Significance
Famine kills millions of people each year. Survival requires the maintenance of blood glucose. Famine depletes body fat, thereby removing a source of energy for hepatic glucose production. Here we used a mouse model of fat depletion to show that growth hormone (GH) maintains blood sugar by stimulating hepatic autophagy, the process by which the liver digests its organelles to provide energy and substrates for producing glucose. When fat-depleted mice are fasted, their stomachs secrete ghrelin, a GH secretagogue. The resultant elevation in GH stimulates hepatic autophagy. In mice lacking ghrelin, GH fails to rise appropriately, hepatic autophagy is reduced, and mice die from hypoglycemia. These studies demonstrate a ghrelin-GH-autophagy axis that is required for survival in famine.
Keywords: ghrelin O-acyltransferase, Goat−/− mice, calorie restriction, hypoglycemia
Abstract
Plasma growth hormone (GH) and hepatic autophagy each have been reported to protect against hypoglycemia in the fasted state, but previous data have not linked the two. Here we demonstrate a connection using a mouse model of fasting in a fat-depleted state. Mice were subjected to 1 wk of 60% calorie restriction, causing them to lose nearly all body fat. They were then fasted for 23 h. During fasting, WT mice developed massive increases in plasma GH and a concomitant increase in hepatic autophagy, allowing them to maintain viable levels of blood glucose. In contrast, lethal hypoglycemia occurred in mice deficient in the GH secretagogue ghrelin as a result of knockout of the gene encoding ghrelin O-acyltransferase (GOAT), which catalyzes a required acylation of the peptide. Fasting fat-depleted Goat−/− mice showed a blunted increase in GH and a marked decrease in hepatic autophagy. Restoration of GH by infusion during the week of calorie restriction maintained autophagy in the Goat−/− mice and prevented lethal hypoglycemia. Acute injections of GH after 7 d of calorie restriction also restored hepatic autophagy, but failed to increase blood glucose, perhaps owing to ATP deficiency in the liver. These data indicate that GH stimulation of autophagy is necessary over the long term, but not sufficient over the short term to maintain blood glucose levels in fasted, fat-depleted mice.
When animals undergo a complete fast, adaptive mechanisms maintain the supply of energy to vital organs. The classic metabolic response to an acute fast was delineated in humans more than 50 y ago, largely through the work of George Cahill and colleagues (1, 2). The initial response to fasting is glycogenolysis, which releases glucose from liver and muscle. At the same time, the liver begins to synthesize and release glucose de novo, initially using lactate, which is returned to the liver from muscle through the Cori cycle. Shortly thereafter, lipolysis is activated in adipose tissue, releasing fatty acids for combustion in muscle and for the supply of energy to fuel gluconeogenesis in the liver. Hepatic fatty acid oxidation also produces the ketone bodies acetoacetate and beta-hydroxybutyrate, which can partially replace glucose for energy in brain and muscle. This entire process is orchestrated by hormones, primarily through a drop in insulin and increases in glucagon, which activates gluconeogenesis in liver (2), and growth hormone (GH), which stimulates lipolysis in adipose tissue (3).
In recent years, another source of gluconeogenic substrates in liver has been identified, namely autophagy (4, 5). Autophagy is the process by which intracellular double-membrane vesicles called autophagosomes ingest cytosolic proteins and organelles (6). Autophagosomes fuse with lysosomes, creating single-membrane bounded autolysosomes that expose the ingested contents to hydrolases that break down the ingested macromolecules. Fasting induces autophagy in liver (4). Some of the autophagic end products are combusted to produce energy, and some are used as substrates for gluconeogenesis. When autophagy is prevented through germline deletion of a required protein, animals develop profound hypoglycemia on fasting (4, 7, 8).
Previous studies of fasting have been conducted in humans or animals that maintain adequate adipose tissue, allowing a continuous supply of fatty acids to liver and muscle. We know little about the maintenance of blood glucose when adipose triglycerides are exhausted and fatty acids are not available. Such a fat-depleted condition occurs in humans subjected to prolonged calorie deprivation through famine, anorexia, or other causes of cachexia (9, 10).
Recently, our laboratory created an experimental model of calorie restriction in fat-depleted C57BL/6 mice (11–13). In this protocol, mice are fed daily with 40% of the chow diet that the mouse would normally consume. We call this 60% calorie restriction. The mice are fed each day at 6:00 PM. Within 1 d, they become ravenously hungry, and consume all of their food by 7:00 PM. They are then fasted for the next 23 h until they are fed once again. This cycle is repeated each day for 7–9 d. We measure blood glucose each day at 5:30 PM, after the 23-h fast and immediately before the next feeding.
Over the first 4 d of this regimen, WT mice lose ∼30% of their body weight, which then stabilizes. By day 4, their body fat, as measured by NMR, constitutes <2% of body weight (11, 13). By day 5 or 6, fasting blood glucose values at 5:30 PM fall to 40–60 mg/dL, and are maintained at that level through day 8. Despite this moderate hypoglycemia, WT mice are alert and active. They have very low concentrations of free fatty acids in plasma (≤0.07 mM) and ketone bodies (≤0.12 mM). The hormonal response includes a marked decrease in insulin, increase in glucagon, and increases in ghrelin and GH, with the latter two rising progressively through 8 d (11, 13).
Survival of these fasted, fat-depleted WT mice requires ghrelin, which acts by stimulating secretion of GH (11). Ghrelin, a 28-aa peptide produced primarily by enteroendocrine cells in the stomach and duodenum, acts in the arcuate nucleus of the hypothalamus and in somatotrophs in the pituitary to release GH (14–16). Ghrelin activity requires ghrelin O-acyltransferase (GOAT), which attaches octanoate, an eight-carbon fatty acid, to serine-3 of ghrelin before secretion (17, 18). Mice with germline deletions of ghrelin or Goat are unable to maintain viable levels of blood glucose when subjected to 60% calorie restriction as described above (11, 13). The calorie-restricted, ghrelin-deficient mice lose body weight and body fat as rapidly as WT mice, and become just as hungry, consuming all of their food within 60 min. The ghrelin-deficient mice are unable to stabilize their blood glucose. By day 7–8, their fasting blood glucose falls below 20 mg/dL. It rises promptly after each meal, only to fall progressively during the next 23-h fast. Profound hypoglycemia can be prevented by chronic infusion of either ghrelin or GH, begun before the onset of calorie restriction and maintained throughout (11, 13).
Tracer experiments demonstrate that hypoglycemia in ghrelin-deficient, fat-depleted mice is caused by reduced production of glucose rather than by enhanced clearance (13). Plasma lactate, the major substrate for gluconeogenesis, falls in parallel with blood glucose (13). On day 7 of calorie restriction, blood glucose can be restored to WT levels by injections of pyruvate, lactate, or alanine, which provide energy and substrates for gluconeogenesis. Blood glucose is also restored by octanoate, a fatty acid that cannot be converted to glucose but can be oxidized to provide energy (13).
Inasmuch as both hepatic autophagy and plasma GH are required to maintain blood glucose during fasting, the present study was designed to test the hypothesis that GH stimulates hepatic autophagy in starved, fat-depleted mice. The results demonstrate that hepatic autophagy is diminished in ghrelin-deficient mice and can be restored acutely by GH infusion. Acute restoration of hepatic autophagy does not prevent hypoglycemia, however. These results raise the possibility that GH-stimulated hepatic autophagy is necessary, but not sufficient, to maintain blood glucose in fasted, fat-depleted mice.
Results
The experiment shown in Fig. 1 was performed to assess the relationships among fat depletion, starvation, plasma GH level, and indicators of autophagy in livers of WT and Goat−/− mice. Sixteen WT and 16 Goat−/− mice were depleted of fat by being placed on a diet containing 40% of the calories that they normally consumed. We previously showed that body fat in both groups falls to <2% of body weight by day 5 on this diet (11, 13). Mice were fed each day at 6:00 PM. As a result of the calorie restriction, the WT and Goat−/− mice were hungry, and they consumed all of their food within 1 h. By 5:30 PM each day, the mice had been fasted for 23 h. At the time points indicated in Fig. 1A, groups of four WT mice and four Goat−/− mice were killed for measurement of blood and liver parameters. As shown in Fig. 1A, at the 5:30 PM time point on day 8, blood glucose in WT mice averaged 45 mg/dL, compared with only 11 mg/dL in the Goat−/− mice. By 8:30 PM, 2.5 h after feeding, blood glucose in both groups had risen to 65 mg/dL. At 2:30 PM on the next afternoon (day 9), blood glucose in the Goat−/− mice was maintained at 51 mg/dL Over the next 3 h, it plummeted to 15 mg/dL. In contrast, the WT mice were able to maintain their blood glucose levels throughout the day in the range of 50–68 mg/dL.
Fig. 1.
Levels of blood glucose (A), plasma GH (B), and hepatic LC3-II (C–E) before and after feeding in calorie-restricted WT and Goat−/− mice. Sixteen male WT and 16 Goat−/− littermates (8 wk old) were subjected to 60% calorie restriction for 8–9 d as described in Materials and Methods. Four mice from each group were killed at the indicated time. (A and B) At 1 h before sacrifice, mice were injected i.p. with leupeptin (15 mg/kg). Immediately before sacrifice, blood was obtained from the retro-orbital sinus for measurement of blood glucose and plasma GH. Each value in A and B represents mean ± SEM of data from four mice. Asterisks denote the level of statistical significance (Student t test) between WT and Goat−/− mice: **P < 0.01; ***P < 0.001. (C–E) Liver supernatants were processed as described in Materials and Methods, after which individual (C) or pooled (D) supernatants were subjected to immunoblotting with the indicated antibody. For immunoblotting of LC3 and GAPDH, 20 μg of liver supernatant was loaded in each lane; for p-STAT5 and t-STAT, 60 μg was loaded. Each pooled sample in D was derived from equal amounts of the four individual samples in C. (E) Relationship between the amounts of LC3II and p-STAT5. The relative amounts of LC3II (normalized to GAPDH) and p-STAT5 (normalized to total STAT5) were quantified by scanning the autoradiograms in D. The data were analyzed with ImageJ.
Plasma GH levels are shown in Fig. 1B. As we reported previously (11, 13), at the 5:30 PM time point on day 8, plasma GH was markedly elevated in WT mice and much lower in Goat−/− mice. After feeding, GH levels fell sharply in WT mice, reaching the same level as in Goat−/− mice. On the next day, GH levels rose progressively in WT mice, reaching a peak at 5:30 PM. The rise in the Goat−/− mice was much less pronounced.
As an index of autophagy in liver, we used immunoblotting to estimate the relative amount of microtubule-associated protein 1A/1B light chain 3 (LC3). This protein exists in two forms: LC3-I, a soluble cytosolic form, and LC3-II, a form coupled covalently to phosphatidylethanolamine and inserted into autophagosomal membranes (19). To prevent lysosomal degradation of LC3-II, we injected all mice with leupeptin, a lysosomal protease inhibitor, at 1 h before sacrifice (4). Under these conditions, the amount of LC3-II reflects the overall rate of autophagy (19). Immunoblotting data for each of the 32 individual mice are shown in Fig. 1C. In addition, equal aliquots from the individual extracts in each of the eight groups were pooled and immunoblotted (Fig. 1D). In WT mice, the LC3-II level was highest at 5:30 PM on both days, and it fell by 8:30 PM (Fig. 1 C and D). Compared with WT mice, Goat−/− mice had a reduced LC3-II level at 5:30 PM, and the level increased at 8:30 PM after feeding (Fig. 1 C and D).
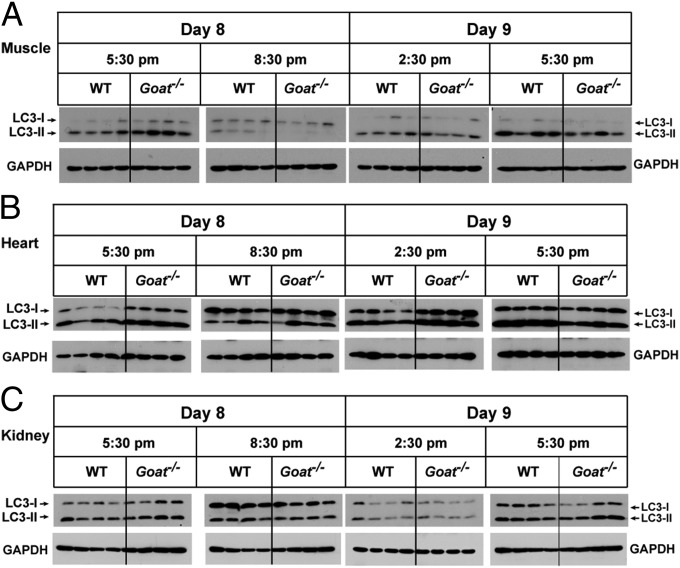
Two closely related genes, LC3A and LC3B, encode LC3-I and LC3-II (20). To determine whether the difference in LC3-II in the liver of WT and Goat−/− mice was the result of transcriptional changes in LC3A and/or LC3B, we checked their mRNA expression levels and found no difference at various time points on days 8 and 9 of calorie restriction (Fig. S1). These data suggest that the decreased LC3-II level in Goat−/− mice is a reflection of a deficiency of autophagy rather than of reduced gene expression. We also checked LC3-II levels in skeletal muscle, heart, and kidney from the same experiment described in Fig. 1. In muscle and kidney, the LC3-II level was highest at 5:30 PM, but there was no difference in level between WT and Goat−/− mice (Fig. 2). In the heart, LC3-II level did not change during the 24-h period, and it was similar in WT and Goat−/− mice.
Fig. 2.
LC3 levels in muscle (A), heart (B), and kidney (C) before and after feeding in calorie-restricted WT and Goat−/− mice. The mice used in this experiment are the same as those used in the experiment shown in Fig. 1. Tissues were processed as described in Materials and Methods, after which 20 µg of each supernatant was subjected to immunoblot analysis with the indicated antibody.
GH signals by inducing phosphorylation of the transcription factor STAT-5 (21). Immunoblotting revealed elevated phospho-STAT-5 (p-STAT5) levels in WT livers at 5:30 PM on both days (Fig. 1 C and D), which correlates with the elevated plasma GH levels at those times (Fig. 1B). In contrast, p-STAT5 was relatively low in Goat−/− livers at all time points. Fig. 1E plots the relative amount of LC3-II vs. the relative amount of p-STAT5 in livers from the eight groups of mice as determined by scans of the immunoblots. The plot reveals a strong correlation between LC3-II level and p-STAT5 level (R2 = 0.90). Inasmuch as p-STAT5 is produced by GH, the data suggest that GH may be responsible for the increase in autophagy in fat-depleted, WT mice subjected to a 23-h fast.
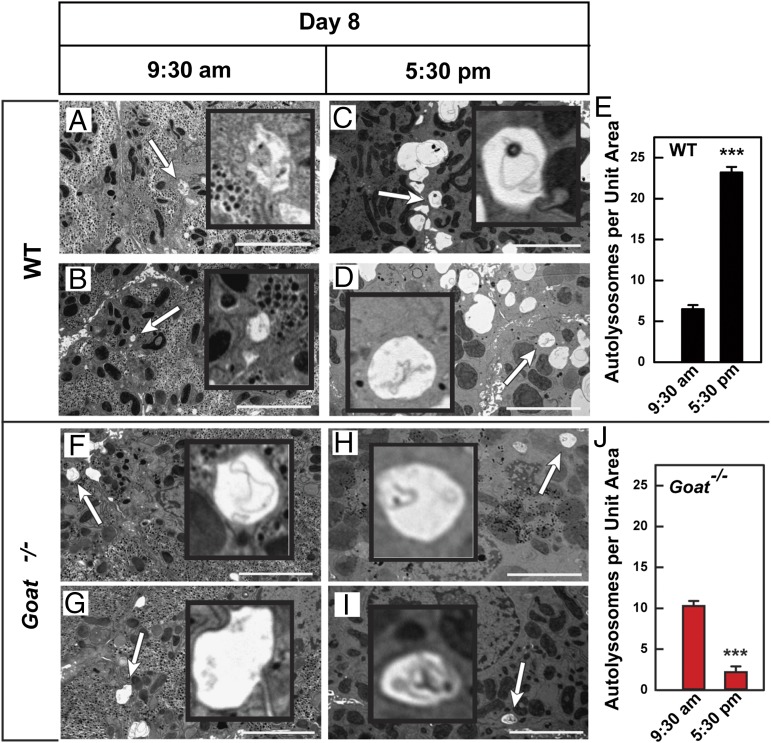
To confirm that the reduction in LC3-II reflects reduced autophagy in livers of calorie-restricted, fat-depleted Goat−/− mice, we used electron microscopy (EM) to examine livers from WT and Goat−/− mice that had been starved for 16 h or 23 h on day 8 of calorie restriction. At 9:30 AM, after 15 h of starvation, livers from WT and Goat−/− mice had very few autolysosomes (Fig. 3 A, B, F, and G). By 5:30 PM, after 23 h of starvation, the number of autolysosomes in WT livers had increased markedly (Fig. 3 C and D) compared with those in Goat−/− livers (Fig. 3 H and I). Autolysosomes (denoted by white arrows) were defined by their characteristic single membranous vacuole- containing amorphous and multilamellar structures (shown in Insets, magnified fivefold). To quantify the data in Fig. 3, we asked three colleagues to count the number of autolysosomes in random EM images. The colleagues were blinded as to the source of the specimens. In the images from WT mice, the observers found that the mean number of autolysosomes per image had increased fourfold, from 6 at 9:30 AM to 23 at 5:30 PM (Fig. 3E). In striking contrast, the mean autolysosome count in the Goat−/− livers decreased by 80%, from 10 autolysosomes per image at 9:30 AM to 2 at 5:30 PM (Fig. 3J).
Fig. 3.
Electron micrographs of liver from calorie-restricted WT (A–D) and Goat−/− (F–I) mice at 9:30 AM and 5:30 PM on day 8. WT and Goat−/− littermates (8 wk old) were subjected to 60% calorie restriction for 8 d. Mice were perfused at 9:30 AM or 5:30 PM on day 8 of calorie restriction, after which the liver was fixed and processed as described in Materials and Methods. Pictures from 20 different unit areas (36 × 26 μm per unit area) from two WT and two Goat−/− livers at each time point were taken at 5,000 amplitude; representative pictures are shown. White arrows denote typical autolysosomes. (Scale bar: 5 μm; magnification, 5,000×.) (Insets) Enlarged images of each arrow-denoted autolysosome (magnified 5×). (E and J) The number of autolysosomes per unit area was determined by three independent examiners as described in Materials and Methods. Each value represents mean ± SEM of data from 20 images. Asterisks denote level of statistical significance (Student t test) between autolysosome numbers at 9:30 AM and 5:30 PM of WT (E) and Goat−/− (J) mice: ***P < 0.001.
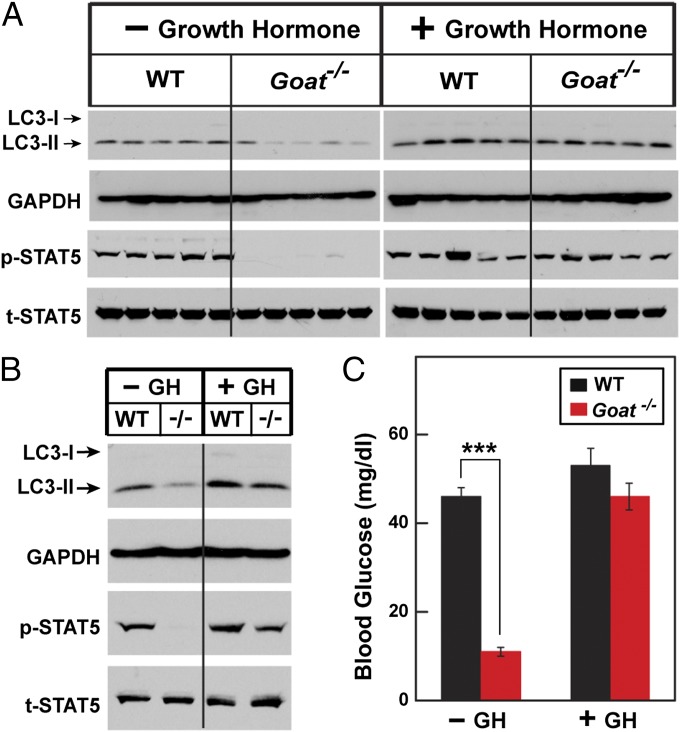
We previously reported that hypoglycemia is prevented in calorie-restricted, fat-depleted Goat−/− mice through GH infusion throughout the period of calorie restriction (11, 13). To determine whether this chronic GH treatment prevents the loss of autophagy, we used an Alzet minipump to administer a constant infusion of GH beginning 3 d before the onset of calorie restriction. At 5:30 PM on day 8 of calorie restriction, blood glucose in vehicle-infused Goat−/− mice was severely reduced compared with WT mice (11 mg/dL vs. 46 mg/dL) (Fig. 4C). The GH infusion had no effect on blood glucose in WT mice (52 mg/dL), but it prevented profound hypoglycemia in Goat−/− mice (46 mg/dL). Mice were killed at 5:30 PM on day 8, and liver extracts were subjected to immunoblotting. In the vehicle-infused Goat−/− mice, LC3-II levels were reduced both in individual mice (Fig. 4A) and in pooled extracts from all of the mice in each group (Fig. 4B). LC3-II levels were nearly as high in Goat−/− mice that received the GH infusion as in WT mice. p-STAT5 levels were low in vehicle-infused Goat−/− mice, and were returned to near-normal levels by GH infusion.
Fig. 4.
Preservation of autophagy in calorie-restricted Goat−/− mice by infusion of GH. Alzet osmotic minipumps containing either vehicle or GH (15 μg/24 h) were implanted at 3 d before calorie restriction as described in Materials and Methods. Mice were then subjected to 60% calorie restriction for 8 d. Mice were dissected at 5:30 PM on day 8, at 1 h after leupeptin was injected i.p. (A and B) Immunoblot analysis with the indicated antibody was carried out on liver supernatants from individual mice (A) or pooled supernatants (B) from the five individual mice as described in Fig. 1. (C) Blood glucose levels were measured at 5:30 PM on day 8. Each value represents mean ± SEM of data from five mice. Asterisks denote level of statistical significance (Student t test) between WT and Goat−/− mice infused with vehicle or GH: ***P < 0.001.
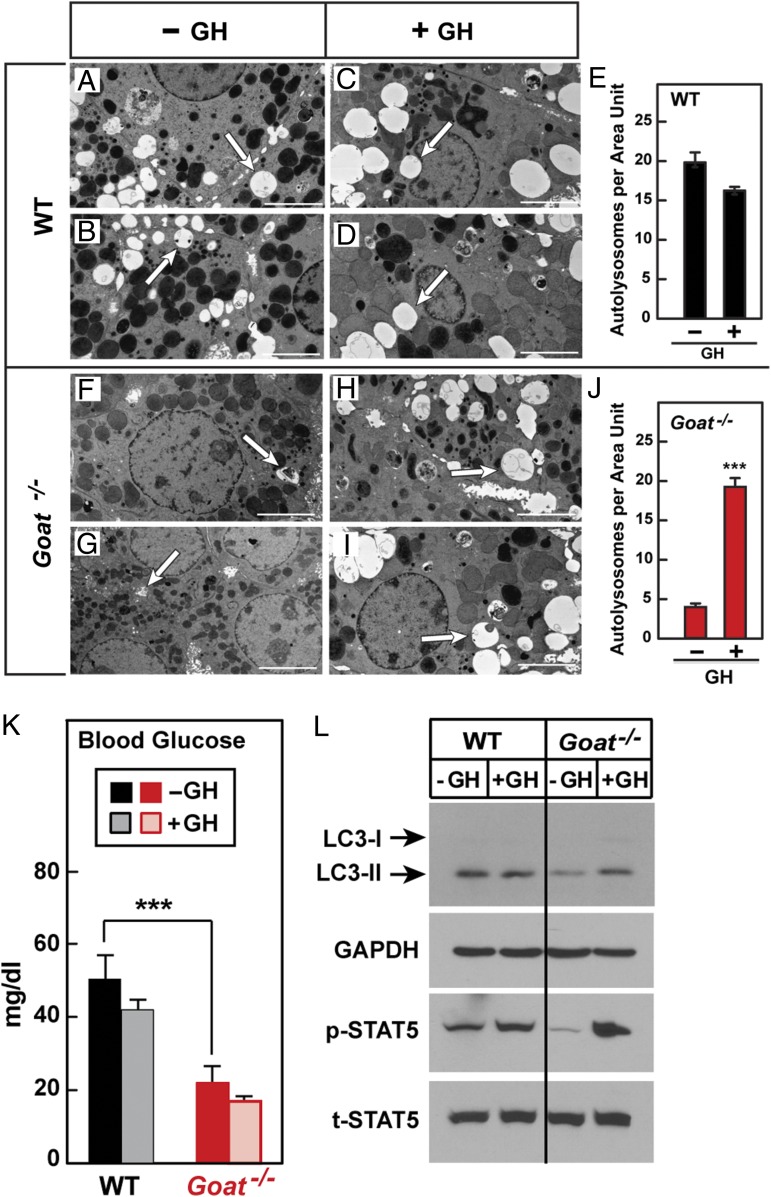
To determine whether acute s.c. injections of GH would have the same effects as chronic infusions, we injected calorie-restricted WT and Goat−/− mice with GH on day 7 at 2:30 PM, 3:30 PM, and 4:30 PM and examined liver autophagy using EM. In the absence of GH injection, WT livers (Fig. 5 A and B) had significantly more autolysosomes than Goat−/− livers (Fig. 5 F and G), consistent with the results shown in Fig. 2. Acute injections of GH had no effect on the number of autolysosomes in WT livers (Fig. 5 A–E), but markedly increased the number of autolysosomes in Goat−/− livers (Fig. 5 F–J). Despite the increase in hepatic autophagy, GH did not restore blood glucose levels in the Goat−/− mice (average values, 20 mg/dL in vehicle-injected mice and 16 mg/dL in GH-injected mice; also see Fig. 5K).
Fig. 5.
(A–J) Electron micrographs of livers from GH-injected, calorie-restricted WT (A–D) and Goat−/− (F–I) mice. Male WT and Goat−/− mice littermates (8 wk old) were subjected to 60% calorie restriction for 8 d. On day 7, each mouse received three s.c. injections of either vehicle or GH (30 µg per mouse) at 2:30 PM, 3:30 PM, and 4:30 PM. At 5:30 PM, blood was obtained for glucose measurements, after which mouse livers were fixed, perfused, and processed as described in Materials and Methods. Pictures from 20 different unit areas (36 × 26 µm per unit area) from two WT and two Goat−/− mice were taken at 5,000 amplitude; representative pictures are shown. White arrows denote typical autolysosomes. (Scale bar: 5 µm; magnification, 5,000×.) (E and J) The number of autolysosomes per unit area was determined by three independent examiners as described in Materials and Methods. Each value represents mean ± SEM of data from 20 images. Asterisks denote level of statistical significance (Student t test) between autolysosome numbers of vehicle or GH-injected WT (E) and Goat−/− (J) mice: ***P < 0.001. (K and L) Failure of acute injections of GH to restore blood glucose in Goat−/− mice. WT and Goat−/− mice (five in each group) were calorie-restricted and treated with three s.c. injections of GH as described above. On day 7, mice were killed at 5:30 PM, at 1 h after i.p. injection of leupeptin (15 mg/kg). Blood was obtained from the retro-orbital sinus for measurement of glucose (K). Supernatants from pooled livers of five mice were immunoblotted for LC3, GAPDH, p-STAT5, and t-STAT5 (L) as described in Fig. 1.
To confirm that the acute injections of GH did not desensitize hepatic GH receptors, we treated WT and Goat−/− mice exactly as shown in Fig. 5 A–J and then assayed the livers for p-STAT5. On day 7 of calorie restriction at 5:30 PM, Goat−/− mice showed a decrease in immunoblottable p-STAT5 that subsequently increased markedly after the three acute injections of GH (Fig. 5L), indicating that hepatic GH receptors were not desensitized by the repeated GH injections.
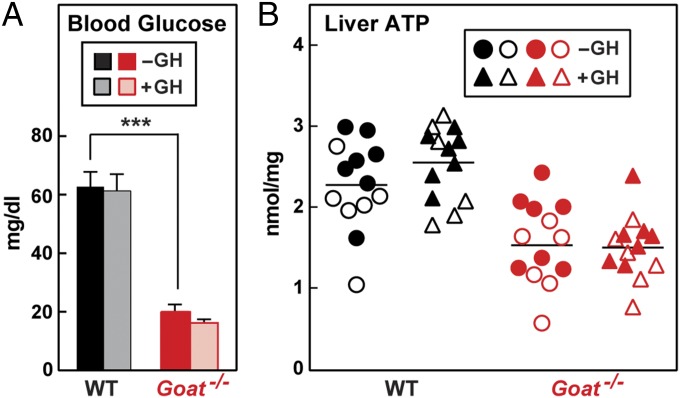
The foregoing data suggest that short-term restoration of hepatic autophagy is not sufficient to restore blood glucose in fat-depleted, ghrelin-deficient mice. To seek an explanation for this failure, we freeze-clamped livers for measurement of ATP. Fig. 6 shows the combined results of two experiments with a total of 13 mice in each group. On day 8 at 5:30 PM, after 23 h of starvation, blood glucose levels were reduced in the Goat−/− mice, and were not restored by three GH injections given at hourly intervals (Fig. 6A). Liver ATP levels were reduced by 30% in Goat−/− mice and were unaffected by GH injections (Fig. 6B). Table 1 presents the numeric data for the adenine nucleotides in the mice used in the experiments shown in Fig. 6. The measured ADP levels tended to be increased in the Goat−/− livers, but the differences were not statistically significant. From these measurements, we calculated the concentration of AMP using the formula of Burgess et al. (22), which assumes that the three adenine nucleotides are in thermodynamic equilibrium. The calculated level of AMP was elevated by twofold to threefold in the Goat−/− livers (P < 0.01), and was unaffected by the GH injections.
Fig. 6.
Failure to restore liver content of ATP in calorie-restricted Goat−/− mice by acute injection of GH. Male WT and Goat−/− mice littermates (8 wk old) were subjected to 60% calorie restriction for 8 d. On day 8, each mouse received three s.c. injections of vehicle (−GH) or 30 µg of GH (+GH) at 2:30 PM, 3:30 PM, and 4:30 PM. Mice were killed at 5:30 PM, liver samples were freeze-clamped, and blood glucose level (A) and hepatic content of ATP (B) were measured as described in Materials and Methods. In A, individual values from two independent experiments (n = 13 mice) were combined (mean ± SEM; ***P < 0.001). In B, each individual value from the two experiments is shown. Results from one experiment are denoted by open symbols; results from the other experiment, by closed symbols.
Table 1.
Liver content of ATP, ADP, and AMP in calorie-restricted WT and Goat−/− mice injected with GH
| Liver content, nmol/mg protein | ||||||
| ATP | ADP | AMP | ||||
| Genotype | −GH | +GH | −GH | +GH | −GH | +GH |
| WT | 2.3 ± 0.2 | 2.6 ± 0.1 | 2.6 ± 0.3 | 2.5 ± 0.3 | 3.5 ± 0.7 | 2.8 ± 0.6 |
| Goat−/− | 1.6 ± 0.1** | 1.5 ± 0.1*** | 3.2 ± 0.4 | 3.3 ± 0.2* | 7.9 ± 1.4** | 8.3 ± 1.3*** |
These data were obtained from the mice described in Fig. 6 (13 mice in each group). Liver content of ATP and ADP was measured as described in Materials and Methods. Liver content of AMP was calculated according to Burgess et al. (22). Each value represents mean ± SEM of data from 13 mice. Asterisks denote level of statistical significance between vehicle or GH-injected WT vs. Goat−/− mice: *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
The present study establishes a link among GH, hepatic autophagy, and maintenance of blood glucose in the setting of long-term calorie restriction in mice. We previously noted that fasting plasma GH rose progressively over 7 d when mice were subjected to 60% calorie restriction, and that this rise required ghrelin (11, 13), a hormone identified originally because of its ability to stimulate GH secretion in the pituitary (14). In response to calorie restriction, ghrelin-deficient mice failed to show the normal increase in GH and failed to maintain blood glucose at viable levels. Chronic infusion of GH, started before the onset of calorie restriction, prevents hypoglycemia in the ghrelin-deficient mice, indicating that the major role of ghrelin is to facilitate the secretion of GH (11).
The link between GH and hepatic autophagy was established by the experiment shown in Fig. 1. After 8 d of calorie restriction in WT mice, GH levels rose during the 23-h period when food was not available, peaking at 5:30 PM just before the animals were fed and declining immediately thereafter. The rise in GH was paralleled by a rise in hepatic p-STAT5, the mediator of GH action (21), and in LC3-II, a marker of autophagy (19). In Goat−/− mice deficient in ghrelin, the fasting-induced rise in GH was blunted, and there were corresponding decreases in p-STAT5 and LC3-II. We found a striking correlation between p-STAT5 and LC3-II levels (R2 = 0.9), supporting the notion that the rise in GH was responsible for the increase in autophagy (Fig. 1E). The changes in LC3-II were mirrored by changes in autophagic vacuoles in liver as assessed by EM (Fig. 3).
Hepatic autophagy was maintained, and hypoglycemia was prevented when the ghrelin-deficient mice were subjected to continuous infusion of GH, started before calorie restriction and continued throughout (Fig. 4). Autophagy was also restored when three injections of GH were given at hourly intervals to the ghrelin-deficient mice (Fig. 5); however, these injections were unable to raise the blood glucose level (Fig. 6A). A possible explanation for this finding is provided by the observation that hepatic ATP was depleted and AMP accumulated on day 8 in the fasted, ghrelin-deficient mice, neither of which was restored to normal by the GH injections (Table 1).
Considered together, the foregoing data suggest that GH maintains blood glucose levels in these calorie-restricted mice in part by activating hepatic autophagy. However, once the animals have become depleted of fat, the increase in hepatic autophagy is not sufficient to restore blood glucose. In addition to autophagy, a source of ATP is required. Indeed, we have shown previously that blood glucose is restored to WT levels when the fasted, fat-depleted, ghrelin-deficient mice are injected acutely with sources of energy that include lactate, pyruvate, alanine, and octanoate (13).
Although GH and hepatic autophagy are recognized to be important factors in maintaining blood glucose level during fasting, we are unaware of previous data linking these two agents. During an ordinary fast, GH is thought to support blood glucose primarily by activating adipose tissue lipolysis, thereby providing fatty acids that the liver can use as a source of energy to fuel gluconeogenesis (3). Our mice represent a different phenomenon, because the week-long calorie restriction depletes fat stores, making lipolysis an inadequate source of energy. Under these fat-depleted conditions, GH-induced hepatic autophagy may assume a greater role in maintaining blood glucose than it does in animals with adequate fat stores.
A major unanswered question is why chronic GH infusion is able to prevent hypoglycemia, whereas acute injections of GH fail to do so, even though both treatments raise hepatic p-STAT5 and increase hepatic autophagy. It may be that maintenance of blood glucose requires the presence of GH during the time when fat stores are being depleted. Perhaps GH acts to preserve some hidden store of energy during this depletion period. Experiments designed to solve this puzzle are underway.
Materials and Methods
The following previously described methods are summarized in SI Materials and Methods: generation of Goat−/− mice, protocol for 60% caloric restriction, GH administration, and measurement of metabolic parameters. Detailed information on electron microscopy for quantifying autolysosomes, immunoblotting, and real-time PCR is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank our colleague Guosheng Liang for helpful suggestions, Bilkish Bajaj and Hayley Ray for excellent technical assistance, and Isis Soto for invaluable help with the animal studies. This work was supported by the National Institutes of Health (Grant HL20948) and the Moss Heart Foundation. Y.Z. is supported by the Medical Scientist Training Program (Grant ST32GM08014).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423643112/-/DCSupplemental.
References
- 1.Cahill GF., Jr Starvation in man. N Engl J Med. 1970;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- 2.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 3.Nørrelund H. The metabolic role of growth hormone in humans with particular reference to fasting. Growth Horm IGF Res. 2005;15(2):95–122. doi: 10.1016/j.ghir.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Ezaki J, et al. Liver autophagy contributes to the maintenance of blood glucose and amino acid levels. Autophagy. 2011;7(7):727–736. doi: 10.4161/auto.7.7.15371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13(5):495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klionsky DJ. Autophagy: From phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8(11):931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 7.Efeyan A, et al. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493(7434):679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuma A, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 9.Svedberg P. How many people are malnourished? Annu Rev Nutr. 2011;31:263–283. doi: 10.1146/annurev-nutr-081810-160805. [DOI] [PubMed] [Google Scholar]
- 10.Singhal V, Misra M, Klibanski A. Endocrinology of anorexia nervosa in young people: Recent insights. Curr Opin Endocrinol Diabetes Obes. 2014;21(1):64–70. doi: 10.1097/MED.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao T-J, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107(16):7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein JL, et al. Surviving starvation: Essential role of the ghrelin-growth hormone axis. Cold Spring Harb Symp Quant Biol. 2011;76:121–127. doi: 10.1101/sqb.2011.76.010447. [DOI] [PubMed] [Google Scholar]
- 13.Li RL, et al. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem. 2012;287(22):17942–17950. doi: 10.1074/jbc.M112.358051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev. 2005;85(2):495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 15.Cruz CRY, Smith RG. The growth hormone secretagogue receptor. Vitam Horm. 2008;77:47–88. doi: 10.1016/S0083-6729(06)77004-2. [DOI] [PubMed] [Google Scholar]
- 16.Nass R, Gaylinn BD, Thorner MO. The role of ghrelin in GH secretion and GH disorders. Mol Cell Endocrinol. 2011;340(1):10–14. doi: 10.1016/j.mce.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez JA, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105(17):6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabeya Y, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He H, et al. Post-translational modifications of three members of the human MAP1LC3 family and detection of a novel type of modification for MAP1LC3B. J Biol Chem. 2003;278(31):29278–29287. doi: 10.1074/jbc.M303800200. [DOI] [PubMed] [Google Scholar]
- 21.Herrington J, Smit LS, Schwartz J, Carter-Su C. The role of STAT proteins in growth hormone signaling. Oncogene. 2000;19(21):2585–2597. doi: 10.1038/sj.onc.1203526. [DOI] [PubMed] [Google Scholar]
- 22.Burgess SC, et al. Carbohydrate-response element-binding protein deletion alters substrate utilization producing an energy-deficient liver. J Biol Chem. 2008;283(3):1670–1678. doi: 10.1074/jbc.M706540200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.