Abstract
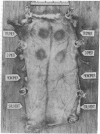
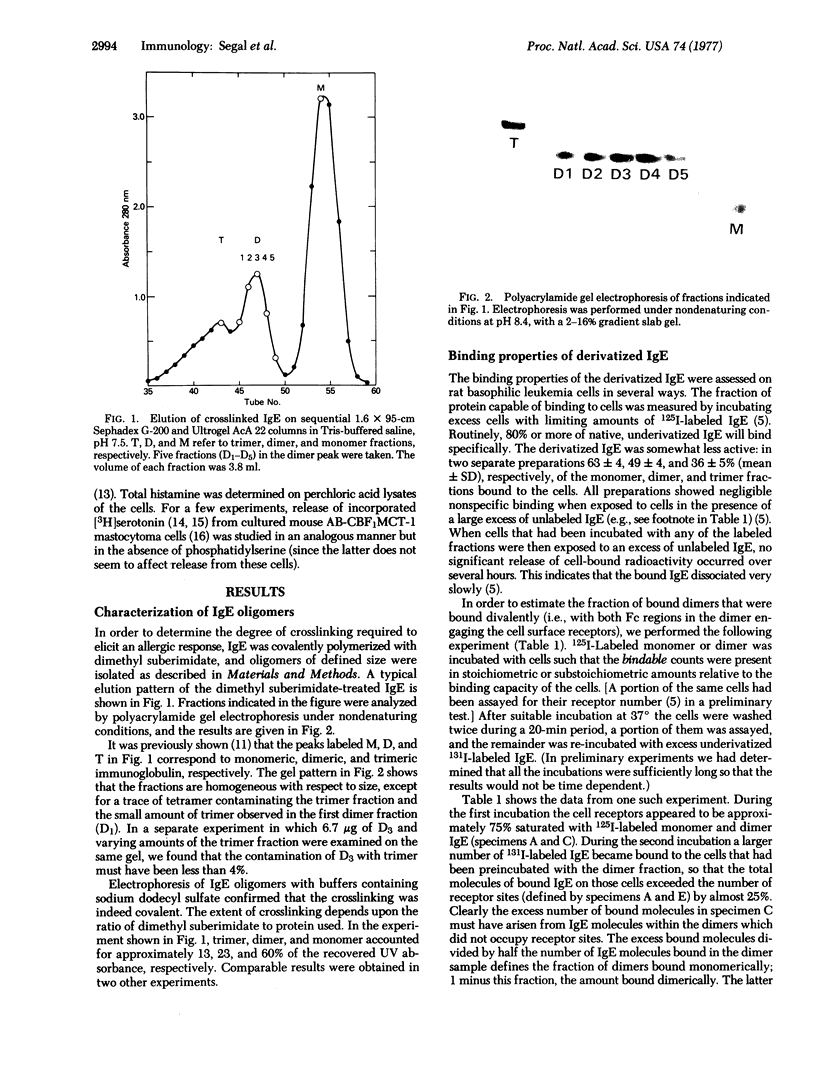
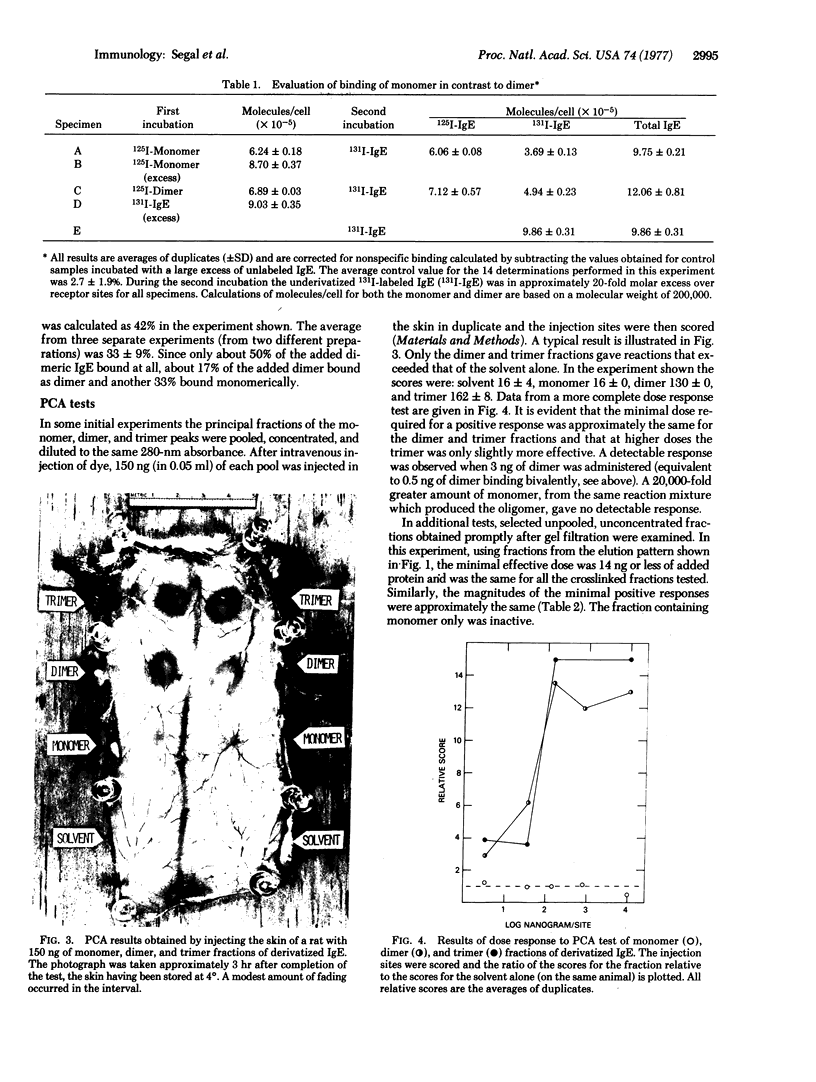
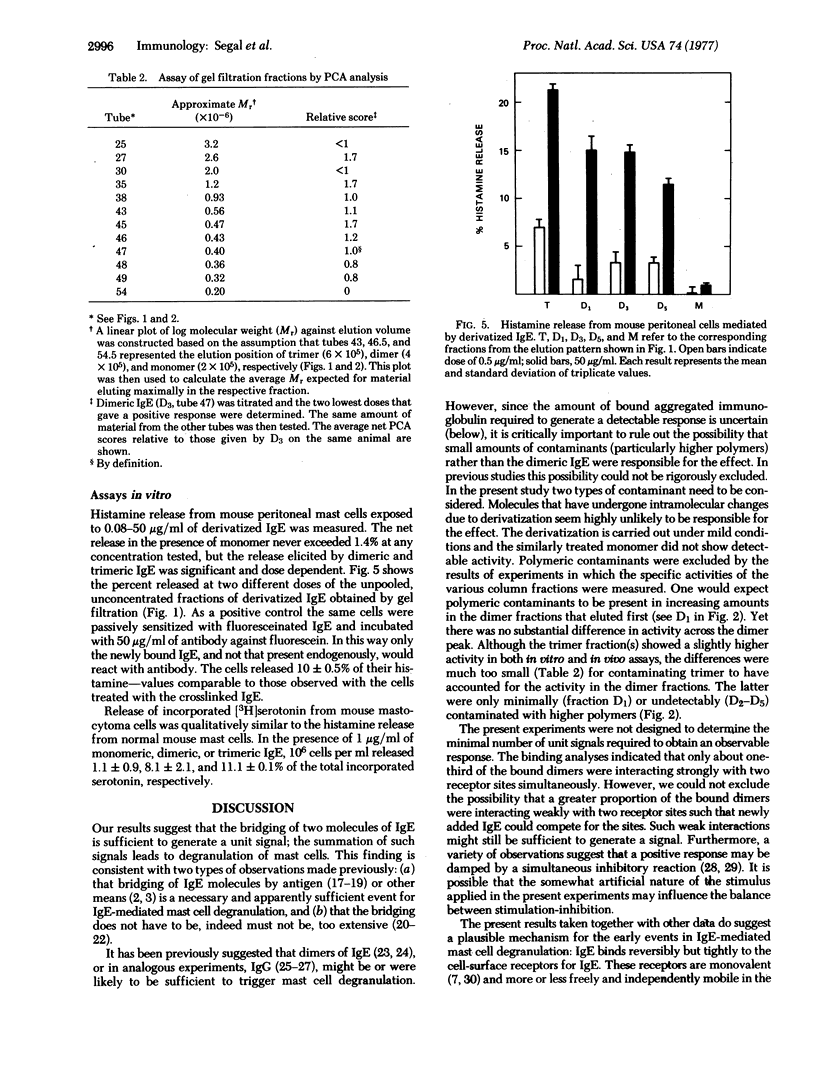
Rat immunoglobulin E (IgE) was treated with a crosslinking reagent, dimethyl suberimidate, and fractionated by gel filtration into monomers, dimers, trimers, and higher polymers. The fractions retained substantial ability to bind specifically to mast cells. About one-third of the cell-bound dimers appeared to bind bivalently. The fractions were assayed in vivo by passive cutaneous anaphylaxis in rats, and for histamine or serotonin release in vitro using normal or tumor mouse mast cells. The monomers showed no activity, while the dimers and higher polymers gave excellent and approximately equivalent responses. We conclude that IgE that has been crosslinked to form dimers prior to the addition to mast cells can serve as a unit signal for triggering IgE-mediated exocytosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazin H., Querinjean P., Beckers A., Heremans J. F., Dessy F. Transplantable immunoglobulin-secreting tumours in rats. IV. Sixty-three IgE-secreting immunocytoma tumours. Immunology. 1974 Apr;26(4):713–723. [PMC free article] [PubMed] [Google Scholar]
- Becker K. E., Ishizaka T., Metzger H., Ishizaka K., Grimley P. M. Surface IgE on human basophils during histamine release. J Exp Med. 1973 Aug 1;138(2):394–409. doi: 10.1084/jem.138.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston E., Leonard B. J., Lowe J. S., Welford H. J. Basophilic leukaemia in the albino rat and a demonstration of the basopoietin. Nat New Biol. 1973 Jul 18;244(133):73–76. doi: 10.1038/newbio244073b0. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Garland L. G. Desensitization in the process of histamine secretion induced by antigen and dextran. J Physiol. 1974 Jun;239(2):381–391. doi: 10.1113/jphysiol.1974.sp010574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth A., Adams H. R., Knoohuizen M. Phosphatidylserine: selective enhancer of histamine release. Science. 1971 Sep 10;173(4001):1034–1035. doi: 10.1126/science.173.4001.1034. [DOI] [PubMed] [Google Scholar]
- ISHIZAKA K., ISHIZAKA T., BANOVITZ J. BIOLOGIC ACTIVITY OF SOLUBLE ANTIGEN-ANTIBODY COMPLEXES. IX. SOLUBLE COMPLEXES OF RABBIT ANTIBODY WITH UNIVALENT AND DIVALENT HAPTENS. J Immunol. 1964 Dec;93:1001–1007. [PubMed] [Google Scholar]
- ISHIZAKA K., ISHIZAKA T., BANOVITZ J. BIOLOGICAL ACTIVITIES OF AGGREGATED GAMMA-GLOBULIN. VII. MINIMUM SIZE OF AGGREGATED GAMMA-GLOBULIN OR ITS PIECE 3 REQUIRED FOR THE INDUCTION OF SKIN REACTIVITY AND COMPLEMENT FIXATION. J Immunol. 1965 Jun;94:825–832. [PubMed] [Google Scholar]
- Isersky C., Metzger H., Buell D. N. Cell cycle-associated changes in receptors for IgE during growth and differentiation of a rat basophilic leukemia cell line. J Exp Med. 1975 May 1;141(5):1147–1162. doi: 10.1084/jem.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Immune mechanisms of reversed type reaginic hypersensitivity. J Immunol. 1969 Sep;103(3):588–595. [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Induction of erythema-wheal reactions by soluble antigen-gammaE antibody complexes in humans. J Immunol. 1968 Jul;101(1):68–78. [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T., Lee E. H. Biologic function of the Fc fragments of E myeloma protein. Immunochemistry. 1970 Aug;7(8):687–702. doi: 10.1016/0019-2791(70)90175-8. [DOI] [PubMed] [Google Scholar]
- Kulczycki A., Jr, Metzger H. The interaction of IgE with rat basophilic leukemia cells. II. Quantitative aspects of the binding reaction. J Exp Med. 1974 Dec 1;140(6):1676–1695. doi: 10.1084/jem.140.6.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE B. B. THE NATURE OF THE ANTIGEN-ANTIBODY COMPLEXES WHICH INITIATE ANAPHYLACTIC REACTIONS. II. THE EFFECT OF MOLECULAR SIZE ON THE ABILITIES OF HOMOLOGOUS MULTIVALENT BENZYLPENICILLOYL HAPTENS TO EVOKE PCA AND PASSIVE ARTHUS REACTIONS IN THE GUINEA PIG. J Immunol. 1965 Jan;94:121–131. [PubMed] [Google Scholar]
- Lawson D., Fewtrell C., Gomperts B., Raff M. Anti-immunoglobulin-induced histamine secretion by rat peritoneal mast cells studied by immunoferritin electron microscopy. J Exp Med. 1975 Aug 1;142(2):391–402. doi: 10.1084/jem.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M. The immediate allergic response: in vitro separation of antigen activation, decay and histamine release. J Immunol. 1971 Oct;107(4):1122–1130. [PubMed] [Google Scholar]
- Magro A. M., Alexander A. Histamine release: in vitro studies of the inhibitory region of the dose-response curve. J Immunol. 1974 May;112(5):1762–1765. [PubMed] [Google Scholar]
- Magro A. M., Alexander A. In vitro studies of histamine release from rabbit leukocytes by divalent haptens. J Immunol. 1974 May;112(5):1757–1761. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mendoza G. R., Metzger H. Disparity of IgE binding between normal and tumor mouse mast cells. J Immunol. 1976 Nov;117(5 Pt 1):1573–1578. [PubMed] [Google Scholar]
- Mendoza G., Metzger H. Distribution and valency of receptor for IgE on rodent mast cells and related tumour cells. Nature. 1976 Dec 9;264(5586):548–550. doi: 10.1038/264548a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Roser J. F., Henson P. M., Cochrane C. G. Activation of rat mast cells by low molecular weight stimuli. J Immunol. 1974 Feb;112(2):573–582. [PubMed] [Google Scholar]
- Mossmann H., Meyer-Delius M., Vortisch U., Kickhöfen B., Hammer D. K. Experimental studies on the bridging hypothesis of anaphylaxis: haptenic determinants required to elicit immediate-type reactions in calf skin by separate or concurrent sensitization with reagins of different specificity. J Exp Med. 1974 Dec 1;140(6):1468–1481. doi: 10.1084/jem.140.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki J. A., Grassick R., Seymour D., Kind L. S. The use of 3H serotonin release from mast cells of the mouse as an assay for mediator liberation. Immunol Commun. 1976;5(1-2):27–39. doi: 10.3109/08820137609020610. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Hurwitz E. Dimers and trimers of immunoglobulin G covalently cross-linked with a bivalent affinity label. Biochemistry. 1976 Nov 30;15(24):5253–5258. doi: 10.1021/bi00669a009. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974 Feb;57(2):383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]