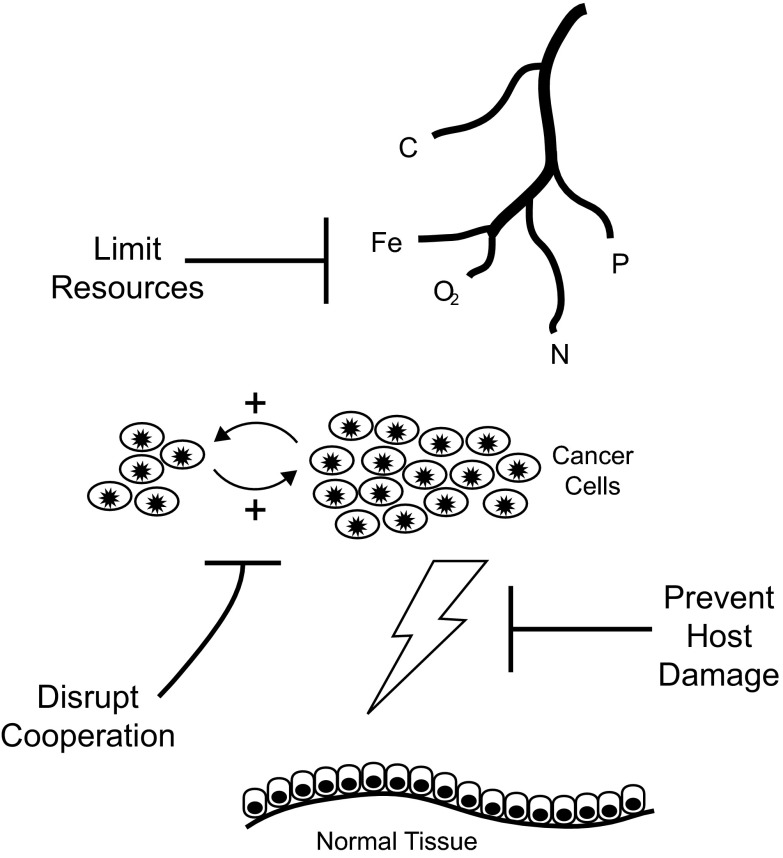
Clinical treatment for metastatic cancer has traditionally entailed administering the highest possible dose in the shortest period, a strategy known as high-dose density therapy. The implicit goal is complete eradication. Unfortunately, a systemic cure for most metastatic cancers remains elusive, and the role of chemotherapy has been reduced to prolonging life and ameliorating symptoms.
Fig. 1.
These three infectious disease-controlling strategies have the potential to be translated into cancer-control measures. They all avoid attempting to completely eradicate tumor cells, instead aiming to (i) limit essential resources to cancer cells, (ii) disrupt cooperation among cancer cells, and (iii) prevent host damage.
Recently, the application of evolutionary and ecological principles to cancer therapy and infectious disease treatment has led to a rethinking of conventional treatments. High dose-density therapies may in fact accelerate the emergence and growth of resistant cell populations in both cancer (1) and infectious disease (2, 3), leading many to question the approach of complete eradication on evolutionary grounds (4–7).
An alternative to the traditional approach is a control-based tactic that focuses on extending patient life and reducing suffering by limiting, rather than eradicating, the growth and spread of cancer. Although control-based approaches have been used in infectious disease, they have not been systematically studied in cancer. Here we describe several strategies that have been effective in controlling bacterial growth and discuss how they might be applied to cancer therapy.
Limiting Resources
Bacterial growth can be controlled by limiting essential resources in the environment, e.g., limiting access to iron through iron homologs, chelators (8), or compounds interfering with siderophore production (9). Iron, glucose, carbon, oxygen, nitrogen, and phosphorus are limiting for cancer cells also (10), suggesting that reducing their in vivo availability may potentially control cancer. Extensive research on manipulation of carbon sources illustrates the promise of the approach of limiting resource supply. But there is an unmet need to identify tumor sources of phosphates, nitrogen, and iron as potential therapeutic targets.
Antiangiogenic drug treatments limit blood vessel growth and could potentially be used in low doses for tumor control (low doses are already being used during therapy to normalize vasculature and enhance drug delivery). A control rather than an eradication approach could prove valuable: administering high doses of antiangiogenic drugs, in hopes of eradicating the tumor, can lead not only to tumor shrinkage but also high rates of metastasis.
Damage Control
Reducing host damage by directly targeting virulence factors (11, 12) has been effective in the case of infectious disease; a similar strategy could be adopted for cancer control. Cancer cells may damage host tissues in a variety of ways including through the byproducts of Warburg metabolism (e.g., lactic acid). Increasing the pH of the tumor microenvironment (13) has been shown to minimize damage resulting from acid byproducts of Warburg metabolism, both reducing tumor growth and invasion (14). However, approaches like this are the exception rather than the rule. In general, cancer therapies are not specifically aimed at minimizing host damage from cancer, but rather limiting host damage from the treatment while killing as many cancer cells as possible. Research focused on understanding the causes of host damage in cancer, and limiting it through targeted approaches, has the potential to extend patient life and improve well-being.
Cooperation Interference
Interfering with cooperation among cells is a promising approach in the treatment of both infectious disease and cancer. Treatments that aim to disrupt cooperation (e.g., by targeting growth factors, angiogenic factors, or immune suppression factors) block cell products that can enhance the fitness of neighboring cells, and hence allow for weaker selection pressures for resistance to the treatment compared with treatments that target cell survival (15, 16). In infectious disease, the use of quorum quenchers, which block the molecules that bacteria use for communication and cooperation, has been successfully deployed to reduce bacterial population sizes and prevent the formation of host-damaging collective phenotypes such as biofilms (17). Promoting avirulent cheaters among bacteria—bacteria that fail to cooperate with their bacterial neighbors by not producing the virulence factors—to outcompete virulent strains (18) is an approach that has been suggested in cancer (19) but not tested.
One promising control-based approach that is currently being tested in animal models is adaptive therapy, i.e., adjusting the drug dose to keep the tumor a stable size. This approach exploits the fitness cost of resistance and explicitly maintains a population of therapy-sensitive cells. Periodically, therapy is withheld so that the sensitive cells (which are fitter than the resistant ones in the absence of drug) are allowed to proliferate and suppress growth of resistant phenotypes. This therapy demonstrably leads to long-term control of tumor size and life extension in mice compared with traditional high-dose therapy (1). However, the effectiveness of adaptive therapy has yet to be replicated or tested in clinical trials with humans. Further research is necessary.
In deciding which tumors to treat with a control-based approach, the genetic and immunological characteristics should be taken into consideration. For example, more heterogeneous tumors might be the best candidates for a more “control”-based approach, because they harbor preexisting resistance mutations that are more likely to cause tumor relapse after high-dose treatment. Implementation of flexible, computationally guided treatment strategies informed by evolutionary principles will undoubtedly need to overcome a variety of practical, regulatory, statistical, and ethical challenges. Most likely this will require a new generation of tumor boards that include not only oncologists, pathologists, surgeons, and immunologists but also applied mathematicians, computer scientists, statisticians versed in adaptive trials, and evolutionary biologists.
Like infectious disease, cancer is a complex adaptive system that can only be controlled by strategies that recognize and respond to its dynamic nature. Control-based approaches represent a shift in thinking away from the traditional, aggressive, high-dose treatment aimed at waging an all-or-nothing war to eliminate cancer (5, 20). Researchers and clinicians need tools for strategic containment and control of cancer, and infectious disease-inspired approaches such as those suggested here represent one promising frontier for the development of new approaches to cancer treatment.
Footnotes
Any opinions, findings, conclusions, or recommendations expressed in this work are those of the authors and do not necessarily reflect the views of the National Academy of Sciences.
References
- 1.Gatenby RA, Silva AS, Gillies RJ, Frieden BR. Adaptive therapy. Cancer Res. 2009;69(11):4894–4903. doi: 10.1158/0008-5472.CAN-08-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Read AF, Day T, Huijben S. The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proc Natl Acad Sci USA. 2011;108(Suppl 2):10871–10877. doi: 10.1073/pnas.1100299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pena-Miller R, et al. When the most potent combination of antibiotics selects for the greatest bacterial load: The smile-frown transition. PLoS Biol. 2013;11(4):e1001540. doi: 10.1371/journal.pbio.1001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9(2):117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 5.Gatenby RA. A change of strategy in the war on cancer. Nature. 2009;459(7246):508–509. doi: 10.1038/459508a. [DOI] [PubMed] [Google Scholar]
- 6.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read AF, Graham AL, Råberg L. Animal defenses against infectious agents: Is damage control more important than pathogen control. PLoS Biol. 2008;6(12):e4. doi: 10.1371/journal.pbio.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross-Gillespie A, Weigert M, Brown SP, Kümmerli R. Gallium-mediated siderophore quenching as an evolutionarily robust antibacterial treatment. Evol Med Public Health. 2014;2014(1):18–29. doi: 10.1093/emph/eou003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imperi F, et al. Repurposing the antimycotic drug flucytosine for suppression of Pseudomonas aeruginosa pathogenicity. Proc Natl Acad Sci USA. 2013;110(18):7458–7463. doi: 10.1073/pnas.1222706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hann H-WL, Stahlhut MW, Blumberg BS. Iron nutrition and tumor growth: Decreased tumor growth in iron-deficient mice. Cancer Res. 1988;48(15):4168–4170. [PubMed] [Google Scholar]
- 11.Allen RC, Popat R, Diggle SP, Brown SP. Targeting virulence: Can we make evolution-proof drugs? Nat Rev Microbiol. 2014;12(4):300–308. doi: 10.1038/nrmicro3232. [DOI] [PubMed] [Google Scholar]
- 12.Nicolosi D, Tempera G, Genovese C, Furneri PM. Anti-adhesion activity of A2-type proanthocyanidins (a cranberry major component) on uropathogenic E. coli and P. mirabilis strains. Antibiotics. 2014;3(2):143–154. doi: 10.3390/antibiotics3020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robey IF, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69(6):2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva AS, Yunes JA, Gillies RJ, Gatenby RA. The potential role of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion. Cancer Res. 2009;69(6):2677–2684. doi: 10.1158/0008-5472.CAN-08-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepper JW. Defeating pathogen drug resistance: Guidance from evolutionary theory. Evolution. 2008;62(12):3185–3191. doi: 10.1111/j.1558-5646.2008.00525.x. [DOI] [PubMed] [Google Scholar]
- 16.Pepper JW. Drugs that target pathogen public goods are robust against evolved drug resistance. Evol Appl. 2012;5(7):757–761. doi: 10.1111/j.1752-4571.2012.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen LD, et al. Synergistic antibacterial efficacy of early combination treatment with tobramycin and quorum-sensing inhibitors against Pseudomonas aeruginosa in an intraperitoneal foreign-body infection mouse model. J Antimicrob Chemother. 2012;67(5):1198–1206. doi: 10.1093/jac/dks002. [DOI] [PubMed] [Google Scholar]
- 18.Rumbaugh KP, et al. Quorum sensing and the social evolution of bacterial virulence. Curr Biol. 2009;19(4):341–345. doi: 10.1016/j.cub.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 19.Maley CC, Reid BJ, Forrest S. Cancer prevention strategies that address the evolutionary dynamics of neoplastic cells: Simulating benign cell boosters and selection for chemosensitivity. Cancer Epidemiol Biomarkers Prev. 2004;13(8):1375–1384. [PubMed] [Google Scholar]
- 20.Aktipis CA, Maley CC, Neuberg SL. 2010 Psychological barriers to evolutionary thinking in medicine. Evolution and Medicine Review. Available at evmedreview.com/?p=231. Accessed December 10, 2014.