocytochemical techniques to identify the presence of intranuclear inclusions, or at least a careful optimization of H&E staining protocols (cf., Willemsen et al. 2003, Fig. 14.2). The reason for these differences is unclear and most likely does not affect the interpretation of the findings in the mouse models; the fundamental differences between species needs to be considered in all studies of comparative neuropathology resultant from the PM. On the other hand, this may be caused by the fact that we study the end stage of the disease in FXTAS patients and the mice we studied might not have reached this stage. These findings highlight the need to study the development of disease progression in the mice instead of focusing solely on the final stage in patients.
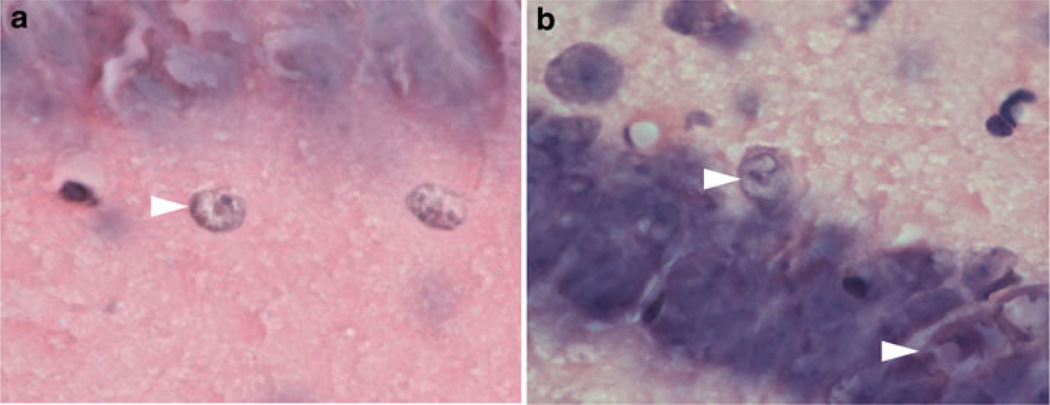
Fig. 14.2.
(a) H&E stained hippocampus demonstrating an interneuron in the stratum radiatum of CA1 with an intranuclear inclusions (arrow head). (b) H&E stained hippocampus demonstrating CA1 pyramidal cells with intranuclear inclusions (arrow heads). Both images are from a 52 week old female CGG KI mouse with 8, 152 CGG repeats
What remains unknown about the role of these neuropathological features in the PM and FXTAS is the developmental time course of inclusion formation as well as the role of these inclusions in cellular processing/toxicity. The first of these questions has been preliminarily addressed, for example, using cellular models (Sellier et al. 2010), but no work to date has evaluated CGG KI or CGG-CCG tissue at ages <12 weeks of age. Such work is necessary to determine a potential age where the brain is free from pathological features to evaluate preventative treatment strategies. However, the Purkinje cell specific transgenic mice (Hashem et al. 2009) very nicely show that the formation of inclusions also occurs when expressing expanded CGG RNA independent of Fmr1 context, suggesting a strong role for tandem CGG repeat containing RNA toxicity in intranuclear inclusion formation.
14.3.4 Modeling Behavioral Sequelae of FXTAS in CGG KI and CGG-CCG Mice
Until recently, the PM was thought to be free of behavioral and molecular sequelae (Hagerman and Hagerman 2004; Cornish et al. 2005, 2008b, 2009). Once it was determined that there were potential aberrant behavioral and psychiatric phenotypes in the PM prior to FXTAS, the study of the mouse models were expanded to model these phenotypes. Unfortunately, neither the CGG KI nor the CGG-CCG mouse shows classic tremor or ataxia on basic behavioral assays (Van Dam et al. 2005; Qin et al. 2011). This lack of a clear motor phenotype suggests that either the mouse models are lacking, or there are differences between species that prevent potential motor phenotypes from being observed (i.e., methodological differences in tests between species, bipedal gait in humans vs. quadrapedal in mice, etc).
The CGG-CCG mouse has only been preliminarily evaluated for a behavioral phenotype. The CGG-CCG mouse has been shown to be slightly hyperactive and shows reduced anxiety in the open field and elevated zero mazes. Furthermore, the CGG-CCG mouse shows impaired passive avoidance learning and a slight reduction in social interaction (Qin et al. 2011). They interpret these results to indicate a subtle deficit similar to those reported in the Fmr1 KO model of FXS.
The CGG KI mouse has been evaluated for the cognitive deficits present in the PM and FXTAS. Van Dam et al. (2005) demonstrated a clear age-related worsening of motor performance on the accelerating rotarod and memory impairments on the water maze. To further characterize these deficits,Hunsaker et al. (2009) evaluated spatial processing in CGG KI mice using tasks designed to more specifically evaluate spatial processing than the water maze. They found that CGG KI mice showed significant deficits in spatial processing compared to littermate control animals as early as 12 weeks of age. On a similar task involving learning the relationship between objects and their location in space, the same mice showed deficits only at 48 weeks of age. Intriguingly, in a separate group of animals,Hunsaker et al. (2009) evaluated the presence of intranuclear inclusions in the dentate gyrus in the hippocampus (which subserves performance in the first task) and the parietal cortex (which subserves performance in the second task) (cf., Goodrich-Hunsaker et al. 2005, 2008). They found that there were inclusions (albeit low in number) in the dentate gyrus of the CGG KI mice as early as 12 weeks of age and progressively more with increasing age. Intranuclear inclusions were only detectable in the parietal cortex at 48 weeks of age. These findings suggest that the development of neuropathology follows a similar time course as the emergence of behavioral dysfunction in the CGG KI mouse, implying a potential neuropathological correlate to the spatial processing deficits.
In a subsequent experiment, female CGG KI mice were tested for their ability to learn and remember short sequences of stimuli. In this task, the mice were presented with three pairs of visual objects for 5 min each separated by 5 min intervals. Afterward, the mice were presented with two tests, one for temporal order, wherein the first object and the last object encountered were presented and the mouse was allowed to preferentially explore. The second test was for novelty, and the first object encountered and a novel, never before seen object was presented. Female CGG KI mice showed a CGG-repeat length-dependent deficit for learning and remembering sequences. Mice with 80–100 CCG trinucleotide repeats performed worse than wild type littermate mice, but performed better than mice with 140–190 CGG repeats. All animals performed the novelty task equally well (Hunsaker et al. 2010). These data suggest that temporal processing is deficient in CGG KI mice. What makes this finding all the more intriguing is that these data were from female mice, who should be 50% as affected as male mice, and thus should show a more subtle phenotype. As such, male mice should show much more profound deficits on the same task; however, this has yet to be assessed.
To better evaluate the cognitive and behavioral phenotypes in CGG KI mice, there is a need to develop a number of novel tasks to more precisely evaluate specific behaviors proposed to be affected by the PM and FXTAS. As it has been suggested previously that the traditional tasks evaluating motor function often miss subtle pathology, task development is needed in this arena.
In order to identify and potentially quantify more subtle motor deficits,Hashem et al. (2009) evaluated mice with expanded CGG repeats expressed from the L7/pcp2 promoter in cerebellar Purkinje cells on the rotarod measure of motor function. They found that these mice showed age-related deficits in the rotarod (i.e., the mice fell from the rod at slower speeds and were unable to stay on the rotating drum as long as controls even at slow speeds).
These findings suggest motor deficits in the mouse models of FXTAS, but to date such robust findings using the rotarod have not been found in the other FXTAS mouse models. However, Van Dam et al. (2005) did find a mild rotarod phenotype in old CGG KI mice. The Purkinje-specific transgenic mice demonstrate that overexpression of the expanded CGG RNA in Purkinje cells is sufficient to cause motor dysfunction.
As the primary tremor present in FXTAS is an intention tremor, it may be worthwhile to evaluate CGG KI and CGG-CCG mice on a skilled forelimb reaching tasks that allow precise quantification of limb use. Such tasks may uncover subtle tremor missed on tests of more gross motor function (Alaverdashvili and Whishaw 2008; Blume et al. 2009; Farr et al. 2006; Farr and Whishaw 2002; Metz and Whishaw 2002, 2009; Ward 1997; Whishaw and Metz 2002; Whishaw et al. 2010). To better model the gait ataxia, skilled walking tasks similar to those used in grid walking paradigms could be applied as they are in models of alcohol intoxication that allow for similarly specific quantification of walking behavior.
Another common cognitive disruption in FXTAS is a sort of dysexecutive syndrome (Brega et al. 2008) involving cognitive control and attentional processing. Although difficult to model in mice, tasks such as the five choice serial reaction time task or biconditional discrimination tasks can be used to model these processes (George et al. 2010; Haddon et al. 2008; Marquis et al. 2007). Similarly, there are attentional tasks in rats that can be modified for mice that can get at specific attentional processes affected in FXTAS (Ward 1997; Ward and Brown 1996).
Furthermore, as the parietal lobe appears to be atrophied in FXTAS, tasks specifically evaluating parietal functions need to be performed in mice [similar to the second task mentioned above from Hunsaker et al. (2009)]. As the time course for the development of neuropathological features has been described in the CGG KI mouse, this mouse provides a unique opportunity to thoroughly evaluate the specific hypotheses concerning the role of molecular factors that may be underlying the neurocognitive deficits present in the PM and FXTAS.
14.4 Utility of CGG KI and CGG-CCG Mice for Interventional Studies
To date, no therapeutic studies have been performed on any of the FXTAS mouse models, primarily because there were no clearly defined behavioral outcome measures and no real biomarkers to speak of. The primary difficulty present in evaluating therapies in the FXTAS mouse models is the fact that FXTAS is defined as a late onset neurodegenerative disorder characterized by a motor phenotype. This means that, in theory, animals have to be set aside for the better part of a year prior to treatment and then the outcome measures (i.e., latency to fall on the accelerating rotarod) are not all that clear cut. One potential solution to this problem is to use the mouse models reported by Hashem et al. (2009) for evaluating treatments of the motor phenotype. In these mice the motor phenotype is specifically exaggerated in those mice at an age earlier than either the CGG KI or CGG-CCG mice; however, these mice are transgenic and express the CGG repeat in Purkinje cells, not all cells, so this model is incomplete from a clinical perspective.
To better dissect the respective roles of different molecular factors for FXTAS disease progression, further/new transgenic mouse models need to be generated to identify the respective roles of different cell types for FXTAS. The development of transgenic mouse models expressing an expanded CGG RNA in different cell populations at higher levels will facilitate the design of experiments evaluating sufficiency, necessity, and timing of disease progression. The generation of inducible mice will facilitate research into treatment options and outcomes, as well as answer questions concerning the potential reversibility of neuropathology and aid in developing pharmaco- and gene-targeted therapies.
The CGG KI mouse develops subtle behavioral phenotypes that appear to be present from ages as early as 12 weeks or earlier [though the animals have not been tested earlier than 12 weeks of age (Hunsaker et al. 2009, 2010)]. This mouse model, however, does not show motor deficits in the rotarod until advanced ages (Van Dam et al. 2005). A combined strategy of using the CGG KI and the transgenic mice expressing CGG repeats in Purkinje cells to model different aspects of the FXTAS disease process may provide valuable insights into the nature of behavioral and motor problems in FXTAS.
Finally, an additional outcome measure may be to evaluate effect or stress responses in the CGG KI mouse. As Brouwer et al. (2008b) showed CGG KI mice exhibit abnormal HPA activity, which correlated with an abnormal stress response in the amygdala. If these findings extend earlier in life similar to the behavioral measures, then reversing a dysfunctional HPA axis/stress response may provide benefit to FXTAS.
14.5 Conclusion
The CGG KI and CGG-CCG mouse models for the fragile X PM and FXTAS provide an invaluable resource for the translational scientist to generate and evaluate hypotheses into the molecular correlates of FXTAS disease onset and progression. These mouse models further provide outcome measures and putative biomarkers that may aid in the development and evaluation of therapeutic interventions.
Acknowledgements
This work was made possible by a Roadmap Initiative grant (UL1 DE019583) from the NIDCR in support of the NeuroTherapeutics Research Institute (NTRI) consortium and Grant RL1 NS064211 from NINDS. This work was also supported by a grant (UL1 RR024146) from the National Center for Research Resources, a component of the National Institute of Health, and NIH Roadmap for Medical Research. This work was also supported by a research grant from the National Fragile X Foundation (RKH).
Contributor Information
Michael R. Hunsaker, Department of Neurological Surgery, University of California, Davis, CA, USA NeuroTherapeutics Research Institute (NTRI), University of California, Davis, CA, USA.
Gloria Arque, NeuroTherapeutics Research Institute (NTRI), University of California, Davis, CA, USA; Department of Psychiatry and Behavioral Sciences, University of California, Davis, CA, USA.
Robert F. Berman, Department of Neurological Surgery, University of California, Davis, CA, USA NeuroTherapeutics Research Institute (NTRI), University of California, Davis, CA, USA.
Rob Willemsen, Email: r.willemsen@erasmusmc.nl, NeuroTherapeutics Research Institute (NTRI), University of California, Davis, CA, USA; Department of Clinical Genetics, Erasmus MC, P.O. Box 2040, 3000 CA Rotterdam, The Netherlands.
Renate K. Hukema, Department of Clinical Genetics, Erasmus MC, P.O. Box 2040, 3000 CA Rotterdam, The Netherlands
References
- Alaverdashvili M, Whishaw IQ. Motor cortex stroke impairs individual digit movement in skilled reaching by the rat. Eur J Neurosci. 2008;28:311–322. doi: 10.1111/j.1460-9568.2008.06315.x. [DOI] [PubMed] [Google Scholar]
- Baskaran S, Datta S, Mandal A, Gulati N, Totey S, Anand RR, Brahmachari V. Instability of CGG repeats in transgenic mice. Genomics. 2002;80:151–157. doi: 10.1006/geno.2002.6813. [DOI] [PubMed] [Google Scholar]
- Blume SR, Cass DK, Tseng KY. Stepping test in mice: a reliable approach in determining forelimb akinesia in MPTP-induced Parkinsonism. Exp Neurol. 2009;219:208–211. doi: 10.1016/j.expneurol.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Bontekoe CJ, de Graaff E, Nieuwenhuizen IM, Willemsen R, Oostra BA. FMR1 premutation allele (CGG)81 is stable in mice. Eur J Hum Genet. 1997;5:293–298. [PubMed] [Google Scholar]
- Bontekoe CJ, Bakker CE, Nieuwenhuizen IM, van der Linde H, Lans H, de Lange D, Hirst MC, Oostra BA. Instability of a (CGG)98 repeat in the Fmr1 promoter. Hum Mol Genet. 2001;10:1693–1699. doi: 10.1093/hmg/10.16.1693. [DOI] [PubMed] [Google Scholar]
- Bourgeois JA, Seritan A, Casillas EM, Hessl D, Schneider A, Yang Y, Kaur I, Cogswell J, Nguyen DV, Hagerman R. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2011;72:175–182. doi: 10.4088/JCP.09m05407blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brega AG, Goodrich G, Bennett RE, Hessl D, Engle K, Leehey MA, Bounds LS, Paulich MJ, Hagerman RJ, Hagerman PJ, Cogswell JB, Tassone F, Reynolds A, Kooken R, Kenny M, Grigsby J. The primary cognitive deficit among males with fragile Xassociated tremor/ ataxia syndrome (FXTAS) is a dysexecutive syndrome. J Clin Exp Neuropsychol. 2008;30:853–869. doi: 10.1080/13803390701819044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Mientjes EJ, Bakker CE, Nieuwenhuizen IM, Severijnen LA, Van der Linde HC, Nelson DL, Oostra BA, Willemsen R. Elevated Fmr1 mRNA levels and reduced protein expression in a mouse model with an unmethylated fragile X full mutation. Exp Cell Res. 2007;313:244–253. doi: 10.1016/j.yexcr.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Huizer K, Severijnen LA, Hukema RK, Berman RF, Oostra BA, Willemsen R. CGG-repeat length and neuropathological and molecular correlates in a mouse model for fragile X-associated tremor/ataxia syndrome. J Neurochem. 2008a;107:1671–1682. doi: 10.1111/j.1471-4159.2008.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Severijnen E, de Jong FH, Hessl D, Hagerman RJ, Oostra BA, Willemsen R. Altered hypothalamus-pituitary-adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology. 2008b;33:863–873. doi: 10.1016/j.psyneuen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Huizer K, Severijnen LA, Hukema RK, Berman RF, Oostra WR. CGG repeat length and neuropathological and molecular correlates in a mouse model for fragile Xassociated tremor/ataxia syndrome. J Neurochem. 2008c;107:1671–1682. doi: 10.1111/j.1471-4159.2008.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Willemsen R, Oostra BA. The FMR1 gene and fragile X-associated tremor/ ataxia syndrome. Am J Med Genet B Neuropsychiatr Genet. 2009a;150:782–798. doi: 10.1002/ajmg.b.30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Willemsen R, Oostra BA. The FMR1 gene and fragile X-associated tremor/ ataxia syndrome. Am J Med Genet B Neuropsychiatr Genet. 2009b;150B:782–798. doi: 10.1002/ajmg.b.30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tassone F, Berman RF, Hagerman P, Hagerman RJ, Willemsen R, Pessah IN. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010;19:196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Kogan C, Turk J, Manly T, James N, Mills A, Dalton A. The emerging fragile X premutation phenotype: evidence from the domain of social cognition. Brain Cogn. 2005;57:53–60. doi: 10.1016/j.bandc.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Cornish K, Turk J, Hagerman R. The fragile X continuum: new advances and perspectives. J Intellect Disabil Res. 2008a;52:469–482. doi: 10.1111/j.1365-2788.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Li L, Kogan CS, Jacquemont S, Turk J, Dalton A, Hagerman RJ, Hagerman PJ. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008b;44:628–636. doi: 10.1016/j.cortex.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Kogan CS, Li L, Turk J, Jacquemont S, Hagerman RJ. Lifespan changes in working memory in fragile X premutation males. Brain Cogn. 2009;69:551–558. doi: 10.1016/j.bandc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Martínez Cerdeno V, Navarro Porras E, Prakash AN, Angelastro JM, Willemsen R, Hagerman PJ, Pessah IN, Berman RF, Noctor SC. Premutation CGG repeat expansion of the Fmr1 gene impairs mouse neocortical development. Hum Mol Genet. 2011;20:64–79. doi: 10.1093/hmg/ddq432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, Heulens I, Brouwer JR, Willemsen R, De Geest N, Reeve SP, De Deyn PP, Hassan BA, Kooy RF. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS) Brain Res. 2009;1253:176–183. doi: 10.1016/j.brainres.2008.11.075. [DOI] [PubMed] [Google Scholar]
- Entezam A, Usdin K. ATR protects the genome against CGG.CCG-repeat expansion in Fragile X premutation mice. Nucleic Acids Res. 2008;36:1050–1056. doi: 10.1093/nar/gkm1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entezam A, Usdin K. ATM and ATR protect the genome against two different types of tandem repeat instability in Fragile X premutation mice. Nucleic Acids Res. 2009;37:6371–6377. doi: 10.1093/nar/gkp666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entezam A, Biacsi R, Orrison B, Saha T, Hoffman GE, Grabczyk E, Nussbaum RL, Usdin K. Regional FMRP deficits and large repeat expansions into the full mutation range in a new fragile X premutation mouse model. Gene. 2007;395:125–134. doi: 10.1016/j.gene.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entezam A, Lokanga AR, Le W, Hoffman G, Usdin K. Potassium bromate, a potent DNA oxidizing agent, exacerbates germline repeat expansion in a fragile X premutation mouse model. Hum Mutat. 2010;31:611–616. doi: 10.1002/humu.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr TD, Whishaw IQ. Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke. 2002;33:1869–1875. doi: 10.1161/01.str.0000020714.48349.4e. [DOI] [PubMed] [Google Scholar]
- Farr TD, Liu L, Colwell KL, Whishaw IQ, Metz GA. Bilateral alteration in stepping pattern after unilateral motor cortex injury: a new test strategy for analysis of skilled limb movements in neurological mouse models. J Neurosci Methods. 2006;153:104–113. doi: 10.1016/j.jneumeth.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Garcia A, Hagerman P. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19:R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19:R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DN, Duffaud AM, Pothuizen HH, Haddon JE, Killcross S. Lesions to the ventral, but not the dorsal, medial prefrontal cortex enhance latent inhibition. Eur J Neurosci. 2010;31:1474–1482. doi: 10.1111/j.1460-9568.2010.07178.x. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behav Neurosci. 2005;119:1307–1315. doi: 10.1037/0735-7044.119.5.1307. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Howard BP, Hunsaker MR, Kesner RP. Human topological task adapted for rats: spatial information processes of the parietal cortex. Neurobiol Learn Mem. 2008;90:389–394. doi: 10.1016/j.nlm.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, LeeheyM A, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Greco CM, Soontrapornchai K, Wirojanan J, Gould JE, Hagerman PJ, Hagerman RJ. Testicular and pituitary inclusion formation in fragile X associated tremor/ataxia syndrome. J Urol. 2007;177:1434–1437. doi: 10.1016/j.juro.2006.11.097. [DOI] [PubMed] [Google Scholar]
- Greco CM, Tassone F, Garcia-Arocena D, Tartaglia N, Coffey SM, Vartanian TK, Brunberg JA, Hagerman PJ, Hagerman RJ. Clinical and neuropathologic findings in a woman with the FMR1 premutation and multiple sclerosis. Arch Neurol. 2008;65:1114–1116. doi: 10.1001/archneur.65.8.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon JE, George DN, Killcross S. Contextual control of biconditional task performance: evidence for cue and response competition in rats. Q J Exp Psychol (Colchester) 2008;61:1307–1320. doi: 10.1080/17470210701515819. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ. The fragile X prevalence paradox. J Med Genet. 2008;45:498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman P, Hagerman RJ. The fragile-X premutation: a maturing perspective. Am J Hum Genet. 2004;74:805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- Hashem V, Galloway JN, Mori M, Willemsen R, Oostra BA, Paylor R, Nelson DL. Ectopic expression of CGG containing mRNA is neurotoxic in mammals. Hum Mol Genet. 2009;18:2443–2451. doi: 10.1093/hmg/ddp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Wenzel HJ, Willemsen R, Berman RF. Progressive spatial processing deficits in a mouse model of the fragile X premutation. Behav Neurosci. 2009;123:1315–1324. doi: 10.1037/a0017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Goodrich-Hunsaker NJ, Willemsen R, Berman RF. Temporal ordering deficits in female CGG KI mice heterozygous for the fragile X premutation. Behav Brain Res. 2010;213:263–268. doi: 10.1016/j.bbr.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F, Hagerman PJ. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, Zhang L, Jardini T, Gane LW, Harris SW, Herman K, Grigsby J, Greco CM, Berry-Kravis E, Tassone F, Hagerman PJ. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediatelength and premutation carriers. Hum Mol Genet. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- Kraff J, Tang HT, Cilia R, Canesi M, Pezzoli G, Goldwurm S, Hagerman PJ, Tassone F. Screen for excess FMR1 premutation alleles among males with parkinsonism. Arch Neurol. 2007;64:1002–1006. doi: 10.1001/archneur.64.7.1002. [DOI] [PubMed] [Google Scholar]
- Lavedan CN, Garrett L, Nussbaum RL. Trinucleotide repeats (CGG)22TGG(CGG)43TGG (CGG)21 from the fragile X gene remain stable in transgenic mice. Hum Genet. 1997;100:407–414. doi: 10.1007/s004390050525. [DOI] [PubMed] [Google Scholar]
- Lavedan C, Grabczyk E, Usdin K, Nussbaum RL. Long uninterrupted CGG repeats within the first exon of the human FMR1 gene are not intrinsically unstable in transgenic mice. Genomics. 1998;50:229–240. doi: 10.1006/geno.1998.5299. [DOI] [PubMed] [Google Scholar]
- Leehey MA, Berry-Kravis E, Min SJ, Hall DA, Rice CD, Zhang L, Grigsby J, Greco CM, Reynolds A, Lara R, Cogswell J, Jacquemont S, Hessl DR, Tassone F, Hagerman R, Hagerman PJ. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007;22:203–206. doi: 10.1002/mds.21252. [DOI] [PubMed] [Google Scholar]
- Leehey MA, Berry-Kravis E, Goetz CG, Zhang L, Hall DA, Li L, Rice CD, Lara R, Cogswell J, Reynolds A, Gane L, Jacquemont S, Tassone F, Grigsby J, Hagerman RJ, Hagerman PJ. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology. 2008;70:1397–1402. doi: 10.1212/01.wnl.0000281692.98200.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis JP, Killcross S, Haddon JE. Inactivation of the prelimbic, but not infralimbic, prefrontal cortex impairs the contextual control of response conflict in rats. Eur J Neurosci. 2007;25:559–566. doi: 10.1111/j.1460-9568.2006.05295.x. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and coordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. The ladder rung walking task: a scoring system and its practical application. J Vis Exp. 2009;12:1204. doi: 10.3791/1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Pessah IN, Gane L, Ono M, Hagerman PJ, Brunberg JA, Tassone F, Bourgeois JA, Adams PE, Nguyen DV, Hagerman R. Early onset of neurological symptoms in fragile X premutation carriers exposed to neurotoxins. Neurotoxicology. 2010;31:399–402. doi: 10.1016/j.neuro.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Entezam A, Usdin K, Huang T, Liu ZH, Hoffman GE, Smith CB. A mouse model of the fragile X premutation: effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiol Dis. 2011;42:85–98. doi: 10.1016/j.nbd.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raske C, Hagerman PJ. Molecular pathogenesis of fragile X-Associated tremor/ataxia syndrome. J Investig Med. 2009;57(8):825–829. doi: 10.231/JIM.0b013e3181be329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellier C, Rau F, Liu Y, Tassone F, Hukema RK, Gattoni R, Schneider A, Richard S, Willemsen R, Elliott DJ, Hagerman PJ, Charlet-Berguerand N. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010;29:1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senturk D, Nguyen DV, Tassone F, Hagerman RJ, Carroll RJ, Hagerman PJ. Covariate adjusted correlation analysis with application to FMR1 premutation female carrier data. Biometrics. 2009;65:781–792. doi: 10.1111/j.1541-0420.2008.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Hagerman P. Expression of the FMR1 gene. Cytogenet Genome Res. 2003;100:124–128. doi: 10.1159/000072846. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Loesch DZ, Lachiewicz A, Taylor AK, Hagerman PJ. Fragile X males with unmethylated, full mutation trinucleotide repeat expansions have elevated levels of FMR1 messenger RNA. Am J Med Genet. 2000a;94:232–236. doi: 10.1002/1096-8628(20000918)94:3<232::aid-ajmg9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000b;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Mills JB, Harris SW, Gane LW, Hagerman PJ. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet. 2000c;91:144–152. doi: 10.1002/(sici)1096-8628(20000313)91:2<144::aid-ajmg14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Garcia-Arocena D, Khandjian EW, Greco CM, Hagerman PJ. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ ataxia syndrome. J Med Genet. 2004a;41:e43. doi: 10.1136/jmg.2003.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS) RNA Biol. 2004b;1:103–105. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C, Li L, Glover K, Bentley D, Hagerman PJ. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007;13:555–562. doi: 10.1261/rna.280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam D, Errijgers V, Kooy RF, Willemsen R, Mientjes E, Oostra BA, De Deyn PP. Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS) Behav Brain Res. 2005;162:233–239. doi: 10.1016/j.bbr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Ward B. Deficits in response initiation, but not attention, following excitotoxic lesions of posterior parietal cortex in the rat. Brain Res. 1997;775:81–90. doi: 10.1016/s0006-8993(97)00915-3. [DOI] [PubMed] [Google Scholar]
- Ward NM, Brown VJ. Covert orienting of attention in the rat and the role of striatal dopamine. J Neurosci. 1996;16:3082–3088. doi: 10.1523/JNEUROSCI.16-09-03082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel HJ, Hunsaker MR, Greco CM, Willemsen R, Berman RF. Ubiquitinpositive intranuclear inclusions in neuronal and glial cells in a mouse model of the fragile X premutation. Brain Res. 2010;1318:155–166. doi: 10.1016/j.brainres.2009.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Metz GA. Absence of impairments or recovery mediated by the uncrossed pyramidal tract in the rat versus enduring deficits produced by the crossed pyramidal tract. Behav Brain Res. 2002;134:323–336. doi: 10.1016/s0166-4328(02)00051-7. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Travis SG, Koppe SW, Sacrey LA, Gholamrezaei G, Gorny B. Hand shaping in the rat: conserved release and collection vs. flexible manipulation in overground walking, ladder rung walking, cylinder exploration, and skilled reaching. Behav Brain Res. 2010;206:21–31. doi: 10.1016/j.bbr.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen LA, Nieuwenhuizen IM, Schrier M, van Unen L, Tassone F, Hoogeveen AT, Hagerman PJ, Mientjes EJ, Oostra BA. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions: implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12:949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]