Significance
In the human brain, from early in development through to adulthood, the superior temporal sulcus is deeper in the right than the left cerebral hemisphere in the area ventral of Heschl’s gyrus. Irrespective of gender, handedness, and language lateralization, and present in several pathologies, this asymmetry is widely shared among the human population. Its appearance early in life suggests strong genetic control over this part of the brain. In contrast, the asymmetry is barely visible in chimpanzees. Thus this asymmetry probably is a key locus to look for variations in gene expression among the primate lineage that have favored the evolution of crucial cognitive abilities sustained by this sulcus in our species, namely communication and social cognition.
Keywords: brain, anatomy, asymmetry, human-specific, STS
Abstract
Identifying potentially unique features of the human cerebral cortex is a first step to understanding how evolution has shaped the brain in our species. By analyzing MR images obtained from 177 humans and 73 chimpanzees, we observed a human-specific asymmetry in the superior temporal sulcus at the heart of the communication regions and which we have named the “superior temporal asymmetrical pit” (STAP). This 45-mm-long segment ventral to Heschl’s gyrus is deeper in the right hemisphere than in the left in 95% of typical human subjects, from infanthood till adulthood, and is present, irrespective of handedness, language lateralization, and sex although it is greater in males than in females. The STAP also is seen in several groups of atypical subjects including persons with situs inversus, autistic spectrum disorder, Turner syndrome, and corpus callosum agenesis. It is explained in part by the larger number of sulcal interruptions in the left than in the right hemisphere. Its early presence in the infants of this study as well as in fetuses and premature infants suggests a strong genetic influence. Because this asymmetry is barely visible in chimpanzees, we recommend the STAP region during midgestation as an important phenotype to investigate asymmetrical variations of gene expression among the primate lineage. This genetic target may provide important insights regarding the evolution of the crucial cognitive abilities sustained by this sulcus in our species, namely communication and social cognition.
Since Geschwind and Levitsky’s (1) first attempt to identify a specifically human cortical landmark, the identification of unique features of the human brain that might explain the cognitive success of the human species has remained elusive so that anatomical targets still do not exist to inform the search for genetic mutations contributing to the human cognitive phenotype. Because hemispheric asymmetry and language processing are fundamental human traits, the perisylvian language areas have been especially scrutinized for such markers, but until now none has been forthcoming. In particular, the reported asymmetries in the planum temporale and the inferior frontal region are not as robust as initially thought (1–3) and also are observed, albeit often less marked, in other primates (4). However, we show here that asymmetry of the superior temporal sulcus (STS), at the core of the human communication system, represents a species-specific perisylvian anatomical marker. This finding is consistent with functional brain imaging studies that have emphasized the importance of STS not only for language processing in the left hemisphere but also for social communication in the right hemisphere (5, 6). Notably, in the left hemisphere a hierarchy of areas sensitive to increased levels of acoustical complexity is observed along superior temporal regions and become specifically linguistic along the STS (7, 8), whereas in the right hemisphere the presence of areas involved in voice and face recognition, gaze perception, and theory of mind confirms the importance of the right superior temporal cortex in social cognition (6).
As is the case for many cortical sulci, the STS appears 1 or 2 wk earlier in the right hemisphere than in the left during gestation (9–11), but unlike other sulci, in which the left hemisphere catches up, a depth asymmetry in the STS also is reported later in life (12–14) with a reproducible location in the posterior STS, at least in young adults and adolescents (10, 14, and 24 y) (15). The principal objectives of the present study were first to define the location and extent of STS asymmetry accurately; second to examine whether the STS asymmetry has the same characteristics from infanthood, when sulcation is on-going, to adulthood; and finally to compare the measurements in humans with the measurements obtained in chimpanzees.
We assessed how widespread STS asymmetry was across the human species by investigating several factors known to affect brain asymmetry. First, because of the critical role of the STS in verbal and nonverbal human communication, analyses were extended to cohorts with atypical communication profiles, namely adults with right hemispheric dominance for language and autistic children who previously have been reported to show anatomical and functional abnormalities in the STS (16). Second, the effects of handedness and sex, which have been shown to modulate asymmetries of both the planum temporale and Heschl’s gyrus in nearby temporal regions (17, 18), were investigated by including patients with Turner syndrome (XO). Third, two general mechanisms have been proposed to explain brain asymmetries, namely a left–right reversed pattern of body-axis orientation and a competition between homotopic regions across hemispheres through the corpus callosum (19), and these potential mechanisms were investigated by studying cohorts of subjects with situs inversus and patients with corpus callosum agenesis (AgCC). In situs inversus, the main organs of the body are flipped relative to their usual position (i.e., the heart is on the right side), and the brain is reported to exhibit reversed petalia (20).
Finally, a detailed characterization of STS asymmetry was attempted via the measurement of the shape of individual sulcal profiles and the assessment of how anatomical interruptions of the sulcus might influence overall STS asymmetry. Because sulcal interruption, also referred to as a “pli de passage,” has been reported to be more frequent in the left hemisphere than in the right (21), we investigated whether sulcal interruption is the underlying cause of STS asymmetry.
Results
For each subject the brain and sulci were segmented on individual T1-weighted (T1w) 3D MR images using the BrainVISA Morphologist pipeline,* normalized to Talairach space, and a mesh representing the STS in each cerebral hemisphere was constructed. When the sulcus was disconnected, sulcal parts were concatenated; and with regard to potential extension of STS into parietal lobe only the caudal rami within the angular gyrus were considered. Subsequently, a new coordinate system was defined along and across the STS (22), with an origin referenced to the location where the planum temporale has the greatest depth (12). Subsequently, depth profiles were computed along the long axis of the sulcus and aligned across hemispheres and subjects (Fig. 1). A depth asymmetry index [AI = 2*(R − L)/(R + L)] was computed, e.g., AI = +20% for right (R) depth = +26 mm, and left (L) depth = +21 mm.
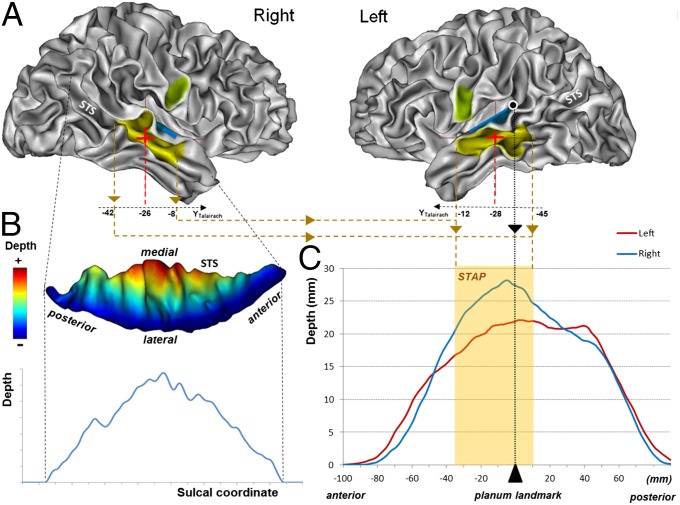
Fig. 1.
(A) Location of the STAP (yellow) relative to Heschl’s gyrus (blue) and the ventral tip of the central sulcus (green) on both left and right inner cortical surfaces of an individual adult brain. The STAP center is shown by a cross. The black dot with a white contour line shows the planum temporale landmark. (B, Upper) Sulcal depth shown by color coding of the sulcal mesh (seen from above). (Lower) Sulcal depth profile in the right hemisphere of an individual subject. (C) Adult sulcal depth profile; STAP anterior and posterior ends as well as the planum landmark are drawn in dotted lines. The light orange overlay illustrates the STAP (deeper on the right), defined as the common asymmetrical segment in the three typical groups (infant, right-handed children, and adults).
Location and Extent of Sulcal Asymmetry in Typical Groups.
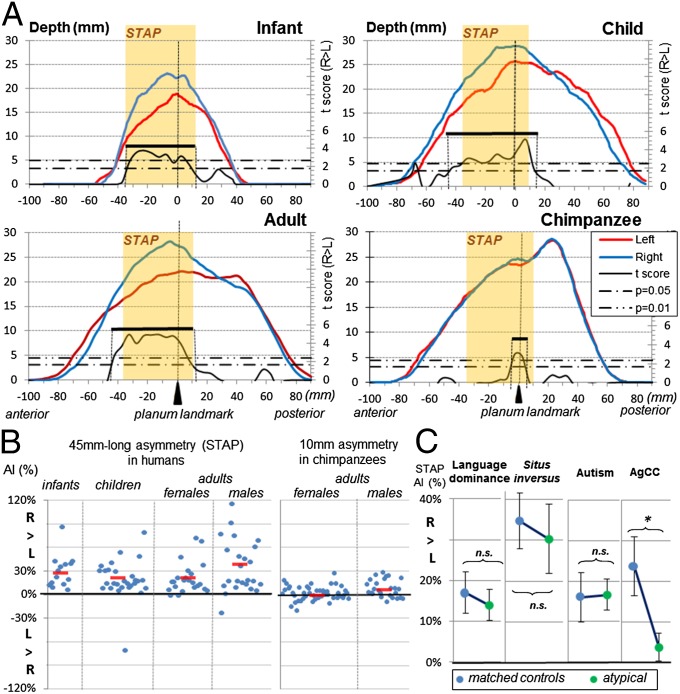
STS asymmetry was measured in 14 infants, 28 right-handed children, and 47 right-handed adults. On average, the right STS was deeper than the left in each age group (Ps ≤ 0.004) (Table 1). By using permutation tests for 5-mm-long intervals along the length of the STS, an asymmetrical sulcal segment was detected in each group. A large region of overlap of these asymmetrical sulcal segments across the three age groups was termed the “superior temporal asymmetrical pit) (STAP) (Figs. 1 and 2). The STAP is a 45-mm-long sulcal segment located at the base of Heschl’s gyrus (mid-STS) with its center slightly forward of the planum landmark. Specifically, the STAP has the following position within Talairach space: left anterior border: x = −59, y = −12, z = −9; right anterior border: +54, −8, −14; left center: −55, −28, −3; right center: +50, −26, −4; left posterior border: −52, −45, +5; right posterior border: +51, −42, +7. Based on criteria previously developed for studies of the planum temporale (23), 96% of subjects had an AI >0, and 74% had an AI >10%. The left–right difference in magnitude was around 29%, 19%, and 28% in infants, children, and adults, respectively, but these potential differences across the lifespan were not significant (F2,86 = 1.5; P = 0.2; one-way ANOVA).
Table 1.
Sulcal depth asymmetry and sulcal interruptions
| Group | Subtype | n | Mean sulcal depth asymmetry | Group-specific asymmetrical segment | STAP region range: −35, +10mm; Center: L: −55, −28, −3; R: 50, −26, −4 | Plis de passage, frequency | ||||||
| AI, % | P (R > L) | Range, mm | AI, % | AI, % | P (R > L) | Subjects R > L, % | L, % | R, % | P (L > R) | |||
| Infants | 14 | +20 (± 17) | < 0.001 | −35, +10 | +29 (± 21) | +29 (± 21) | Na | 100 | 29 | 7 | Ns | |
| Right-handed children | 28 | +8 (± 15) | 0.004 | −45, +15 | +18 (± 21) | +19 (± 25) | Na | 96 | 50 | 7 | * | |
| Right-handed adults | All | 47 | +13 (± 17) | < 0.001 | −45, +10 | +23 (± 21) | +28 (± 28) | Na | 94 | 53 | 6 | * |
| Males | 24 | +11 (± 18) | 0.004 | −35, +5 | +25 (± 32) | +35 (± 33) | Na | 96 | 67 | 13 | * | |
| Females | 23 | +15 (± 15) | < 0.001 | −45, +20 | +20 (± 17) | +20 (± 19) | Na | 91 | 39 | 0 | * | |
| Left-handed adults | All | 48 | +12 (± 13) | < 0.001 | −45, +10 | +18 (± 16) | +19 (± 19) | < 0.001 | 96 | 54 | 17 | * |
| Males | 14 | +20 (± 12) | < 0.001 | −35, +30 | +26 (± 13) | +29 (± 16) | < 0.001 | 100 | 79 | 14 | * | |
| Females | 34 | +8 (± 13) | < 0.001 | −45, 5 | +16 (± 17) | +15 (± 18) | < 0.001 | 94 | 44 | 18 | * | |
| LLg | 17 | +10 (± 12) | 0.002 | −15, +30 | +15 (± 18) | +17 (± 21) | 0.002 | 94 | 35 | 12 | Ns | |
| RLg | 17 | +6 (± 13) | 0.03 | −30, 0 | +18 (± 18) | +14 (± 16) | 0.001 | 94 | 53 | 24 | Ns | |
| Other humans | ASD | 15 | +7 (± 16) | 0.05 | −35, 0 | +16 (± 10) | +17 (± 15) | < 0.001 | 93 | 60 | 27 | Ns |
| Turner | 14 | +14 (± 13) | 0.001 | −35; +15 | +32 (± 20) | +33 (± 21) | < 0.001 | 100 | 64 | 7 | * | |
| AgCC | 5 | +2 (± 15) | Ns (0.4) | Na (small sample size) | +4 (± 8) | Ns (0.2) | 80 | 20 | 20 | Ns | ||
| SI | 6 | +16 (± 19) | 0.05 | Na (small sample size) | +30 (± 21) | 0.008 | 100 | 50 | 0 | Ns | ||
| Chimpanzees | All | 73 | +1 (± 9) | Ns (0.3) | −5, +5 | +4 (± 10) | +1 (± 8) | Ns (0.2) | 56 | 10 | 7 | Ns |
| Males | 26 | +2 (± 10) | Ns (0.2) | −5, +5 | +7 (± 11) | +1 (± 7) | Ns (0.2) | 62 | 8 | 4 | Ns | |
| Females | 47 | 0 (± 8) | Ns (0.4) | None | 0 (± 9) | Ns (0.4) | 53 | 11 | 9 | Ns | ||
The sulcal AI was assessed in each group using Student’s paired t test. Average values and SDs (in parenthesis) are expressed as percentages. The group-specific segment is the most asymmetrical sulcal part within each group as defined using permutation tests. All human groups of suitable size (≥14) had only one asymmetrical segment except for left-handed female adults with left lateralization for language (only the largest segment is given for this group; see text for details). Range is given in sulcal coordinates. The STAP region is the asymmetrical segment overlapping across typical groups (three first rows). The center of the STAP region is given in Talairach space; percent of subjects having right deeper than left STAP is shown. To avoid circularity, STAP statistics are not given for the first three groups, which were used to define the STAP. Finally, the occurrence of sulcal interruptions, plis de passage, has been computed across the STAP; left greater than right number of plis de passage was tested using Fisher’s exact test. LLg, adults with left-hemispheric language dominance; Na, not applicable; Ns, not significant; RLg, adults with right-hemispheric language dominance; SI, adults with situs inversus.
P < 0.05.
Fig. 2.
(A) Left (red line) and right (blue line) STS depth profiles from the temporal pole to its parietal caudal end. Depth profiles are shown for infants, right-handed children, adults, and chimpanzees. The asymmetrical part of the STS is computed for each group by permutation tests over 5-mm-long intervals along the sulcus. Two statistical thresholds (Pcorr = 0.05; Pcorr = 0.01) are shown by horizontal dashed-and-dotted lines. The extent of the asymmetrical segment is given by the range of the Student t variable (black line) above the lower threshold and is identified by a black bar (see also Table 1). The extent of the common region across the three typical human groups (STAP) is shown in light orange overlay. (B) STAP per cent in individual typical humans and chimpanzees. Mean group values are shown by a short red line. (C) STAP in atypical human groups, i.e., adults with right lateralization for language, adults with situs inversus (reversed petalia), children with autism spectrum disorders, and children with AgCC. *P < 0.05. n.s., not significant.
Effects of Handedness and Sex.
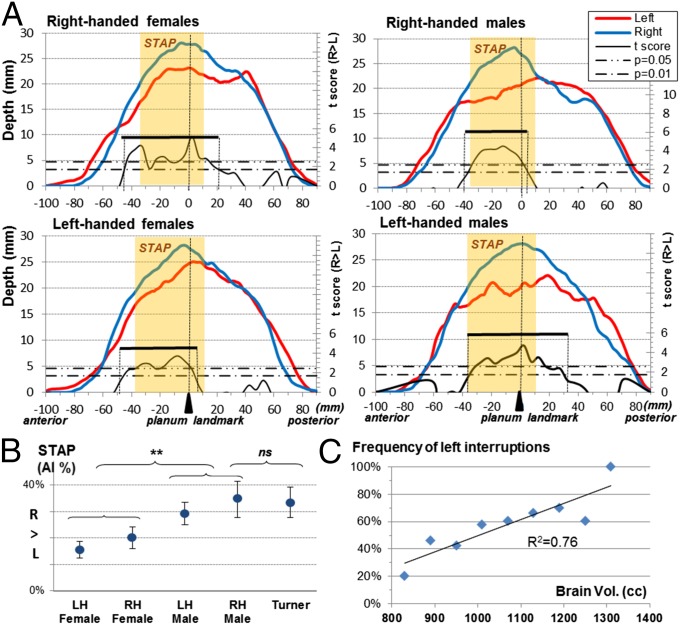
The effect of handedness and sex on STS asymmetry was investigated in 95 adults (Fig. 3 and Table 1). The sulcus was found to be asymmetrical in both male and female left-handers (Ps ≤ 0.001) (Table 1). Using permutation statistics, an asymmetrical segment was found in each group, which largely overlapped with the STAP region (≥89%). The STAP was present in 96% of left-handers (AI >0) with a mean amplitude of 19%. When the asymmetry magnitude was compared between group-specific asymmetrical segments with sex and handedness as between-subject factors, a trend for a sex effect, but no significant handedness effect, was observed (sex: F1,92 = 3.3, P = 0.07; handedness: F1,92 <1; handedness × sex: F1,92 <1; two-way ANOVA). When the analysis was restricted to the STAP region, the handedness factor remained not significant, but the sex effect was strengthened, with males having a larger AI than females (sex: F1,92 = 8.3; P = 0.001; handedness: F1,92 = 1; P = 0.3; two-way ANOVA) (Fig. 3B).
Fig. 3.
The STAP in relation to sex and handedness in adults. (A) Left (red line) and right (blue line) STS depth profiles. The extent of the asymmetrical segment is given by the range of the Student t variable (black line) above the lower threshold and is identified by a black bar (see also Table 1). The extent of the STAP region is shown in light orange overlay. (B) STAP statistics (mean and SE) in healthy right-handers and left-handers and in patients with Turner syndrome. (C) Frequency of left sulcal interruptions in relation to brain volume. LH, left-handed; n.s., not significant; RH, right-handed. **P < 0.01.
Because of this sexual dimorphism, we analyzed 14 females with Turner syndrome i.e., who have only one X chromosome (Table 1). The STAP was present in every subject, and, unexpectedly, its magnitude was as large as in typical male adults (Turner AI = 33 ± 9%, typical males: AI = 35 ± 7%; P = 0.9; Welch t test).
Species-Specific Asymmetry.
In comparison with the complex, twisted shape of human STS, chimpanzee STS is nearly linear. No significant STS asymmetry was observed in chimpanzees when either the whole sulcus or the STAP was considered (STS AI = 1 ± 9%; P = 0.3; STAP AI = 1 ± 8%; P = 0.2) (Table 1). A small asymmetrical segment within the STAP was barely deeper on the right side than the left in 56% of chimpanzees (AI >0) (Fig. 2). It was 10 mm long in normalized space i.e., 6 mm in the native space. However, this right–left difference was smaller than in every human typical group (AI = 4 ± 10%; Ps < 0.002; Tukey’s test). Notably, the asymmetrical segment was present in male but not in female chimpanzees (47 females: AI = 1% ± 7%; P = 0.1; 26 males: AI = 7 ± 11%; P = 0.001); The asymmetry magnitude was not correlated with brain volume (Pearson’s P = 0.9) although male brains were larger than female brains (female brains: 280 ± 30 cm3; male brains: 304 ± 36 cm3; males > females: P < 0.01; Welch t test).
Atypical Communication Systems: Right Lateralization for Language and Autism.
Because the STS is at the heart of the human communication system, we first investigated whether the STAP is affected by language dominance. In particular, two groups of 17 young adults with either left or right cerebral hemisphere dominance for language, matched in age and handedness (24), were studied. In each group, permutation tests revealed asymmetrical segments along the sulcus which overlapped with the STAP region. In particular, in subjects with right lateralization for language one 30-mm-long segment fully included in the STAP was recognized, whereas in left-lateralized subjects two segments (one 15-mm-long anterior segment with 66% overlap and one 45-mm-long posterior segment with 56% overlap) were recognized. When restricted to the STAP, asymmetry was present (AI > 0) in 94% of subjects with right lateralization for language and was not significantly different between groups (left language dominance: AI = 17 ± 21%; right language dominance: AI = 14 ± 16%; left lateral vs. right lateral dominance: P = 0.6; Welch t test) (Fig. 2C and Table 1).
Next 15 autistic boys, matched with the group of typical right-handed boys for age, handedness, and developmental quotients, were studied. Rightward asymmetry of the STAP region was similar in autistic children and their matched controls (autistic children: AI = 17 ± 15%; controls: AI = 16 ± 26%; autistic boys vs. controls: P = 0.9; Welch t test).
General Structural Factors: Hemispheric Asymmetry, Body-Axis Orientation, and Interhemispheric Interaction.
Because sulcal asymmetry potentially may be explained by a more general brain asymmetry, the volume asymmetry of the hemispheres was computed. Right-handed children and adults had a slightly bigger right than left hemisphere, on average (Ps ≤ 0.01), but left-handed adults did not (Ps ≥ 0.7). However, no correlation was found between brain asymmetry and any asymmetrical STS segment in these groups (Ps ≥ 0.15).
Six subjects (five men) with situs inversus for whom a reverse pattern of brain petalia had been shown were studied also. Despite the small sample size, the right STS again was deeper than the left in the STAP (AI = 30 ± 21%; P = 0.008), with a magnitude similar to that in the right-handed adult male group used as controls (controls: AI = 28 ± 2 8%; situs inversus vs. controls; P = 0.7; Welch t test).
Finally, five girls with complete or partial AgCC were studied. The STAP was present (AI >0) in four of the five patients, although it was smaller than in the right-handed girls used as controls (AgCC: AI = 4 ± 8%; P = 0.2; controls: AI = 24 ± 23%; AgCC vs. controls: P = 0.03; Welch t test).
Sulcal Interruptions.
Because it has been shown previously in adults that sulcal interruptions (plis de passage) occur more frequently on the left than on the right STS (21), a post hoc analysis was run over all human subjects to assess the effect of these interruptions on depth asymmetry. An interruption can either be full (when the sulcus is made of several disconnected parts) or partial (when a transverse gyrus is buried in the depth of the sulcus) (Fig. S1). We computed the occurrence and size of plis de passage in each hemisphere in each group over the STAP. By using Fisher’s exact test, more sulcal interruptions were identified in the left than the right hemisphere in typical children and in adult groups irrespective of sex or handedness (far right column of Table 1). Notably, patients with AgCC had few sulcal interruptions on the left (20%). In chimpanzees, there were few sulcal interruptions, and the number of interruptions was not significantly different in the left and right STS.
An assessment was made to determine whether an asymmetry of sulcal interruptions might explain STS depth asymmetry. Accordingly, all human subjects were divided into two groups: one group of 100 subjects who had an interruption in one or other hemisphere within the STAP and another group with continuous sulci in both hemispheres (77 subjects). As expected, in the former group the left–right difference in interruption size was correlated with the STAP (R2 = 0.45, P < 0.001) (Fig. S2A). The larger the plis de passage in the left hemisphere, the larger the STAP. However, the STAP remained significant in 97% of subjects without plis de passage (Fig. S2B). Thus, the STAP is explained only in part by a greater number of interruptions in the left than right cerebral hemisphere.
Finally, because a larger STAP had been observed in males than in females, sulcal interruptions were compared across sex in the adult groups. There were more interruptions in the left hemisphere in males than in females (females: 42%; males: 71%; P = 0.004; Fisher exact test), whereas no sex effect on AI was found in adults without any interruption (30 females and 9 males; females: AI = 12 ± 10%; males: AI = 12 ± 5%; P = 1; Welch t test). Because this sexual dimorphism might be explained by differences in brain size (brain volume in females: 978 ± 94 cm3; in males: 1,094 ± 106 cm3; males > females: P < 0.001; Welch t test), we computed the frequency of interruptions as a function of brain size intervals. We found a linear relationship between the number of interruptions and brain size (R2 = 0.76, P = 0.001) using 60-cm3-wide intervals in the range of 800–1,340 cm3 (Fig. 3C).
Discussion
A significant depth asymmetry in STS ventral to the Heschl’s gyrus is identified as a feature widely shared among the human population but scarcely visible in chimpanzees. To a large extent this STAP region overlaps with other asymmetrical areas previously reported in the STS (figure 5 in ref. 9, figure 6 in ref. 25, figure 2 in ref. 14, figure 13 in ref. 13, and both middle and posterior segments in figure 3 in ref. 15). In many respects the STAP is as important as the widely studied asymmetry of the planum temporale. First, results obtained in infants, children, and adults, together with studies at other age periods, e.g., in fetuses (10), toddlers (14), and adolescents (15), suggest that the STAP has its origin during midgestation and is present throughout the human lifespan. Second, the STAP, like planum temporale asymmetry, is present irrespective of sex (26), handedness (17), and language lateralization (27). Third, the prevalence of STAP in adults (72% using the criterion defined in ref. 23) is similar to that of planum temporale asymmetry (28). Finally, because both planum temporale asymmetry and the STAP are preserved in subjects with situs inversus (20, 29), these temporal asymmetries may have an origin distinctly different from visceral and petalia asymmetries. Despite these common features, however, notable differences suggest that the STAP might be a more species-distinctive feature than the planum. Atypical asymmetry (i.e., to the other side) is larger for the planum temporale [AI >10% in 15% of subjects (28)] than for the STS (AI ≤10% in 2% of typical subjects in our study). Most importantly, the small spatial extent and weak magnitude of STS asymmetry in chimpanzees might constitute a major difference with the planum temporale, for which asymmetry also has been reported in chimpanzees (4, 30).
The sexual dimorphism of the STS asymmetry found in both chimpanzees and humans encourages a search for a relationship with the sex chromosomes, which also have diverged rapidly between the two species (31). Furthermore, XY aneuploidies often are associated with verbal or social cognition deficits and anomalies of the temporal regions (32). As a first approach, we examined females with Turner syndrome (XO). The STAP in this group was similar to that in typical males. Studies of other types of XY aneuploidies are required to understand better how these chromosomes affect the STAP, but we emphasize that most of the sexual dimorphism observed in humans might be related simply to differences in brain size in the human species. Indeed, we found a close relationship between the STAP, the number of sulcal interruptions, and the brain size, consistent with a previous report of an increase of gyrification patterns in larger cortices (33). Males’ larger brain size increases the number of sulcal interruptions in the left hemisphere and subsequently the STAP magnitude. Thus, a human universal STAP, present in adults with no pli de passage or in small brains (infants), might be enhanced in bigger brains (usually males) by a left pli de passage (15, 21).
The relationship between the STAP and sulcal interruptions may shed light on the biological mechanisms responsible for this asymmetry. A recent study has shown that the number of pits, and subsequently interruptions, increases in the left STS between birth and 2 y of age (34). This increase of sulcal interruptions might correspond to the growth of a transverse gyrus in the depth of the left sulcus. According to models of cortical folding, gyral growth can be explained by variations of mechanical properties of the cortex and/or constraints of the underlying white matter (35, 36). Several findings suggest a large connectivity in this brain region: A dense local connectivity between the many areas useful for the correct encoding of phonemes (37) intermingles with many long-range tracts (38)—the arcuate fasciculus, the middle longitudinal fasciculus, the inferior occipito-frontal fasciculus, and transcallosal fibers. These last fibers might be an important factor, because both reduced STAP magnitude and fewer plis de passage were observed in patients with AgCC. The arcuate fasciculus might participate also, because it shares several features with the STAP: It is much larger in humans than in chimpanzees; it expands near the STAP region to a larger extent on the left hemisphere than on the right (39); and it is asymmetric from early in life (40) and irrespective of language lateralization (41). These observations suggest that the STAP might be related to a dense and asymmetrical development of the underlying white matter in the superior temporal region.
To understand the functional significance of the STAP, a human distinctive anatomical marker, two groups of subjects with atypical communication systems were investigated, namely, autistic children and adults with right-hemispheric language dominance. Dysfunction of the STS region, including decreased gray matter density, hypoperfusion at rest, and decreased activations in tasks involving voice and face recognition, gaze perception, and social cognition in general, has been reported in autism (16). However, in the present study no alteration in STS asymmetry was observed in this group. Similarly, the present study has revealed that STS asymmetry is not related to language lateralization. However, the STAP region is at a key location along the linguistic ventral pathway mapping sounds to meaning (7, 42, 43). The classical criteria of hemispheric language dominance based on functional lateralization in Broca’s area during a word-production task (44) may not reflect the functional role of the STAP region closer to the language-receptive areas. Indeed independent analysis by Greve et al. (27) of the database investigated in the present study found no difference in the asymmetry of the planum temporale between groups of subjects with opposite hemispheric dominance for language. Apart from communication systems, the STS is involved in several other processes, including audiovisual integration and biological motion related to social cognition (6), which should be investigated also. The search for the STAP function would be greatly facilitated by a more detailed description of the cytoarchitectonic organization in this brain region.
In conclusion, a robust asymmetry in the depth of the STS is present at the base of Heschl’s gyrus in the great majority of human subjects. Because STS asymmetry is barely visible in chimpanzees and likely is absent in macaques (45), the presence of the STAP is interpreted as a recent evolutionary change. Furthermore, that this asymmetry is present in infants and even fetuses (10) suggests an early genetically driven mechanism and stimulates the search for genes of recent evolution expressed differently in the superior temporal region during midgestation (46). Although observed in all human groups, the magnitude of the STAP asymmetry is modulated by sex, perhaps because male brains are larger than female brains. Because children with corpus callosum dysgenesis were the least asymmetric human group in our study, further studies should examine how the dense underlying fiber pathways (38) act upon this region. Understanding how evolution shaped this cortical area differently in each hemisphere most likely would highlight a critical feature of one of the several cognitive networks involved in this region (5, 38, 47).
Material and Methods
See SI Materials and Methods for further details on subjects, scanning parameters, brain segmentation, sulcal identification, measurement of interruptions, and landmarks. Informed consent was obtained in each experiment and these experiments were approved by the local ethical committee. MRI acquisition parameters are given in Table S1.
Subjects.
Three typical subject groups were defined, namely, infants, right-handed children, and right-handed adults: 14 healthy full-term infants (mean age = 11.1 ± 3.9 wk; nine males, five females), whose data have already been published (12); 18 right-handed boys (mean age = 10.6 ± 1.3 y) from the Kennedy Krieger Institute, as part of the Autism Brain Imaging Data Exchange (ABIDE) project; 10 right-handed girls (mean age = 9.6 ± 0.4 y) imaged at the NeuroSpin center [Office of Atomic Energy and Alternative Energies (CEA), Gif sur Yvette, France]; and 47 young right-handed adults (mean age = 21 ± 1.2 y; 23 females, 24 males) imaged at the NeuroSpin center (CEA, Gif sur Yvette, France).
The following atypical human groups were studied also: 48 young left-handed adults from a study by Van der Haegen et al. (24), consisting of 14 males (mean age = 22 ± 4 y), 17 females with left hemisphere lateralization for language (mean age = 20 ± 1.6 y), and 17 females with right hemisphere lateralization for language (mean age = 20.2 ± 1.5 y); 15 autistic right-handed boys (mean age = 10.0 ± 1.5 y) from the Kennedy Krieger Institute recruited as part of the ABIDE project matched with the typically developing child group defined above; five right-handed girls with prenatal diagnosis of isolated AgCC (mean age = 11.4 ± 1.5 y) imaged at the NeuroSpin center (CEA, Gif sur Yvette, France) (48); six adults with situs inversus from the Situs Inversus Project managed by N.R.; and 14 patients with Turner syndrome (mean age 24.5 ± 6 y) from a study by Molko et al. (49).
Finally, 73 adult chimpanzees (47 females, 26 males; mean age = 23 y ± 12 y) from the Division of Developmental and Cognitive Neuroscience at the Yerkes National Primate Research Center, Atlanta, GA (45) were studied.
Computing and Aligning Sulcal Depth Profiles.
A model-driven parameterization (22) was used to define a coordinate system along the length and depth of the sulcus. Once parameterized, local sulcal depth may be computed as the geodesic distance between the most and least superficial sulcal locations at each length coordinate. A depth profile is the curve made of all these local depth measurements along the sulcus. A common landmark was applied to define the origin of the depth profile in all subjects. As in Glasel et al. (12), the landmark was provided by the deepest location of the planum temporale, which is located in the posterior part of the insula posterior to the medial tip of Heschl’s gyrus. This location has been shown to be relatively stable across subjects (12). When Heschl’s gyrus was duplicated, the anterior border of the planum temporale was set posterior to the most anterior transverse gyrus (23). Finally, the planum landmark was projected onto the STS along the dorso–ventral axis to set the profile origin. Depth profiles both within and across groups of subjects were aligned according to this common origin.
Location and Extent of Asymmetry Using Permutation Tests.
As in Glasel et al. (12), the asymmetrical part of the STS was identified by applying permutation tests to groups of suitable size (≥14), i.e., infants, normally developing and autistic children, all adult groups except for subjects with situs inversus, and chimpanzees. Paired t tests were applied between right and left depth profiles at each sulcal location using a sliding window, and the most asymmetrical segment that provided the maximum tmax score was determined. Next, 5,000 random inversions of right and left profiles across subjects were performed to estimate the random distribution of t-score maximal values. Finally, the measured tmax was compared with this distribution to obtain the P value. This procedure was performed in Talairach space with a 5-mm-wide sliding window and enabled 5-mm-long segments with significant asymmetry to be identified along the sulcus. Adjacent segments were concatenated to define the extent of asymmetry in each group (Table 1). We applied Bonferonni correction to take into account the number of sulcal locations. Despite this restrictive criterion, we confirmed that one asymmetrical segment could still be found in all groups but LLg left-handed females and that typical groups had one asymmetrical segment in common within the STAP region.
Supplementary Material
Acknowledgments
We thank the ABIDE initiative, the Laboratory for Neurocognitive and Imaging Research (Kennedy Krieger Institute, Baltimore, MD), and Roberto Toro for the dataset related to normally developing and autistic children; specifically, from the Laboratory for Neurocognitive and Imaging Research, we thank Anita Barber, Rebecca Buhlman, Brian Caffo, Deana Crocetti, Suresh Joel, John Muschelli, Carrie Nettles, James Pekar, Kristie Sweeney, Michelle Talley, Mary Beth Nebel, and Stewart Mostofsky. We also thank Nicolas Molko and Stanislas Dehaene for the dataset related to Turner syndrome. Finally, we are grateful to the NeuroSpin teams for their help in the acquisition of infants and children data. This work was supported by the McDonnell and Bettencourt–Schueller Foundations.
Footnotes
The authors declare no conflict of interest.
*Fischer C, The 18th Annual Meeting of the Organization for Human Brain Mapping, June 10–14, 2012, Beijing, China.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412389112/-/DCSupplemental.
References
- 1.Geschwind N, Levitsky W. Human brain: Left-right asymmetries in temporal speech region. Science. 1968;161(3837):186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 2.Dorsaint-Pierre R, et al. Asymmetries of the planum temporale and Heschl’s gyrus: Relationship to language lateralization. Brain. 2006;129(Pt 5):1164–1176. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- 3.Keller SS, Crow T, Foundas A, Amunts K, Roberts N. Broca’s area: Nomenclature, anatomy, typology and asymmetry. Brain Lang. 2009;109(1):29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins WD, Nir TM. Planum temporale surface area and grey matter asymmetries in chimpanzees (Pan troglodytes): The effect of handedness and comparison with findings in humans. Behav Brain Res. 2010;208(2):436–443. doi: 10.1016/j.bbr.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: Processing of mutual and averted social gaze in the superior temporal sulcus. Psychol Sci. 2004;15(9):598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 6.Hein G, Knight RT. Superior temporal sulcus—It’s my area: Or is it? J Cogn Neurosci. 2008;20(12):2125–2136. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- 7.DeWitt I, Rauschecker JP. Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci USA. 2012;109(8):E505–E514. doi: 10.1073/pnas.1113427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dehaene-Lambertz G, et al. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc Natl Acad Sci USA. 2006;103(38):14240–14245. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois J, et al. Structural asymmetries of perisylvian regions in the preterm newborn. Neuroimage. 2010;52(1):32–42. doi: 10.1016/j.neuroimage.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Habas PA, et al. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cereb Cortex. 2012;22(1):13–25. doi: 10.1093/cercor/bhr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1(1):86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- 12.Glasel H, et al. A robust cerebral asymmetry in the infant brain: The rightward superior temporal sulcus. Neuroimage. 2011;58(3):716–723. doi: 10.1016/j.neuroimage.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 14.Li G, et al. Mapping longitudinal hemispheric structural asymmetries of the human cerebral cortex from birth to 2 years of age. Cereb Cortex. 2014;24(5):1289–1300. doi: 10.1093/cercor/bhs413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonte M, et al. Development from childhood to adulthood increases morphological and functional inter-individual variability in the right superior temporal cortex. Neuroimage. 2013;83:739–750. doi: 10.1016/j.neuroimage.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Zilbovicius M, et al. Autism, social cognition and superior temporal sulcus. Open J Psychiatr. 2013;3(2A):46–55. [Google Scholar]
- 17.Steinmetz H, Volkmann J, Jäncke L, Freund HJ. Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers. Ann Neurol. 1991;29(3):315–319. doi: 10.1002/ana.410290314. [DOI] [PubMed] [Google Scholar]
- 18.Good CD, et al. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 19.Cook ND. Callosal inhibition: The key to the brain code. Behav Sci. 1984;29(2):98–110. doi: 10.1002/bs.3830290203. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy DN, et al. Structural and functional brain asymmetries in human situs inversus totalis. Neurology. 1999;53(6):1260–1265. doi: 10.1212/wnl.53.6.1260. [DOI] [PubMed] [Google Scholar]
- 21.Ochiai T, et al. Sulcal pattern and morphology of the superior temporal sulcus. Neuroimage. 2004;22(2):706–719. doi: 10.1016/j.neuroimage.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Coulon O, et al. 2006. pp. 29–185. Cortical localization via surface parameterization: a sulcus-based approach. Neuroimage 31(Suppl 1):
- 23.Galaburda AM, Corsiglia J, Rosen GD, Sherman GF. Planum Temporale Asymmetry, Reappraisal since Geschwind and Levitsky. Neuropsychologia. 1987;25(6):853–868. [Google Scholar]
- 24.Van der Haegen L, Cai Q, Brysbaert M. Colateralization of Broca’s area and the visual word form area in left-handers: fMRI evidence. Brain Lang. 2012;122(3):171–178. doi: 10.1016/j.bandl.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Hill J, et al. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. 2010;30(6):2268–2276. doi: 10.1523/JNEUROSCI.4682-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer IE, Aleman A, Somers M, Boks MP, Kahn RS. Sex differences in handedness, asymmetry of the planum temporale and functional language lateralization. Brain Res. 2008;1206:76–88. doi: 10.1016/j.brainres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Greve DN, et al. A surface-based analysis of language lateralization and cortical asymmetry. J Cogn Neurosci. 2013;25(9):1477–1492. doi: 10.1162/jocn_a_00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: Asystematic, quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev. 1999;29(1):26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 29.Ihara A, et al. Neuroimaging study on brain asymmetries in situs inversus totalis. J Neurol Sci. 2010;288(1-2):72–78. doi: 10.1016/j.jns.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: Humanlike pattern of Wernicke's brain language area homolog. Science. 1998;279(9 January):220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- 31.Hughes JF, et al. Chimpanzee and human Y chromosomes are remarkably divergent in structure and gene content. Nature. 2010;463(7280):536–539. doi: 10.1038/nature08700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raznahan A, et al. 2014. Globally divergent but locally Convergent X- and Y-chromosome influences on cortical development. Cereb Cortex, in press.
- 33.Germanaud D, et al. Larger is twistier: Spectral analysis of gyrification (SPANGY) applied to adult brain size polymorphism. Neuroimage. 2012;63(3):1257–1272. doi: 10.1016/j.neuroimage.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 34.Meng Y, Li G, Lin W, Gilmore JH, Shen D. Spatial distribution and longitudinal development of deep cortical sulcal landmarks in infants. Neuroimage. 2014;100:206–218. doi: 10.1016/j.neuroimage.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu G, et al. Axons pull on the brain, but tension does not drive cortical folding. J Biomech Eng. 2010;132(7):071013. doi: 10.1115/1.4001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toro R, Burnod Y. A morphogenetic model for the development of cortical convolutions. Cereb Cortex. 2005;15(12):1900–1913. doi: 10.1093/cercor/bhi068. [DOI] [PubMed] [Google Scholar]
- 37.Mesgarani N, Cheung C, Johnson K, Chang EF. Phonetic feature encoding in human superior temporal gyrus. Science. 2014;343(6174):1006–1010. doi: 10.1126/science.1245994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turken AU, Dronkers NF. The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández-Miranda JC, et al. Asymmetry, connectivity, and segmentation of the arcuate fascicle in the human brain. Brain Struct Funct, in press. 2014 doi: 10.1007/s00429-014-0751-7. [DOI] [PubMed] [Google Scholar]
- 40.Dubois J, et al. Structural asymmetries in the infant language and sensori-motor networks. Cereb Cortex. 2009;19(2):414–423. doi: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]
- 41.Vernooij MW, et al. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: A combined fMRI and DTI study. Neuroimage. 2007;35(3):1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 42.Liebenthal E, et al. Specialization along the left superior temporal sulcus for auditory categorization. Cereb Cortex. 2010;20(12):2958–2970. doi: 10.1093/cercor/bhq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Striem-Amit E, Hertz U, Amedi A. Extensive cochleotopic mapping of human auditory cortical fields obtained with phase-encoding FMRI. PLoS ONE. 2011;6(3):e17832. doi: 10.1371/journal.pone.0017832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van der Haegen L, Cai Q, Seurinck R, Brysbaert M. Further fMRI validation of the visual half field technique as an indicator of language laterality: A large-group analysis. Neuropsychologia. 2011;49(10):2879–2888. doi: 10.1016/j.neuropsychologia.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Bogart SL, et al. Cortical sulci asymmetries in chimpanzees and macaques: A new look at an old idea. Neuroimage. 2012;61(3):533–541. doi: 10.1016/j.neuroimage.2012.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson MB, et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62(4):494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yovel G, Belin P. A unified coding strategy for processing faces and voices. Trends Cogn Sci. 2013;17(6):263–271. doi: 10.1016/j.tics.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bénézit A, et al. Organising white matter in a brain without corpus callosum fibres. Cortex. 2015;63:155–171. doi: 10.1016/j.cortex.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 49.Molko N, et al. Brain anatomy in Turner syndrome: Evidence for impaired social and spatial-numerical networks. Cereb Cortex. 2004;14(8):840–850. doi: 10.1093/cercor/bhh042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.