Significance
The paradox of selective hepatic insulin resistance, wherein the insulin-resistant liver fails to suppress glucose production but continues to produce triglycerides, is central to the pathophysiology of type 2 diabetes. We hypothesized that hepatic triglyceride synthesis is regulated mostly by fatty acid delivery to the liver and independent of changes in hepatic insulin signaling. To examine this hypothesis, we used a novel LC-MS/MS method to measure rates of hepatic fatty acid esterification in vivo. In contrast to hepatic de novo hepatic lipogenesis, rates of hepatic esterification of fatty acids into triglyceride was primarily dependent on fatty acid delivery and independent of hepatic insulin action, providing an explanation for increased hepatic triglyceride synthesis in the presence of hepatic insulin resistance.
Keywords: nonalcoholic fatty liver disease, hepatic insulin resistance, lipogenesis, esterification, mass spectrometry
Abstract
A central paradox in type 2 diabetes is the apparent selective nature of hepatic insulin resistance—wherein insulin fails to suppress hepatic glucose production yet continues to stimulate lipogenesis, resulting in hyperglycemia, hyperlipidemia, and hepatic steatosis. Although efforts to explain this have focused on finding a branch point in insulin signaling where hepatic glucose and lipid metabolism diverge, we hypothesized that hepatic triglyceride synthesis could be driven by substrate, independent of changes in hepatic insulin signaling. We tested this hypothesis in rats by infusing [U-13C] palmitate to measure rates of fatty acid esterification into hepatic triglyceride while varying plasma fatty acid and insulin concentrations independently. These experiments were performed in normal rats, high fat-fed insulin-resistant rats, and insulin receptor 2′-O-methoxyethyl chimeric antisense oligonucleotide-treated rats. Rates of fatty acid esterification into hepatic triglyceride were found to be dependent on plasma fatty acid infusion rates, independent of changes in plasma insulin concentrations and independent of hepatocellular insulin signaling. Taken together, these results obviate a paradox of selective insulin resistance, because the major source of hepatic lipid synthesis, esterification of preformed fatty acids, is primarily dependent on substrate delivery and largely independent of hepatic insulin action.
The phenomenon of selective hepatic insulin resistance, in which hepatic glucose metabolism becomes unresponsive to insulin but hepatic lipogenesis continues unabated, is a longstanding paradox in type 2 diabetes (T2D) (1, 2). Selective hepatic insulin resistance has been proposed to explain the common clinical phenotype of hyperglycemia, hyperlipidemia, and nonalcoholic fatty liver disease (NAFLD) in T2D patients and begs the question of whether treating T2D patients with insulin might in turn exacerbate NAFLD.
Attempts to resolve the mechanism by which triglyceride content paradoxically increases in the insulin-resistant liver have focused on the regulation of de novo lipogenesis (DNL) by the insulin-dependent sterol regulatory element binding protein 1c (SREBP-1c) (3, 4). Evidence for the importance of DNL in insulin-resistant hepatic triglyceride synthesis includes reports of increased hepatic DNL in both humans with insulin resistance and humans with NAFLD (5–7), as well as in leptin-deficient insulin-resistant ob/ob mice (8). To address the paradox at the level of hepatic insulin signaling, several investigators have hypothesized the existence of a branch point in the insulin signaling cascade to separate insulin's anabolic signal into discrete pathways controlling gluconeogenesis (through the transcription factor FoxO1) and DNL (through SREBP-1c). Differential expression or activation of insulin receptor substrate-2 (IRS-2) has been implicated as such a branch point; cultured hepatocytes exposed to chronically elevated insulin displayed reduced IRS-2 and increased SREBP-1c expression (9), and short hairpin RNA (shRNA) injection studies in mice supported differential effects of IRS-1 and IRS-2 on glucose and lipid metabolism (10). The mTORC1 complex is another potential branch point in insulin signaling: rapamycin treatment in rat hepatocytes revealed that, although insulin-mediated SREBP-1c induction requires mTORC1 activity, insulin-mediated phosphoenolpyruvate carboxykinase (PEPCK) transcriptional repression does not (1). Additionally, experiments in genetically modified mice led to the proposal that Notch can regulate DNL through mTORC, independent of regulation of FOXO1 (11). A third recently proposed branch point suggests that Akt2 phosphorylation at serine 473 is required for Akt activity toward some, but not all, of its substrates (12). Although insulin stimulation of Akt2 Ser473 phosphorylation has been shown to be impaired in the insulin-resistant liver, biochemical evidence for this hypothesis is absent thus far.
However, this proposed model of a branch point in the insulin signaling cascade leading to increased SREBP-1c–driven DNL and decreased suppression of hepatic gluconeogenesis is not consistent with studies of cellular mechanisms of lipid-induced insulin resistance. These studies have implicated inhibition of insulin signaling at the insulin receptor kinase by lipid-mediated activation of protein kinase C-ε (PKCε) (13), thus precluding the possibility of more distal branch points. Furthermore, the role of insulin in the regulation of DNL is difficult to discern from the role of lipogenic substrate supply for DNL in vivo; individuals with selective insulin resistance in skeletal muscle but normal hepatic insulin sensitivity demonstrate elevated rates of hepatic DNL, which could be attributed to increased diversion of DNL precursors away from skeletal muscle to liver (14, 15). The same concerns exist with regard to the relationship between insulin levels and DNL measurements in the leptin-deficient ob/ob mouse. Decreasing expression of the carbohydrate response element binding protein (ChREBP) by shRNA completely normalizes rates of DNL without a significant reduction in circulating insulin concentrations (16), suggesting that insulin is not the key mediator of increased DNL in leptin-deficient animals.
Hepatic triglyceride synthesis has two components: (i) de novo synthesis of fatty acids from acetyl CoA (DNL) and (ii) esterification of fatty acids from all sources (DNL, fatty acids from adipose lipolysis or chylomicron lipolysis, and re-esterification of intrahepatic lipid). As an alternative explanation for the apparently paradoxical excess lipid synthesis seen in the insulin-resistant state, we examined the hypothesis that fatty acid substrate supply may regulate hepatic lipid synthesis in an insulin-independent manner. This hypothesis is particularly appealing given that NAFLD often occurs in the setting of nutrient oversupply (e.g., obesity or lipodystrophy). Furthermore, studies of insulin-resistant humans with and without fatty liver indicate that hepatic DNL typically accounts for less than 20% of hepatic VLDL triglyceride synthesis, that more than 60% of liver triglyceride is derived from the esterification of plasma fatty acids, and that these re-esterified lipids can contribute to ∼60% of hepatic VLDL triglyceride (TG) synthesis in individuals with T2D (6, 14, 15, 17–19).
Although most studies have determined that esterification of preexisting fatty acids is the predominant lipogenic flux in human liver, prior investigators have been unable to definitively determine in vivo the insulin dependence or insulin independence of fatty acid esterification. In perfused livers, increased insulin led to increased esterification of fatty acids present in the perfusate (20, 21), whereas in tissue culture, insulin did not alter rates of fatty acid re-esterification from the intrahepatocellular lipid pool (22).
To examine the hypothesis that rates of hepatic triglyceride synthesis from fatty acid esterification are dependent on substrate flux and independent of circulating plasma insulin concentrations, we developed a novel method to assess rates of hepatic esterification of fatty acids into TG. We infused [U-13C] palmitate in awake rats, assessed precursor and product pools by LC-MS/MS spectrometry, and thereby calculated rates of fatty acid esterification into hepatic TG. The discrete effects of increased fatty acid supply, hyperinsulinemia, and hepatic insulin signaling on rates of hepatic esterification were assessed in control rats and rats made insulin resistant from high fat feeding or acute knockdown of hepatic insulin receptor expression.
Results
We developed an acute lipid infusion model to test the discrete roles of substrate (fatty acid) delivery and insulin action on hepatic lipid synthesis in an awake free-ranging rat model. Endogenous insulin secretion was suppressed with somatostatin to match plasma insulin concentrations in low and high lipid infusion groups. Rats were infused with either saline or Intralipid (Baxter Healthcare) and either low-dose (0.5 mU/kg⋅min) or high-dose (4.0 mU/kg⋅min) insulin. High-dose insulin infusion raised plasma insulin concentrations 7- to 10-fold relative to the low-dose insulin infusion (Table 1). Infusion of Intralipid with heparin led to increased plasma fatty acid levels and plasma TG levels. Although Intralipid infusion rates were matched between basal insulin and high insulin groups, hyperinsulinemia blunted the rise in plasma fatty acid and TG concentration with Intralipid infusion.
Table 1.
Plasma insulin, nonesterified fatty acid (NEFA), and triglyceride levels during 70-min Intralipid (vs. saline) infusions
| Saline infusion | Intralipid infusion | |||
| Regular chow-fed rats | Low insulin | High insulin | Low insulin | High insulin |
| Insulin (µU/mL) | 10.5 ± 0.7 | 99.2 ± 4.8 | 9.0 ± 3.3 | 77.7 ± 3.3 |
| NEFA (mM) | 0.76 ± 0.04 | 0.14 ± 0.09 | 2.9 ± 0.2 | 1.4 ± 0.1 |
| Triglycerides (mg/dL) | 30.7 ± 2.0 | 16.6 ± 1.6 | 268 ± 14 | 153 ± 12 |
| High Fat Diet Fed Rats | ||||
| Insulin (µU/mL) | 10.1 ± 0.8 | 79.1 ± 3.8 | 8.5 ± 1.0 | 56.4 ± 3.6 |
| NEFA (mM) | 0.74 ± 0.03 | 0.29 ± 0.07 | 2.0 ± 0.1 | 1.1 ± 0.09 |
| Triglycerides (mg/dL) | 27.2 ± 2.9 | 14.3 ± 1.9 | 200 ± 16 | 109 ± 6 |
| IRASO-treated rats | Saline infusion | Intralipid infusion | ||
| Insulin (µU/mL) | 28.4 ± 1.9 | 42.2 ± 5.0 | ||
| NEFA (mM) | 1.15 ± 0.08 | 3.8 ± 0.2 | ||
| Triglycerides (mg/dL) | 44.9 ± 3.2 | 281 ± 30 | ||
Data are reported as mean ± SEM.
Insulin-Independent Fatty Acid Esterification into Hepatic Triglyceride.
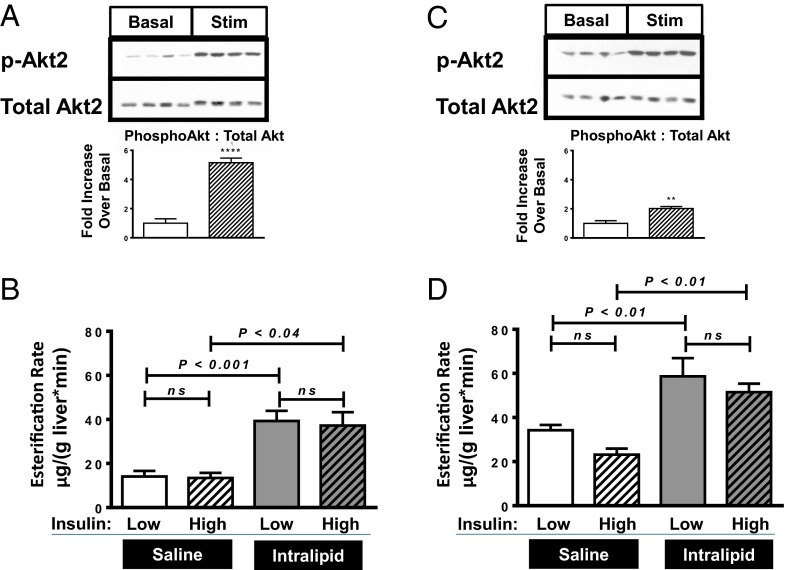
In regular chow-fed, insulin sensitive (Fig. 1A) rats, rates of U13C palmitate esterification into hepatic TG (Fig. 1B) were nearly tripled with Intralipid infusion. In contrast, rates of fatty acid esterification were unaffected by hyperinsulinemia. These results support the hypothesis that plasma fatty acids can promote hepatic TG synthesis independent of increases in plasma insulin concentrations.
Fig. 1.
Infusion studies with insulin-sensitive regular chow-fed rats and insulin-resistant fat-fed rats. (A and B) Regular chow-fed rats. (C and D) Fat-fed rats. (A and C) Hepatic Akt2 phosphorylation immunoblots, comparing basal insulin infused (Basal) vs. high insulin infused (Stim) groups. Bar graphs represents quantitation of Western blot bands, ratio phosphorylated Akt2 to total Akt2, normalized to the basal group. (B and D) Esterification rates. Bar graph symbols—Western blot quantitation:  , basal group;
, basal group;  , high insulin (Stim) group. **P < 0.01 and ****P < 0.0001 comparing insulin stimulated to basal. Esterification rates:
, high insulin (Stim) group. **P < 0.01 and ****P < 0.0001 comparing insulin stimulated to basal. Esterification rates:  , low insulin/saline infusion;
, low insulin/saline infusion;  , high insulin/saline infusion;
, high insulin/saline infusion;  , low insulin/Intralipid infusion;
, low insulin/Intralipid infusion;  , high insulin/Intralipid infusion. Data reported as mean ± SEM.
, high insulin/Intralipid infusion. Data reported as mean ± SEM.
To evaluate whether this insulin-independent substrate-dependent esterification occurs in insulin-resistant animals with hepatic steatosis, we measured rates of hepatic esterification of fatty acids into TG in insulin-resistant (Fig. 1C) high fat-fed rats (Fig. 1D). Although basal esterification rates were already higher in high fat-fed animals vs. regular chow-fed animals, infusion of Intralipid further doubled hepatic esterification rates in high fat-fed rats. In contrast, hyperinsulinemia per se did not alter esterification rates in either chow-fed or high fat-fed rats. These experiments further support our hypothesis that hepatic TG synthesis from fatty acids is largely a substrate-dependent, but insulin-independent, process.
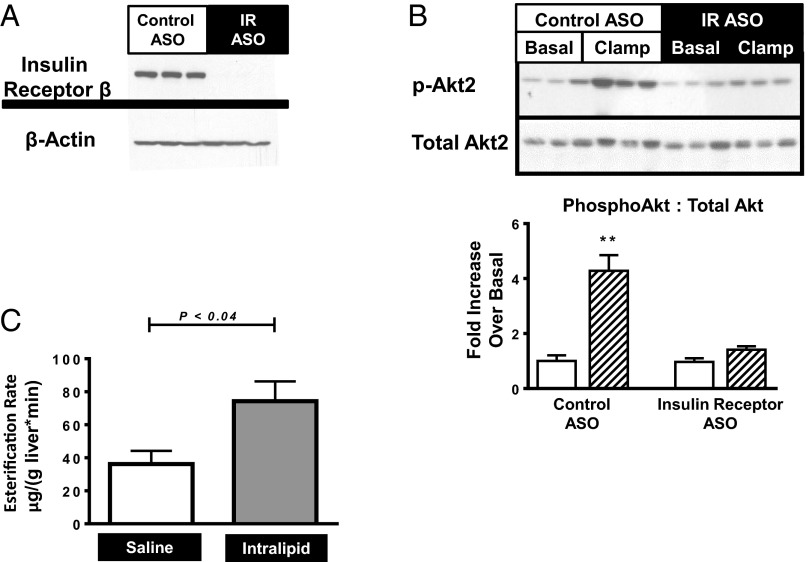
Insulin Receptor 2′-O-Methoxyethyl Chimeric Antisense Oligonucleotide Treatment.
To further discern the role of insulin signaling on hepatic lipid esterification, we acutely decreased insulin receptor content in regular chow-fed rats using an insulin receptor antisense oligonucleotide (IRASO). IRASO effectively decreased hepatic IR protein expression by 99% (Fig. 2A) and prevented insulin-stimulated Akt phosphorylation (Fig. 2B). Hepatic esterification rates were measured in IRASO-treated rats infused with either Intralipid or saline (Fig. 2C). Rates of hepatic esterification in saline-infused animals were similar to rates seen in high fat-fed rats. Despite the near-complete absence of hepatic insulin receptor, Intralipid infusion significantly increased the rates of fatty acid esterification into TG. These data support the hypothesis that hepatocellular insulin signaling is not required for fatty acid-mediated esterification of fatty acids into hepatic TG.
Fig. 2.
Infusion studies with IRASO-treated rats. (A) Hepatic insulin receptor immunoblots, comparing control ASO vs. IRASO groups. (B) Hepatic Akt2 phosphorylation in control ASO treated vs. IRASO treated rats. Overnight fasted rats (Basal) compared with rats subject to a 20-min hyperinsulinemic clamp (Clamp). Bar graphs represent the ratio of phosphorylated Akt2 to total Akt2.  , basal group;
, basal group;  , clamp group. **P < 0.01. (C) Esterification rates, saline infusion vs. Intralipid infusion.
, clamp group. **P < 0.01. (C) Esterification rates, saline infusion vs. Intralipid infusion.  , saline infusion;
, saline infusion;  , Intralipid infusion. Data reported as mean ± SEM.
, Intralipid infusion. Data reported as mean ± SEM.
DNL.
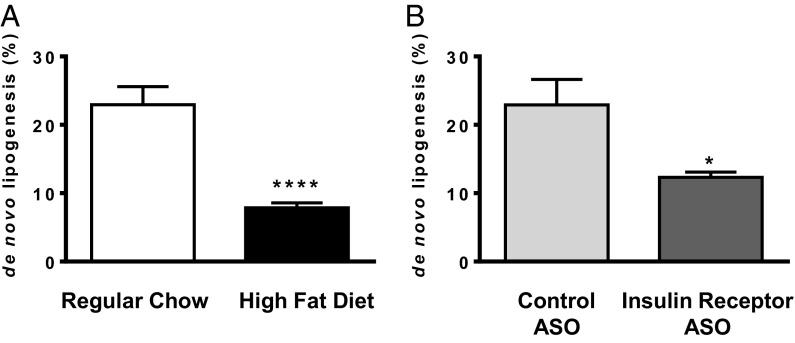
We also assessed DNL in control, high-fat fed, and IRASO-treated rats using a deuterated water method (23–25). DNL was compared in two separate experiments: first in fat-fed rats and regular chow-fed rats and second between IRASO-treated rats and control ASO-treated rats. In contrast to the observed increases in hepatic esterification rates observed in high fat-fed and IRASO-treated rats with increased substrate delivery, net DNL was reduced by 66% and 46%, respectively, in high fat-fed and IRASO-treated rats (Fig. 3). These data are consistent with prior measurements of DNL in fat-fed animals and hepatic insulin receptor KO mice (26, 27) and consistent with the hypothesis that, in contrast to hepatic reesterification, hepatic DNL is an insulin-dependent process.
Fig. 3.
De novo hepatic lipogenesis in control vs. IRASO-treated rats. (A) Regular chow-fed vs. high fat-fed animals. (B) Control ASO treatment vs. IRASO treatment. *P < 0.04; ****P < 0.0001. Data reported as mean ± SEM.
Role of Lipogenic Genes.
One mechanism whereby insulin can regulate DNL is by altering the hepatic expression of key lipogenic enzymes (28). Consistent with this hypothesis, IRASO reduced fasting hepatic mRNA expression of ATP citrate lyase, fatty acid synthase (Fasn), and steroyl CoA desaturase (Scd1) (Fig. S1 A–D). Fat-fed rats demonstrated a significant reduction in Fasn and a trend toward reduction in Scd1. Both total and phosphorylated acetyl CoA carboxylase (ACC) protein abundance were assessed by Western blot (Fig. S1E); total ACC was markedly suppressed in IRASO-treated rats. Hepatic expression of the regulatory element Srebp-1c was also significantly reduced in IRASO-treated rats (Fig. S1I).
Fatty acid esterification may also be regulated by altering expression of key enzymes. IRASO suppressed diacylglycerol acyltransferase (Dgat) expression, whereas there was no increase in any of the key acyltransferases in either the IRASO- or fat-fed cohorts (Fig. S1 F–H). Additionally, we did not observe increased expression of the fatty acid translocase CD36 or key long chain acyl CoA synthetases Acsl1, Acsl3, and Acsl5 (Fig. S1 J–M). Taken together these data are consistent with a model in which substrate flux, not gene expression, is the dominant regulator of hepatic TG synthesis via esterification of preformed fatty acids.
Discussion
In this study, we sought to address the paradox of persistent TG synthesis despite hepatic insulin resistance. We hypothesized that, in contrast to hepatic DNL, hepatic TG synthesis by esterification is substrate dependent and independent of hepatic insulin action. The role of insulin in the regulation of fatty acid esterification in hepatic TG has been controversial due to the difficulties in measuring this flux (20, 21, 29, 30). To address this problem, we used a novel LC-MS/MS method to directly measure rates of hepatic fatty acid esterification from hepatic fatty acyl CoA precursors into hepatic TG in vivo. Using this approach, we found that fatty acyl CoA esterification into hepatic TG was dependent on increases in plasma fatty acid supply and occurred independently of alterations in plasma insulin concentrations. We repeated these experiments in insulin-resistant high fat-fed rats and found that, although basal rates of esterification were increased with respect to the insulin sensitive rat model, esterification rates were dependent on fatty acid flux and did not change with changing plasma insulin concentrations. Finally, we confirmed these findings in a model of acutely ablated hepatic insulin signaling: the insulin receptor 2′-O-methoxyethyl chimeric antisense oligonucleotide-treated rat. In all models studied, rates of hepatic lipogenesis increased with increased fatty acid flux independent of increases in plasma insulin concentrations and hepatocellular insulin signaling.
Our data support the hypothesis that NAFLD can develop independently of insulin action in the liver, and the increase in liver fat observed in insulin-resistant and T2D patients can be explained by increased substrate delivery to the liver, without invoking insulin-driven hepatic DNL. Furthermore these results implicate increased rates of adipocyte lipolysis, and not an increase in insulin stimulation of hepatic lipogenesis, as the major culprit in the pathogenesis of NAFLD. These findings are consistent with studies demonstrating increased TG synthesis from dietary fatty acids in fat-fed mice (31, 32). Our hypothesis would also explain the apparently paradoxical finding of unchanged or decreased hepatic fat content in T2D patients treated with insulin (33–35). If insulin was the primary driving force for hepatic TG synthesis in disorders of hepatic insulin resistance, then one would expect insulin therapy to potentially exacerbate NAFLD rather than having a neutral or beneficial effect (33–35). Our data, demonstrating the insulin-independent substrate-dependent nature of hepatic TG synthesis, explain the beneficial effect of insulin in the progression of hepatic steatosis in insulin-resistant patients (34, 36). Insulin therapy protects the liver from excess fatty acid delivery, both by increasing fat delivery to peripheral stores through increased synthesis and secretion of lipoprotein lipase and plasma membrane translocation of the FATP1 fatty acid transporter in the white adipose tissue and through the suppression of adipose tissue lipolysis (37).
Previous studies have indicated that dietary fat and circulating fatty acids may make the greatest contributions to the hepatic steatosis and dyslipidemia in obesity and T2D (6, 17). In this context, our results suggest a multifaceted approach to treating disorders of dysregulated hepatic lipogenesis. Decreasing caloric intake will both allow for negative energy balance and decrease postprandial lipid loading of the liver. A second approach is to pharmacologically target the adipose tissue, reducing fasting circulating fatty acids by suppression of adipose lipolysis and increasing postprandial peripheral fatty acid uptake. Taken together, these data demonstrate that, in contrast with hepatic DNL, hepatic esterification of fatty acids into hepatic TG is primarily dependent on substrate delivery and largely independent of hepatic insulin action, providing an explanation for the apparent paradox of selective hepatic insulin resistance.
Methods
See SI Methods for additional description of methods, including those for short hyperinsulinemic clamps, in vivo DNL studies, biochemical analyses, quantitative PCR, immunoblotting, and statistical analysis.
All procedures were approved by the Institutional Animal Care and Use Committee of the Yale University School of Medicine.
Animals.
Male Sprague–Dawley rats (Charles River Laboratories) were housed at the animal care facility at the Yale University Animal Research Center and maintained under controlled temperature (22 ± 2 °C) and lighting (12 h of light, 0700–1900 hours; 12 h of dark, 1900–0700 hours) with free access to water and food. Rats weighing 200–275 g underwent surgical arterial and venous catheterization and were allowed 6–21 d to recover before experiments were begun. Rats were maintained on standard regular chow (Harlan Teklad 2108S: 24% protein/58% carbohydrate/18% fat); rats in high-fat diet groups were placed on a high-fat diet (Dyets 112245: 26% carbohydrate, 59% fat, 15% protein calories; Dyets) for 10–14 d before infusions. Rats treated with IRASO (38) were injected intraperitoneally with either control ASO or ASO against insulin receptor β at a dose of 100 mg/kg twice in 1 wk: 7 and 4 d before experiments. All infusions were done after a 14- to 16-h overnight fast.
Infusion Protocols.
Four treatment groups were used in the studies of regular chow-fed insulin-sensitive rats and fat-fed insulin-resistant rats: (i) saline + basal insulin; (ii) Intralipid + basal insulin; (iii) saline + high insulin; and (iv) Intralipid (Baxter Healthcare) + high insulin. Somatostatin was infused to suppress endogenous insulin, insulin was infused at rates to achieve targeted plasma concentrations, and Intralipid and heparin were infused as a source of fatty acids. Glucose was infused to maintain euglycemia in the hyperinsulinemic groups. U13C-palmitate was used as a tracer for the esterification of fatty acids into triglyceride. Infusions began with somatostatin [Bachem, 1:1 mix of soma-14 and soma-28 in normal saline (NS)] at 4 μg/kg⋅min, insulin (0.5 mU/kg⋅min for the low insulin group; 4 mU/kg⋅min for the high insulin group), and 20% (wt/vol) glucose as needed to maintain euglycemia in the hyperinsulinemic groups. After 30 min of insulin and somatostatin infusion, an infusion (50 µL/kg⋅min) of Intralipid or saline was begun in combination with an infusion of U13C palmitate (75 µg/kg⋅min) and heparin (7.2 U/kg⋅h). Blood samples were collected in the basal state and throughout the experiment; between samples, lines were locked with 4 U/mL heparinized normal saline. After 100 min, the animals were anesthetized with pentobarbital, and their livers were removed and freeze-clamped in liquid nitrogen. IRASO-treated rats were divided into two groups, saline vs. Intralipid infused, and treated with a protocol identical to the two basal insulin groups above, although these animals did not achieve basal insulin levels during the 100 min of somatostatin + insulin infusion.
Isotopes and Infusate Preparation.
Human insulin (Novo Nordisk) was diluted in saline with a small amount of BSA [10 μL of 10% (wt/vol) BSA in 10 mL saline] to prevent adhesion to tubing. Uniformly labeled 13C-potassium palmitate (U13C-palmitate; Cambridge Isotope Labs) was prepared at a concentration of 2.5 mg/mL in 5% (wt/vol) BSA in normal saline with 4 U heparin/mL. This was achieved by heating dry U13C-palmitate in purified water to 65 °C and then adding 10% albumin in saline followed by 8 U heparin/mL in saline.
Long Chain Fatty Acid Analysis from Hepatic Triglycerides.
Hepatic TGs were extracted into chloroform (SI Methods). Each sample was spotted onto a silica gel 60 plate, and TLC was performed with a mobile phase of 80:20:1 hexane:diethyl ether:acetic acid. Plates were developed with 0.005% primuline in 80:20 acetone:water; the purified samples were collected while adsorbed to silica and eluted with diethyl ether. TG fatty acids were analyzed by GC-MS (Agilent Technologies 5975CI) as fatty acid methyl esters following derivitization with methanolic boron trifluoride.
Long Chain Fatty Acid Analysis from Hepatic Acyl CoAs.
Hepatic long chain fatty acyl-CoA extractions were performed using 100 mg of frozen liver powder and extracted as previously described. Samples were analyzed using LC-MS/MS (AB Sciex) (39).
Calculations.
-
i)
The concentrations of 13C-labeled TG in the liver following the infusions ([13C-TG]) was calculated as the product of the total milligrams of TG and the fraction of 13C in the liver TG (13C-atom percent enrichment/100), taking into account the fraction of palmitate in the hepatic fatty acid pool.
-
ii)
Next, the rate of production of 13C-labeled triglyceride is the concentration of 13C-labeled triglyceride divided by the time of the experiment.
-
iii)
13C-labeled hepatic TG in this experiment arises primarily from esterification of 13C-labeled long chain CoA, so the enrichment of the long chain CoA pool can be used to calculate the total amount of TG synthesized from esterification. Thus, the rate of 13C-labeled TG production is divided by the atom percent enrichment of the long chain CoA pool to give the esterification rate.
Supplementary Material
Acknowledgments
We thank Fitsum Guebre-Egziabher, Emin Akgul, Rasmus Rabol, Blas Guigni, Dongyan Zhang (Yale University School of Medicine), Helen Chen, and Morris Birnbaum (University of Pennsylvania School of Medicine) for helpful discussions. We thank Diana Costa, Mario Kahn, Yanna Kosover, Julie Serr, John Stack, Maria Batsu, the Yale Diabetes Research Core, and Jianying Dong for excellent technical support. We thank Susan Murray, Sanjay Bhanot, and Isis Pharmaceuticals for kindly providing the insulin receptor 2′-O-methoxyethyl chimeric antisense oligonucleotide. This work was in part supported by US Public Health Service Grants R01 DK-40936, R24 DK-085836, T32 DK-007058, U24 DK-59635, and P30 DK-45735, an American Diabetes Association Mentor-Based Postdoctoral Fellowship award, and the Novo Nordisk Foundation Center for Basic Metabolic Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423952112/-/DCSupplemental.
References
- 1.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA. 2010;107(8):3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown MS, Goldstein JL. Selective versus total insulin resistance: A pathogenic paradox. Cell Metab. 2008;7(2):95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Brown MS, Goldstein JL. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 4.Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99(5):838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77(1):43–50. doi: 10.1093/ajcn/77.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly KL, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiegman CH, et al. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes. 2003;52(5):1081–1089. doi: 10.2337/diabetes.52.5.1081. [DOI] [PubMed] [Google Scholar]
- 9.Shimomura I, et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6(1):77–86. [PubMed] [Google Scholar]
- 10.Taniguchi CM, Ueki K, Kahn R. Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J Clin Invest. 2005;115(3):718–727. doi: 10.1172/JCI23187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Pajvani UB, et al. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med. 2013;19(8):1054–1060. doi: 10.1038/nm.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Chen K, Williams KJ. The role of pathway-selective insulin resistance and responsiveness in diabetic dyslipoproteinemia. Curr Opin Lipidol. 2012;23(4):334–344. doi: 10.1097/MOL.0b013e3283544424. [DOI] [PubMed] [Google Scholar]
- 13.Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen KF, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104(31):12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabøl R, Petersen KF, Dufour S, Flannery C, Shulman GI. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci USA. 2011;108(33):13705–13709. doi: 10.1073/pnas.1110105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dentin R, et al. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55(8):2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 17.Vedala A, Wang W, Neese RA, Christiansen MP, Hellerstein MK. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J Lipid Res. 2006;47(11):2562–2574. doi: 10.1194/jlr.M600200-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab. 2006;91(4):1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- 19.Hellerstein MK, Neese RA, Schwarz JM. Model for measuring absolute rates of hepatic de novo lipogenesis and reesterification of free fatty acids. Am J Physiol. 1993;265(5 Pt 1):E814–E820. doi: 10.1152/ajpendo.1993.265.5.E814. [DOI] [PubMed] [Google Scholar]
- 20.Poledne R, Mayes PA. Lipolysis and the regulation of fatty acid metabolism in the liver. Biochem J. 1970;119(5):47P–48P. doi: 10.1042/bj1190047pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topping DL, Mayes PA. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972;126(2):295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiggins D, Gibbons GF. The lipolysis/esterification cycle of hepatic triacylglycerol. Its role in the secretion of very-low-density lipoprotein and its response to hormones and sulphonylureas. Biochem J. 1992;284(Pt 2):457–462. doi: 10.1042/bj2840457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diraison F, Pachiaudi C, Beylot M. In vivo measurement of plasma cholesterol and fatty acid synthesis with deuterated water: Determination of the average number of deuterium atoms incorporated. Metabolism. 1996;45(7):817–821. doi: 10.1016/s0026-0495(96)90152-3. [DOI] [PubMed] [Google Scholar]
- 24.Lee WN, et al. In vivo measurement of fatty acids and cholesterol synthesis using D2O and mass isotopomer analysis. Am J Physiol. 1994;266(5 Pt 1):E699–E708. doi: 10.1152/ajpendo.1994.266.5.E699. [DOI] [PubMed] [Google Scholar]
- 25.Wadke M, Brunengraber H, Lowenstein JM, Dolhun JJ, Arsenault GP. Fatty acid synthesis by liver perfused with deuterated and tritiated water. Biochemistry. 1973;12(14):2619–2624. doi: 10.1021/bi00738a011. [DOI] [PubMed] [Google Scholar]
- 26.Lemonnier D, Suquet JP, Aubert R, De Gasquet P, Pequignot E. Metabolism of the mouse made obese by a high-fat diet. Diabete Metab. 1975;1(2):77–85. [PubMed] [Google Scholar]
- 27.Haas JT, et al. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 2012;15(6):873–884. doi: 10.1016/j.cmet.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horton JD, Goldstein JL, Brown MS. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topping DL, Mayes PA. Insulin and non-esterified fatty acids. Acute regulators of lipogenesis in perfused rat liver. Biochem J. 1982;204(2):433–439. doi: 10.1042/bj2040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seböková E, Klimes I. Molecular and cellular determinants of triglyceride availability. Ann N Y Acad Sci. 1997;827:200–214. doi: 10.1111/j.1749-6632.1997.tb51835.x. [DOI] [PubMed] [Google Scholar]
- 31.Duarte JA, et al. A high-fat diet suppresses de novo lipogenesis and desaturation but not elongation and triglyceride synthesis in mice. J Lipid Res. 2014;55(12):2541–2553. doi: 10.1194/jlr.M052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunengraber DZ, et al. Influence of diet on the modeling of adipose tissue triglycerides during growth. Am J Physiol Endocrinol Metab. 2003;285(4):E917–E925. doi: 10.1152/ajpendo.00128.2003. [DOI] [PubMed] [Google Scholar]
- 33.Anderwald C, et al. Effects of insulin treatment in type 2 diabetic patients on intracellular lipid content in liver and skeletal muscle. Diabetes. 2002;51(10):3025–3032. doi: 10.2337/diabetes.51.10.3025. [DOI] [PubMed] [Google Scholar]
- 34.Juurinen L, Tiikkainen M, Häkkinen AM, Hakkarainen A, Yki-Järvinen H. Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E829–E835. doi: 10.1152/ajpendo.00133.2006. [DOI] [PubMed] [Google Scholar]
- 35.Shah PK, et al. Effects of intensive insulin therapy alone and in combination with pioglitazone on body weight, composition, distribution and liver fat content in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(6):505–510. doi: 10.1111/j.1463-1326.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lingvay I, Roe ED, Duong J, Leonard D, Szczepaniak LS. Effect of insulin versus triple oral therapy on the progression of hepatic steatosis in type 2 diabetes. J Investigative Med. 2012;60(7):1059–1063. doi: 10.231/JIM.0b013e3182621c5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voshol PJ, Rensen PC, van Dijk KW, Romijn JA, Havekes LM. Effect of plasma triglyceride metabolism on lipid storage in adipose tissue: Studies using genetically engineered mouse models. Biochim Biophys Acta. 2009;1791(6):479–485. doi: 10.1016/j.bbalip.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Buettner C, et al. Severe impairment in liver insulin signaling fails to alter hepatic insulin action in conscious mice. J Clin Invest. 2005;115(5):1306–1313. doi: 10.1172/JCI23109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neschen S, et al. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2(1):55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.