Abstract
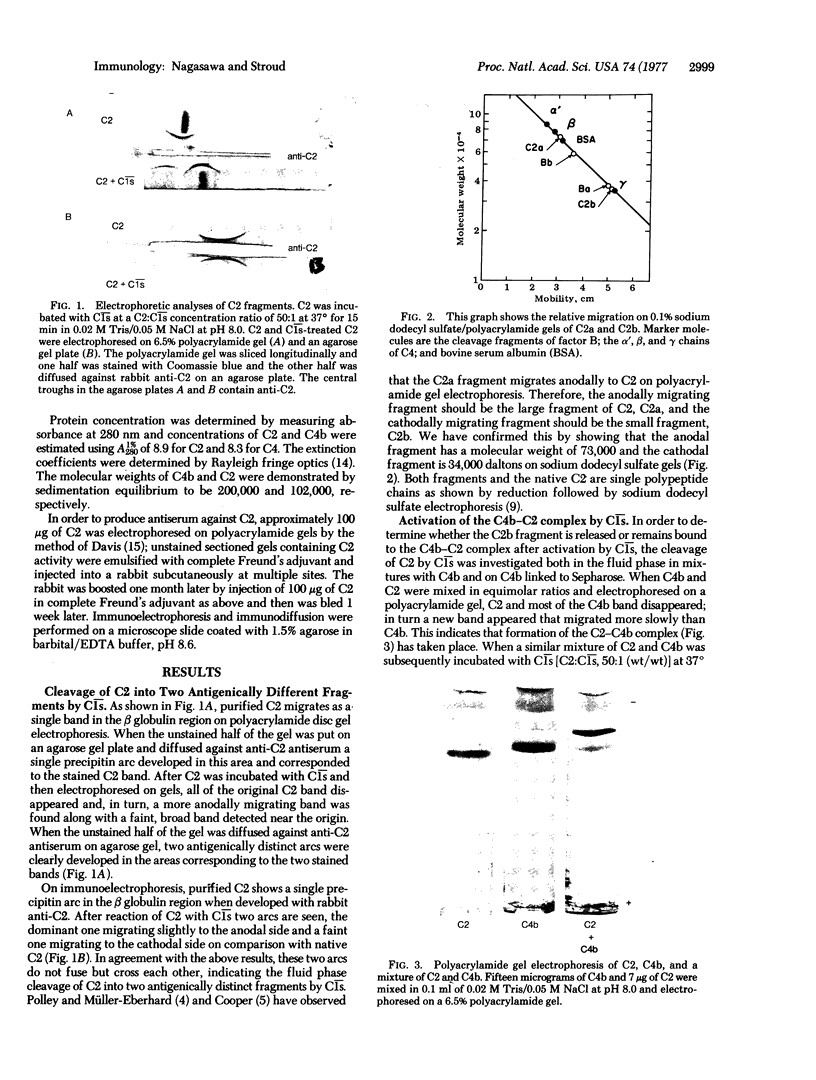
The activation of complement component C2 by C1̄s̄ is a major reaction step leading to the assembly of two related macromolecular enzymes in the classical complement pathway C3 convertase and C5 convertase. The present studies clearly document the smaller fragment, C2b, that results when human C2 reacts with C1̄s̄. We have identified and characterized C2b (34,000 daltons) as a single protein on disc electrophoresis and immunoelectrophoresis. C2a (73,000 daltons), the larger fragment from this reaction, has a more acidic nature and C2b is more basic. These fragments can also be detected by their different antigenic determinants. When the C2-C4b complex is activated in the fluid phase by C1̄s̄ and allowed to decay, it dissociates into C2a and the C2b-C4b complex. Furthermore, when C2 is bound to C4b-Sepharose and then reacted with C1̄s̄, only the C2a fragment is released from the solid phase C2-C4b-Sepharose into the fluid phase, and the C2b fragment remains noncovalently bound to C4b-Sepharose. These results suggest that the C2b portion of C2 contains a stable binding site for C4b and, after the decay release of C2a from this C3 convertase, the C2b fragment remains bound. Thus, the decay release of C2a may represent a temperature-dependent dissociation from C2b.
Keywords: complement, classical C3 convertase, enzyme activation
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Babul J., Stellwagen E. Measurement of protein concentration with interferences optics. Anal Biochem. 1969 Apr 4;28(1):216–221. doi: 10.1016/0003-2697(69)90172-9. [DOI] [PubMed] [Google Scholar]
- Cooper N. R. Enzymatic activity of the second component of complement. Biochemistry. 1975 Sep 23;14(19):4245–4251. doi: 10.1021/bi00690a015. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Goldlust M. B., Shin H. S., Hammer C. H., Mayer M. M. Studies of complement complex C5b,6 eluted from--EAC-6: reaction of C5b,6 with EAC4b,3b and evidence on the role of C2a and C3b in the activation of C5. J Immunol. 1974 Sep;113(3):998–1007. [PubMed] [Google Scholar]
- Medicus R. G., Götze O., Müller-Eberhard H. J. The serine protease nature of the C3 and C5 convertases of the classical and alternative complement pathways. Scand J Immunol. 1976;5(9):1049–1055. doi: 10.1111/j.1365-3083.1976.tb03056.x. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Mayer M. M. On the cleavage of C'2 by C'la: immunological and physical comparisons of C'2ad and C'2a-i. Proc Soc Exp Biol Med. 1968 Oct;129(1):127–130. doi: 10.3181/00379727-129-33266. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Polley M. J., Calcott M. A. Formation and functional significance of a molecular complex derived from the second and the fourth component of human complement. J Exp Med. 1967 Feb 1;125(2):359–380. doi: 10.1084/jem.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. The second component of human complement: its isolation, fragmentation by C'1 esterase, and incorporation into C'3 convertase. J Exp Med. 1968 Sep 1;128(3):533–551. doi: 10.1084/jem.128.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitomer G., Stroud R. M., Mayer M. M. Reversible adsorption of C'2 by EAC'4: role of Mg2+, enumeration of competent SAC'4, two-step nature of C'2a fixation and estimation of its efficiency. Immunochemistry. 1966 Jan;3(1):57–69. doi: 10.1016/0019-2791(66)90282-5. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., Mayer M. M., Miller J. A., McKenzie A. T. C'2ad, an inactive derivative of C'2 released during decay of EAC'4,2a. Immunochemistry. 1966 May;3(3):163–176. doi: 10.1016/0019-2791(66)90182-0. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nagasawa S., Koyama J. The NH-2-terminal sequences of a subunit of the first component of human complement, C1s, and its activated form, C1s. FEBS Lett. 1975 Feb 15;50(3):330–333. doi: 10.1016/0014-5793(75)80521-7. [DOI] [PubMed] [Google Scholar]