When my parents bought our first color television in the early 1970s, they measured off 6 ft from the screen and insisted that we not watch from any closer distance. They feared blinding radiation emanating from the color cathode tube (indeed, in 1967 General Electric did recall 90,000 televisions that produced X-rays at thousands of times the recommended exposure level, but other than with this model, there was no demonstrable risk). Fast forward 40 y, and I see my teenage child spending most free moments in the evenings staring at her computer screen, phone, or tablet at close range. Is there risk in this? In PNAS, Chang et al. (1) provide compelling data that there may indeed be unappreciated effects and perhaps dangers of evening exposure to electronic screens, specifically demonstrating a negative effect on sleep in young adults following evenings spent reading from a tablet-based eReader.
Photons of visible light are ligands for multiple receptors with powerful effects on physiology. Rhodopsin is a canonical G protein-coupled receptor with amplification mechanisms in mammalian rods that allow for cell-level detection of single photons (2). The three cone opsin photopigments, with their remarkable spectral tuning of responses to different photon wavelengths, produce our rich perceptions of color (3). One of the most interesting developments in vision science in the last 20 y has been the discovery of a small population of several thousand intrinsically photosensitive retinal ganglion cells (ipRGCs) in the inner retina that use another opsin family member, melanopsin, for photoreception (4). These cells provide information on light intensity, via a dedicated retinohypothalamic tract, to the suprachiasmatic nuclei (SCNs) of the hypothalamus. The SCNs, in turn, are the brain loci responsible for coordinating the body’s circadian pacemakers, including those controlling the rest-activity rhythm. Mice in which ipRGCs have been genetically deleted can no longer synchronize their clocks to the outside world, and thus free run under light–dark conditions (5, 6).
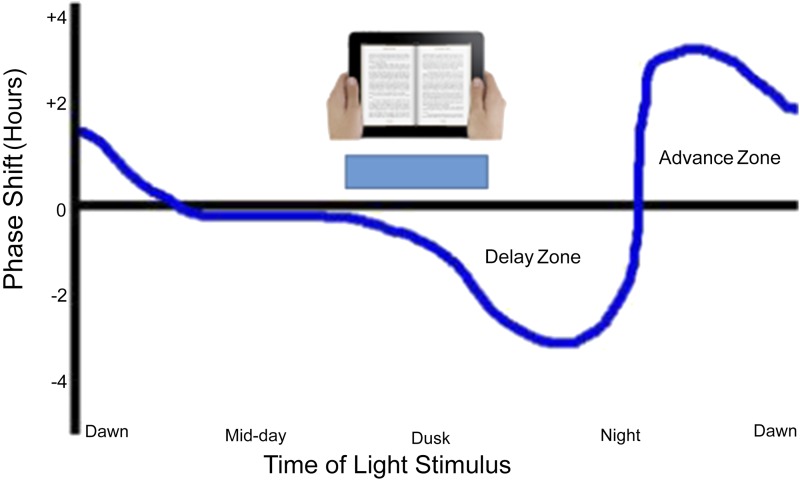
The circadian clock is the major determinant of the timing of sleep and wakefulness. The circadian clocks of most animals have a natural period, in the absence of light–dark cycles, of nearly, but not exactly, 24 h. The human circadian clock, for instance, has a free-running period of ∼24.2 h (7). Circadian clocks thus require daily synchronization or entrainment to the exactly 24-h solar light–dark cycle to remain useful. Entrainment is accomplished by small phase delays and advances mediated by light at dusk and dawn, respectively. By exposing free-running animals to short light pulses at different times in the circadian day, circadian researchers over the last 50 y have elucidated a circadian phase response curve that is common among nearly all organisms (Fig. 1) (8). The circadian clock is insensitive to light pulses occurring during its subjective day, undergoes phase delays to light in the early subjective evening, and phase advances in the late subjective evening. Work in mice has demonstrated that ipRGCs mediate all circadian information reaching the clock and that melanopsin (which has a peak light sensitivity of ∼480 nm) is sufficient for mediating the phase shifting effect of light pulses (9, 10).
Fig. 1.
Typical phase response curve of the circadian clock. Light exposure during the subjective day does not result in phase shifts in the circadian clock, whereas light in the early portion of the subjective night delays the phase of the clock and light in the late night advances the clock. Exposure of the clock to light from tablet-based eReaders appears sufficient to phase delay the clock, leading to disruption of sleep and subsequent daytime sleepiness on the following day. Modified with permission from J. Randall Owens/Wikipedia.
The circadian clock has evolved in a world of fixed spectrum (i.e., the spectrum of outdoor light) and fixed solar timing. In natural conditions, animals are never exposed to bright or continuous light during the night. In the last century, widespread use of electric artificial lighting has, for the first time, created a situation in which humans are routinely exposed to bright light at times in the subjective night, when the clock is susceptible to phase shifts. However, the historical use of tungsten light during much of this time probably protected us from sleep disruption, as the circadian system is relatively insensitive to the yellow-red light of tungsten filaments (11, 12).
Enter the tablet. The backlights of laptop and computer screens are based on light-emitting diodes (LEDs) rich in blue light (as measured in Chang et al., with a peak of 452 nm), which are near the maximal absorption of melanopsin (at 480 nm). The brightness of these devices at their used distances from the eye also falls well within the melanopsin activity range at ∼2 × 1013 photons/cm2 per second (13, 14). The question asked by Chang et al. is whether nightly exposure to light from tablet computers (in the course of reading) is sufficient to phase shift the circadian clock and disrupt the timing and quality of sleep.
To test this, the group enlisted young adults and enrolled them in a constant routine protocol, in which the subjects live in a tightly controlled environment with proscribed times for meals, activities, and sleep under controlled lighting conditions. In the cross-over design, subjects were asked to read the same books from either a paper volume under dim light or from an iPad eReader for 4 h before bedtime each evening for five evenings. Subjects were then retested after five additional nights using the other device. To assay the phase of the circadian clock, the investigators used the well-established proxy of plasma melatonin levels, which reflect the phase of the SCN clock in their nightly rise. The authors also measured sleep by electroencephalography, and subjective sleepiness on the subsequent day. The results clearly showed a substantial phase delay in the rising arm of the melatonin rhythm when subjects read from the tablet compared with the same subjects reading from the printed book using a dim light source. This was accompanied by longer sleep latency (by nearly 10 min), reduced rapid eye movement (REM) sleep, and decreased alertness the following morning. In aggregate, these results suggest that prolonged evening exposure to light from the eReader phase delays the circadian clock and results in reduced quality sleep, as well as subsequent sleepiness the following day.
One might suppose that 4 h of reading on a tablet is a long exposure that is not achieved in daily routine, but surveys of young people suggest that this might reflect real-world conditions when activities besides book reading (such as computer use, social networking, or video gaming) are accounted for (15). As such, this work has substantial public health implications, suggesting that night-time tablet use (and likely also computer use) may be contributing significantly to the already substantial aggregate sleep
The current study by Chang et al. adds to a growing literature suggesting that ambient and occupational lighting have significant effects on human function and human health.
debt in developed countries, particularly among adolescents (16).
In mice, the classical rod and cone photoreceptors (or melanopsin) are each sufficient to signal through ipRGCs to the SCN. It will be worthwhile to determine whether the mechanism of tablet light circadian phase delay is mediated through melanopsin or the classical visual photopigments (or both) by studying the spectral sensitivity of this effect. If mediated through melanopsin, one would predict that either dimmer screens or red-shifted spectrum (for instance by using blue-blocking lenses) would mitigate the effect of light on the clock (17). If the latter, the effect will be more pernicious, as multiple pigments may contribute to this mode of signaling.
The current study by Chang et al. adds to a growing literature suggesting that ambient and occupational lighting have significant effects on human function and human health. Such effects include potential positive cognitive enhancement (18), increased alertness (19), and antidepressive effects (20) of blue light; these effects are counterbalanced by the negative effects of this portion of the spectrum when presented in the evening. Further study of these phenomena will be essential for informed policy on questions such as appropriate spectra of LED-based lighting and computer monitors.
Acknowledgments
This work was supported by an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
The author declares no conflict of interest.
See companion article on page 1232.
References
- 1.Chang A-M, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, ciradian timing, and next-morning alertness. Proc Natl Acad Sci USA. 2015;112:1232–1237. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baylor DA, Lamb TD, Yau KW. Responses of retinal rods to single photons. J Physiol. 1979;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- 3.Hofer H, Carroll J, Neitz J, Neitz M, Williams DR. Organization of the human trichromatic cone mosaic. J Neurosci. 2005;25(42):9669–9679. doi: 10.1523/JNEUROSCI.2414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sexton T, Buhr E, Van Gelder RN. Melanopsin and mechanisms of non-visual ocular photoreception. J Biol Chem. 2012;287(3):1649–1656. doi: 10.1074/jbc.R111.301226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Güler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatori M, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3(6):e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czeisler CA, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 8.Johnson CH. Forty years of PRCs—What have we learned? Chronobiol Int. 1999;16(6):711–743. doi: 10.3109/07420529909016940. [DOI] [PubMed] [Google Scholar]
- 9.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301(5632):525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 11.Brainard GC, et al. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535(Pt 1):261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 14.Tu DC, et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48(6):987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Gradisar M, et al. The sleep and technology use of Americans: Findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9(12):1291–1299. doi: 10.5664/jcsm.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham MG, editor. Sleep Needs, Patterns, and Difficulties of Adolescents: Summary of a Workshop. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- 17.van der Lely S, et al. Blue blocker glasses as a countermeasure for alerting effects of evening light-emitting diode screen exposure in male teenagers. J Adolesc Health. 2015;56(1):113–119. doi: 10.1016/j.jadohealth.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Lehrl S, et al. Blue light improves cognitive performance. J Neural Transm. 2007;114(4):457–460. doi: 10.1007/s00702-006-0621-4. [DOI] [PubMed] [Google Scholar]
- 19.Ekström JG, Beaven CM. Effects of blue light and caffeine on mood. Psychopharmacology (Berl) 2014;231(18):3677–3683. doi: 10.1007/s00213-014-3503-8. [DOI] [PubMed] [Google Scholar]
- 20.Strong RE, et al. Narrow-band blue-light treatment of seasonal affective disorder in adults and the influence of additional nonseasonal symptoms. Depress Anxiety. 2009;26(3):273–278. doi: 10.1002/da.20538. [DOI] [PubMed] [Google Scholar]