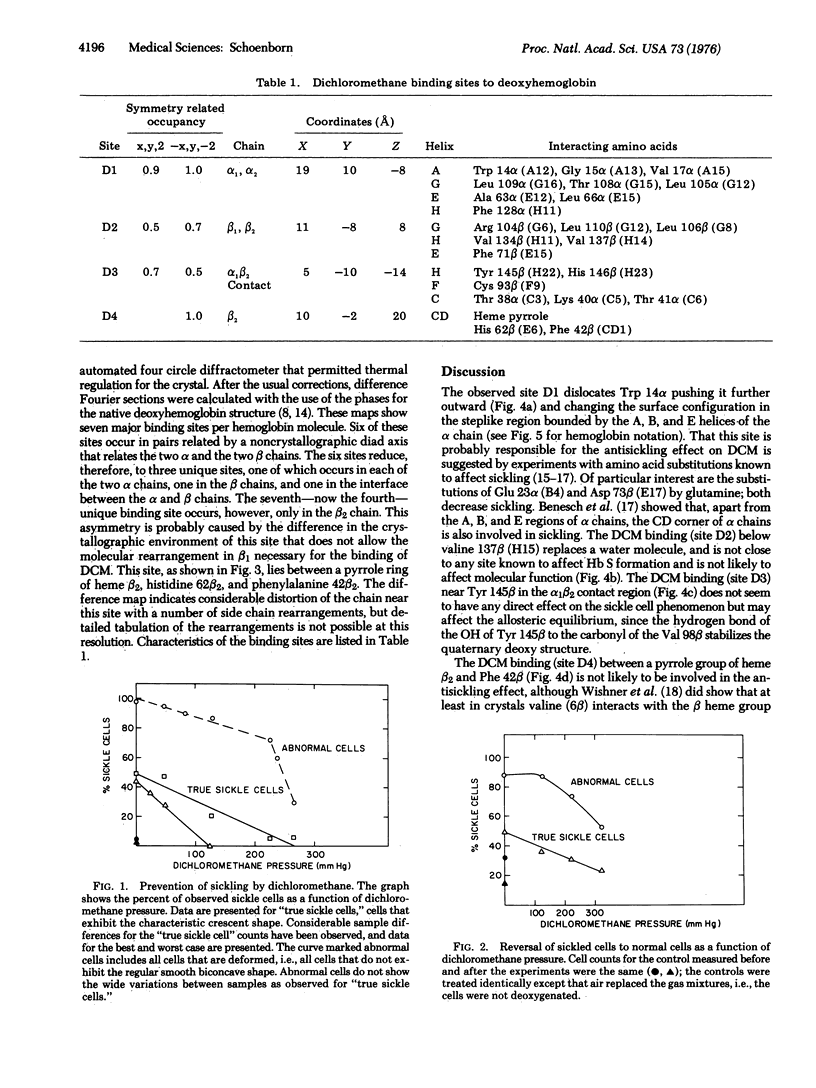
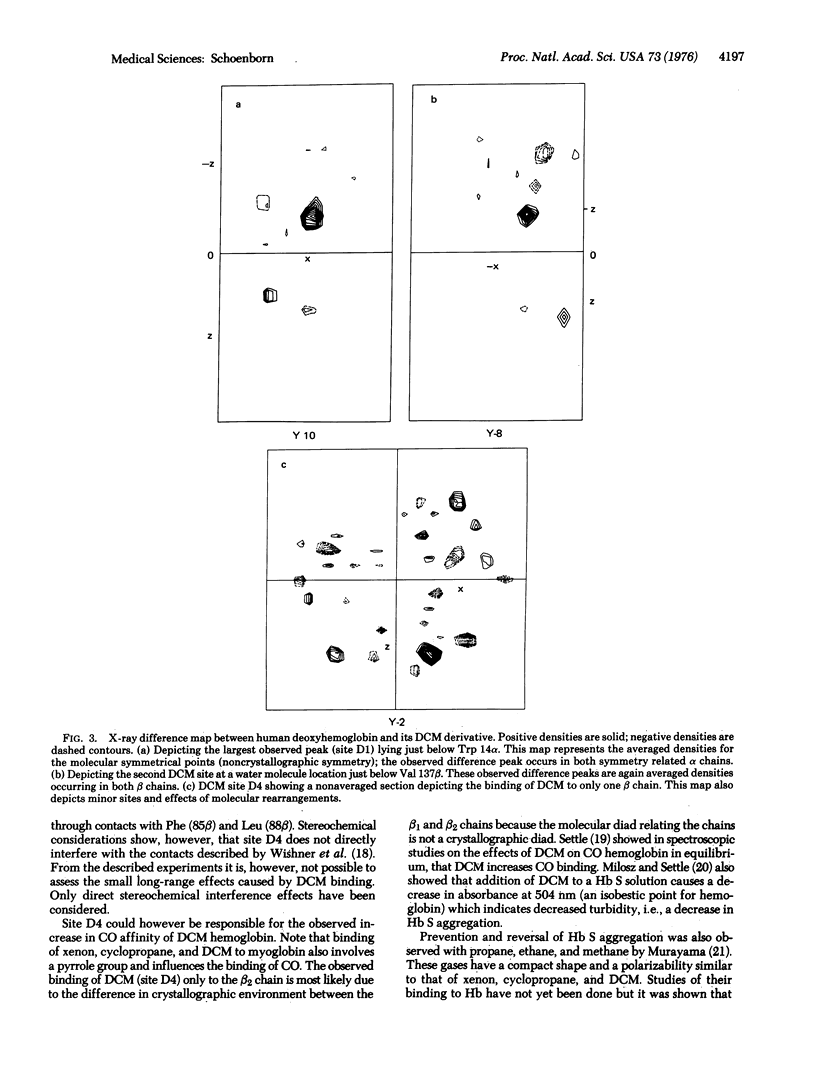
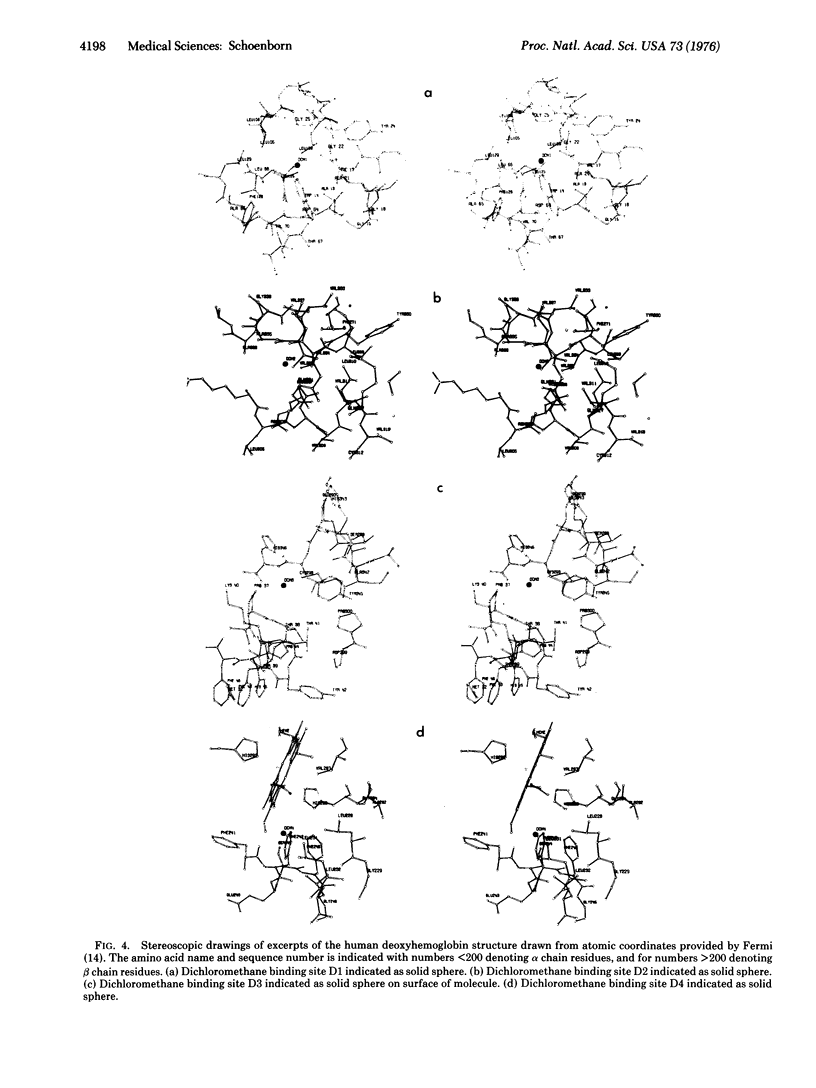
Abstract
Microscopic studies of red cells from homozygous sickle cell patients show that dichloromethane does prevent sickle cell formation in vitro and does cause reversion of sickled cells to normal after exposure to dichloromethane. X-ray structural analysis of human deoxyhemoglobin crystals exposed to dichloromethane shows four unique binding sites. Arguments are presented to suggest that the binding site close to tryptophan 14alpha prevents the formation of helical polymers, i.e., prevent sickling.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benesch R. E., Yung S., Benesch R., Mack J., Schneider R. G. alpha-Chain contacts in the polymerisation of sickle haemogloblin. Nature. 1976 Mar 18;260(5548):219–221. doi: 10.1038/260219a0. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L., Ranney H. M. The effect of beta 73 Asn on the interactions of sickling hemoglobins. Biochim Biophys Acta. 1970 Nov 17;221(2):373–375. doi: 10.1016/0005-2795(70)90279-5. [DOI] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Perutz M. F., Bertles J. F., Döbler J. Structure of sickled erythrocytes and of sickle-cell hemoglobin fibers. Proc Natl Acad Sci U S A. 1973 Mar;70(3):718–722. doi: 10.1073/pnas.70.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM V. M. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957 Aug 17;180(4581):326–328. doi: 10.1038/180326a0. [DOI] [PubMed] [Google Scholar]
- Josephs R., Jarosch H. S., Edelstein S. J. Polymorphism of sickle cell hemoglobin fibers. J Mol Biol. 1976 Apr 15;102(3):409–426. doi: 10.1016/0022-2836(76)90324-7. [DOI] [PubMed] [Google Scholar]
- MURAYAMA M. A MOLECULAR MECHANISM OF SICKLED ERYTHROCYTE FORMATION. Nature. 1964 Apr 18;202:258–260. doi: 10.1038/202258a0. [DOI] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Swerdlow P. H., Bertles J. F. Intermolecular organization of deoxygenated sickle haemoglobin determined by x-ray diffraction. Nature. 1972 Sep 22;239(5369):217–219. doi: 10.1038/239217a0. [DOI] [PubMed] [Google Scholar]
- Milosz A., Settle W. A new approach to the treatment of sickle cell anemia. Res Commun Chem Pathol Pharmacol. 1975 Sep;12(1):137–146. [PubMed] [Google Scholar]
- Muirhead H., Cox J. M., Mazzarella L., Perutz M. F. Structure and function of haemoglobin. 3. A three-dimensional fourier synthesis of human deoxyhaemoglobin at 5.5 Angstrom resolution. J Mol Biol. 1967 Aug 28;28(1):117–156. doi: 10.1016/s0022-2836(67)80082-2. [DOI] [PubMed] [Google Scholar]
- Nunes A. C., Schoenborn B. P. Dichloromethane and myoglobin function. Mol Pharmacol. 1973 Nov;9(6):835–839. [PubMed] [Google Scholar]
- Perutz M. F., TenEyck L. F. Stereochemistry of cooperative effects in hemoglobin. Cold Spring Harb Symp Quant Biol. 1972;36:295–310. doi: 10.1101/sqb.1972.036.01.040. [DOI] [PubMed] [Google Scholar]
- Ranney H. M. The interactions of sickle hemoglobin. Biochimie. 1972;54(5):633–638. doi: 10.1016/s0300-9084(72)80154-8. [DOI] [PubMed] [Google Scholar]
- Schoenborn B. P. Binding of xenon to horse haemoglobin. Nature. 1965 Nov 20;208(5012):760–762. doi: 10.1038/208760a0. [DOI] [PubMed] [Google Scholar]
- Schoenborn B. P., Watson H. C., Kendrew J. C. Binding of xenon to sperm whale myoglobin. Nature. 1965 Jul 3;207(992):28–30. doi: 10.1038/207028a0. [DOI] [PubMed] [Google Scholar]
- Wishner B. C., Ward K. B., Lattman E. E., Love W. E. Crystal structure of sickle-cell deoxyhemoglobin at 5 A resolution. J Mol Biol. 1975 Oct 15;98(1):179–194. doi: 10.1016/s0022-2836(75)80108-2. [DOI] [PubMed] [Google Scholar]


