Significance
The use of light-emitting electronic devices for reading, communication, and entertainment has greatly increased recently. We found that the use of these devices before bedtime prolongs the time it takes to fall asleep, delays the circadian clock, suppresses levels of the sleep-promoting hormone melatonin, reduces the amount and delays the timing of REM sleep, and reduces alertness the following morning. Use of light-emitting devices immediately before bedtime also increases alertness at that time, which may lead users to delay bedtime at home. Overall, we found that the use of portable light-emitting devices immediately before bedtime has biological effects that may perpetuate sleep deficiency and disrupt circadian rhythms, both of which can have adverse impacts on performance, health, and safety.
Keywords: sleep, chronobiology, phase-shifting, digital media, electronics
Abstract
In the past 50 y, there has been a decline in average sleep duration and quality, with adverse consequences on general health. A representative survey of 1,508 American adults recently revealed that 90% of Americans used some type of electronics at least a few nights per week within 1 h before bedtime. Mounting evidence from countries around the world shows the negative impact of such technology use on sleep. This negative impact on sleep may be due to the short-wavelength–enriched light emitted by these electronic devices, given that artificial-light exposure has been shown experimentally to produce alerting effects, suppress melatonin, and phase-shift the biological clock. A few reports have shown that these devices suppress melatonin levels, but little is known about the effects on circadian phase or the following sleep episode, exposing a substantial gap in our knowledge of how this increasingly popular technology affects sleep. Here we compare the biological effects of reading an electronic book on a light-emitting device (LE-eBook) with reading a printed book in the hours before bedtime. Participants reading an LE-eBook took longer to fall asleep and had reduced evening sleepiness, reduced melatonin secretion, later timing of their circadian clock, and reduced next-morning alertness than when reading a printed book. These results demonstrate that evening exposure to an LE-eBook phase-delays the circadian clock, acutely suppresses melatonin, and has important implications for understanding the impact of such technologies on sleep, performance, health, and safety.
The use of electronic devices for reading, communication, and entertainment has greatly increased in recent years. Greater portability, convenience, and ease of access to reading materials in electronic form add to the popularity of these devices. The use of light-emitting devices immediately before bedtime is a concern because light is the most potent environmental signal that impacts the human circadian clock and may therefore play a role in perpetuating sleep deficiency (1). The circadian-timing system synchronizes numerous internal physiological and biochemical processes, including the daily rhythm of sleep propensity (2), to external environmental time cues. For optimal sleep duration and quality, the timing of the sleep episode must be appropriately aligned with the timing of the circadian clock. In humans, exposure to light in the evening and early part of the night, even at low intensity, suppresses the release of the sleep-facilitating hormone melatonin (3–5) and shifts the circadian clock to a later time (3, 6), both of which make it more difficult to fall asleep at night. Light exposure in the biological evening/night also acutely increases alertness (7, 8), but not much is known about its impact on alertness the following day. Here we present results from a randomized study comparing the effects of reading before bedtime using a light-emitting eReader (LE-eBook) with reading a printed book by reflected light. We examined circadian timing and suppression of melatonin, polysomnographic (PSG) recordings of sleep, and subjective and objective measures of sleepiness both in the evening while reading and the following morning.
Results
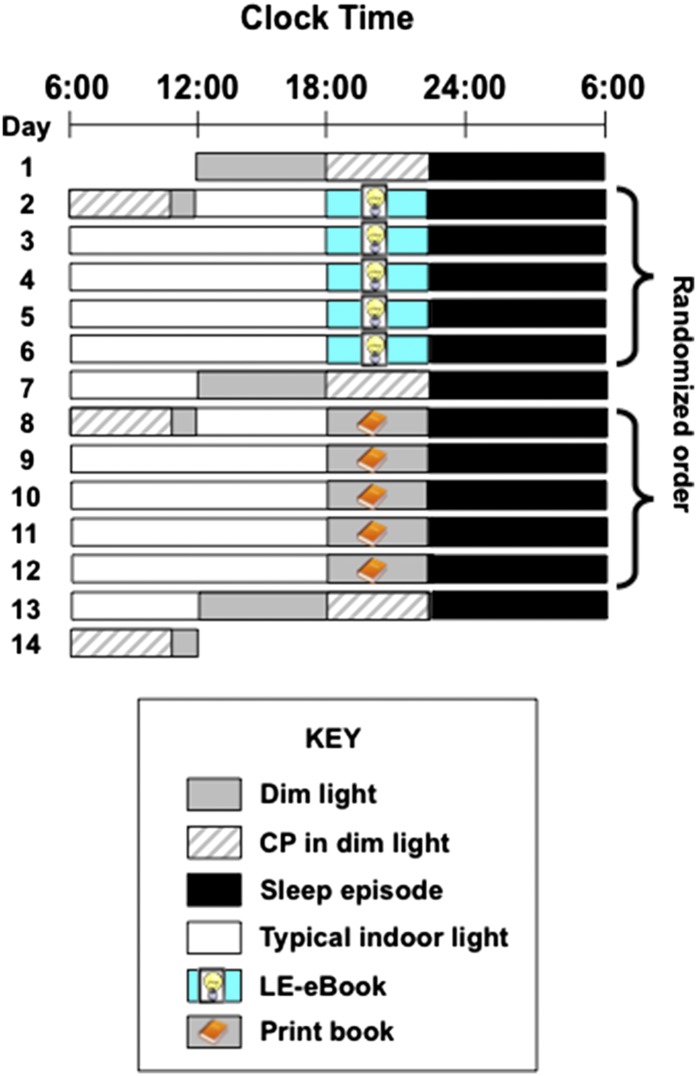
Twelve healthy young adults (mean ± SD: 24.92 ± 2.87 y; six women) completed a 14-d inpatient protocol. The randomized, crossover design (shown in Fig. 1) consisted of two conditions: (i) reading an LE-eBook in otherwise very dim room light for ∼4 h before bedtime for five consecutive evenings, and (ii) reading a printed book in the same very dim room light for ∼4 h before bedtime for five consecutive evenings. All participants completed both reading conditions but were randomized to the order. Hourly blood samples were collected during portions of the study for assessment of plasma melatonin concentrations. Sleep latency (i.e., interval between lights-out and the timing of sleep onset) was assessed from PSG recordings on the fourth and fifth nights of each condition. In addition, we assessed total sleep time (TST), sleep efficiency (the percentage of time in bed spent asleep), and the time spent in each sleep stage. Participants rated their sleepiness using a computerized Karolinska Sleepiness Scale (KSS) (9) every evening and morning, and waking electroencephalogram (EEG) measures were recorded on two evenings and two mornings of each reading condition. More detailed methods are described in Materials and Methods.
Fig. 1.
Representative raster plot of the 14-d study protocol. Black bars indicate the 10:00 PM–6:00 AM sleep episode in darkness. Gray bars denote dim room light (∼3 lx of white light in the angle of gaze; Materials and Methods), and white bars denote typical indoor room light (∼90 lx in the angle of gaze). Striped bars show the constant posture (CP) procedures. Reading sessions are marked either by the LE-eBook or the print-book and symbols. Participants were randomized to the order of reading condition. Ambient room light level for all reading sessions was dim (∼3 lx).
LE-eBook Effects on Levels and Circadian Timing of Melatonin.
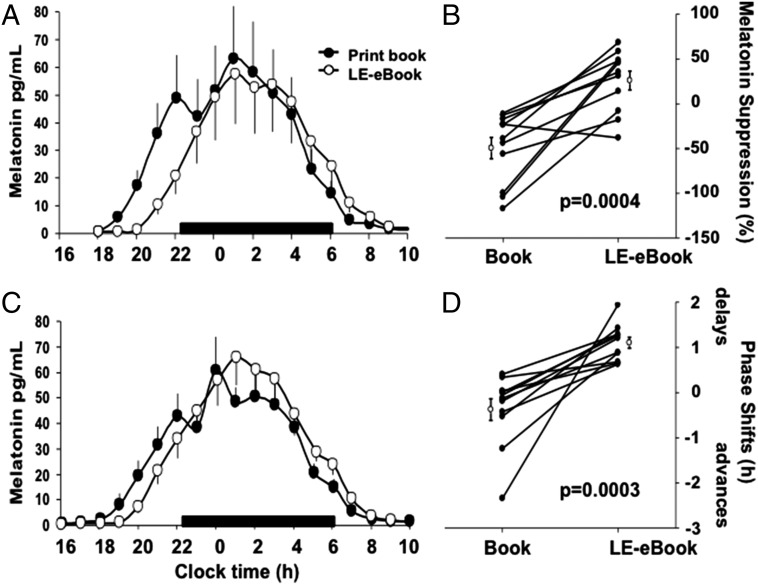
The LE-e-book condition suppressed evening levels of melatonin by 55.12 ± 20.12%, whereas the print-book condition showed no suppression (−18.77 ± 39.57%) as measured during the fifth night (P < 0.001; Fig. 2 A and B). Dim light melatonin onset was >1.5 h later on the day following the LE-eBook condition (22:31 ± 0:42) than in the print-book condition (21:01 ± 0:49; P < 0.001; Fig. 2 C and D).
Fig. 2.
Melatonin suppression (A and B) and phase shifting (C and D) during and after the LE-eBook and print book reading conditions. (A) Average waveforms of melatonin (±SEM) during the fifth night of each reading condition. The black bar denotes the scheduled sleep episode (22:00–06:00). (B) Percent suppression for each condition for each participant (filled symbols) and group average (±SEM; open symbols). (C) Average waveforms of melatonin (±SEM) on the evening/night after each reading condition. (D) Average phase shift of melatonin onset for each condition for each participant (filled symbols) and group average (±SEM; open symbols). The main effect of Condition was significant (P < 0.05, mixed model).
Impact of Reading Condition on Sleep.
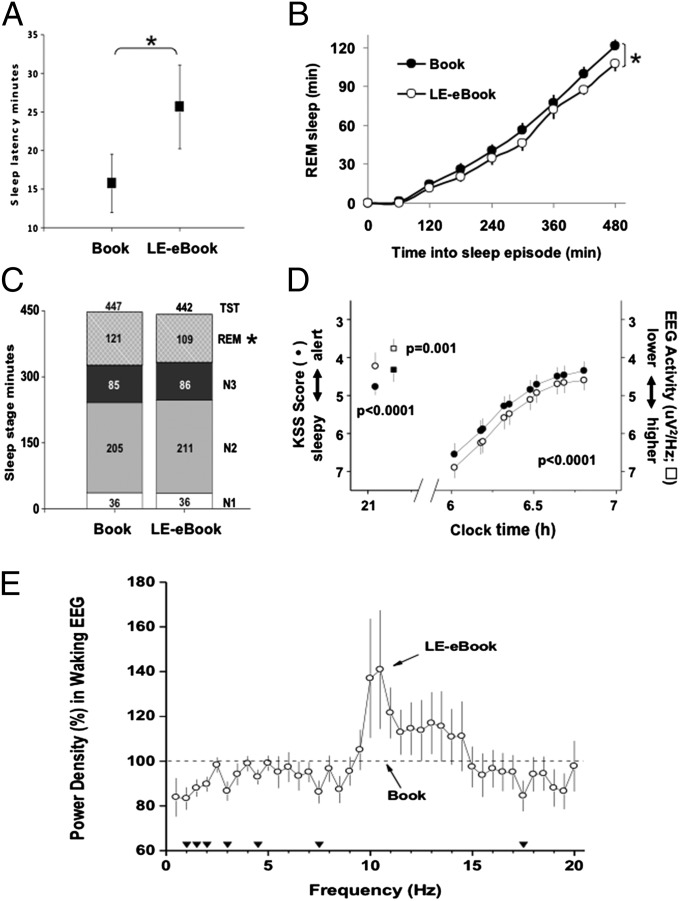
In the LE-eBook condition, participants averaged nearly 10 min longer to fall asleep than in the print-book condition (mean ± SD, 25.65 ± 18.78 min vs. 15.75 ± 13.09 min; P = 0.009; mixed model; Fig. 3A). Participants also had significantly less rapid eye movement (REM) sleep following the LE-eBook condition (109.04 ± 26.25 min vs. 120.86 ± 25.32 min in the print-book condition; P = 0.03; Fig. 3 B and C), reflecting a lower average rate of accumulation of REM sleep during sleep (Fig. 3B). There was no difference between conditions in TST, sleep efficiency, or the duration of non-REM sleep (stages 1–3; Fig. 3C) in the sleep episode, which were scheduled for eight hours each.
Fig. 3.
Sleep and sleepiness/alertness measures during and after the print-book and LE-eBook reading conditions. (A) Mean (±SEM) sleep latency to stage N2 in minutes for each reading condition. *P = 0.009, mixed model. (B) Mean (±SEM) accumulation of REM across 8-h sleep episode for each condition. *P = 0.029. (C) Mean duration (in minutes) of sleep stages N1 (white), N2 (light gray), N3 (dark gray), and REM (patterned), and total sleep time (TST; numbers at top of bar) for each reading condition. *P = 0.029. (D) Mean (±SEM) alertness ratings (circles) during and on the morning after each reading condition with respect to clock hour. Mean delta/theta activity in the waking EEG, power density in the 1.0–7.5 Hz range (squares), that was derived from C3/M2 during the fourth and fifth reading sessions of each condition is also shown. (E) Power density in the waking EEG during the LE-eBook condition (open circles) expressed as a percentage of the printed-book condition (100%; dashed line). Two-way mixed-model ANOVA on log-transformed absolute power densities per 0.5-Hz was significant for condition (P < 0.04). Filled triangles at the bottom indicate EEG frequency bins for which the difference between conditions was significant (P < 0.05, post hoc paired t tests).
Effects on Acute Evening Alertness and Morning Sleepiness.
Reading the LE-eBook was associated with decreased sleepiness in the evening. An hour before bedtime, study participants rated themselves as less sleepy (P < 0.01; Fig. 3D), and their EEG showed less power within the delta/theta frequency range (1.0–7.5 Hz; Fig. 3 D and E) in the LE-eBook condition. The following morning, however, the results for self-reported sleepiness were reversed, with participants feeling sleepier the morning after reading an LE-eBook the prior evening (P < 0.001; Fig. 3D). Furthermore, not only did they awaken feeling sleepier, it took them hours longer to fully “wake up” and attain the same level of alertness than in the printed book condition.
Many eReaders Emit Short-Wavelength Light.
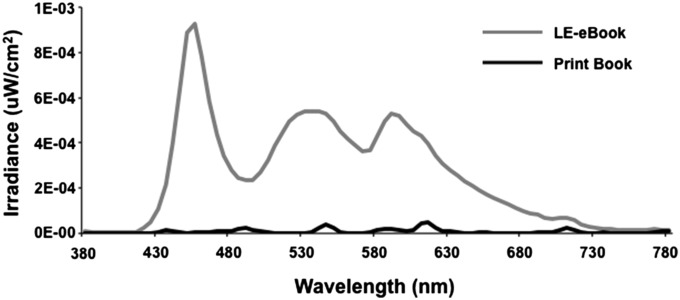
Full spectral profiles for the LE-eBook used by the study participants in the current study and for the incident reflected light in the print book conditions are shown in Fig. 4. Table 1 displays the illuminance measures (cyanopic, melanopic, rhodopic, chloropic, and erythropic lux in comparison with photopic lux) for both the LE-eBook and the reflected light of the print book, using the recently proposed light measurement strategy that takes into account non–image-forming retinal responses to light (see Methods). Light readings for the LE-eBook as well as from several light-emitting and non–light-emitting eReaders and other electronic devices are shown in Table S1. Light from the LE-eBook is short-wavelength–enriched, with a peak at 452 nm in the blue light range, compared with broad-spectrum light (white light), with a peak at 612 nm. As shown in Table S1, measurements from several other light-emitting devices are also enriched for short-wavelength light.
Fig. 4.
Spectral radiometric profile of the LE-eBook device (gray) and incident light reflected by the printed book (black). The peak irradiance for the LE-eBook eReader is ∼450 nm and for the reflected light is 612 nm.
Table 1.
Photopic illuminance and human retinal photopigment-weighted illuminance measures from the LE-eBook device and light reflected by the printed book
| Device | Photopic lux | Cyanopic lux | Melanopic lux | Rhodopic lux | Chloropic lux | Erythropic lux |
| Print book* | 0.91 | 0.45 | 0.65 | 0.68 | 0.81 | 0.92 |
| LE-eBook | 31.73 | 31.64 | 31.03 | 31.68 | 31.84 | 30.69 |
Radiometric light readings were taken in the same dim background room light conditions and from the same distance (38–40 cm). The LE-eBook was set to the maximum brightness setting.
Illuminance in the printed book condition was measured from the ambient room light emitted by the ceiling fixtures and reflected by the book, using the recently proposed light measurement strategy that takes into account non–image-forming retinal responses to light (see Methods).
Discussion
We found that, compared with reading a printed book in reflected light, reading a LE-eBook in the hours before bedtime decreased subjective sleepiness, decreased EEG delta/theta activity, and suppressed the late evening rise of pineal melatonin secretion during the time that the book was being read. We also found that, compared with reading a printed book, reading an LE-eBook in the hours before bedtime lengthened sleep latency; delayed the phase of the endogenous circadian pacemaker that drives the timing of daily rhythms of melatonin secretion, sleep propensity, and REM sleep propensity; and impaired morning alertness. These results indicate that reading an LE-eBook in the hours before bedtime likely has unintended biological consequences that may adversely impact performance, health, and safety. The results of this study are of particular concern, given recent evidence linking chronic suppression of melatonin secretion by nocturnal light exposure with the increased risk of breast, colorectal, and advanced prostate cancer associated with night-shift work (for review, see ref. 10), which has now been classified as a probable carcinogen by the World Health Organization (11, 12). Moreover, the observation that the endogenous circadian melatonin phase was 1.5 h later when reading an LE-eBook compared with reading from a printed book suggests that using a light-emitting device in the hours before bedtime is likely to increase the risk of delayed sleep-phase disorder and sleep onset insomnia (13), especially among individuals living in society who self-select their bedtimes and wake times. Induction of such misalignment of circadian phase is likely to lead to chronic sleep deficiency (1).
The decreased sleepiness before bedtime and longer sleep latency we observed in the LE-eBook condition is likely due to both an acute alerting effect of light and a delay of the circadian timing system. Suppression of melatonin by exposure to evening light may be an underlying mechanism by which light acutely increases alertness, as seen in the present study and in previous reports (14–19). Other studies, however, have not found a relationship between alertness and melatonin levels during light exposure (20, 21) or have shown changes in alertness induced by light exposure during the day, when melatonin levels are at low or undetectable levels (22–24). The circadian-phase delay, as marked by the endogenous melatonin rhythm, probably also contributed to the delay of sleep onset that occurred after study participants were reading the LE-eBook. The significant difference in sleep latency occurred even though the scheduled bedtime was fixed at 10:00 PM each night during the study protocol to ensure an 8-h sleep opportunity in bed. Thus, these results likely underestimate the impact that use of these devices in the hours before bedtime has on self-selected sleep timing and duration.
The effects of the LE-eBook on sleepiness the following morning, however, cannot be due to the acute effects of light observed the previous evening. Individuals were sleepier the morning after reading in the LE-eBook condition than after reading a printed book the evening before; however, the light levels in the morning were identical for both reading conditions. Therefore, the difference in morning sleepiness between the conditions is most likely due to differences in the prior sleep episode and/or the circadian-phase delay. Indeed, it did take longer for participants to fall asleep after the LE-eBook condition, but there was no difference in average sleep duration and the magnitude of the difference in sleep latency is unlikely to account for the effect on alertness observed 8 h later. The difference in REM sleep between the conditions may have contributed to the difference in morning sleepiness ratings. Given that the majority of REM sleep occurs in the latter portion of the sleep episode (25) (i.e., closer to wake time), participants had significantly less REM sleep in the LE-eBook condition. Because most spontaneous awakenings occur out of REM sleep (26, 27), this reduction in REM sleep in the LE-eBook condition may have also impacted sleepiness upon awakening. The significant phase delay after the LE-eBook condition suggests that the evening light from the LE-eBook phase delayed the circadian clock, delaying the nadir of the circadian rhythm of sleep propensity (2) and thereby resulting in a robust, albeit indirect, effect on morning sleepiness. A phase delay of the circadian clock is consistent with the slower rise in the rate of accumulation of REM sleep. The change in the timing of REM sleep likely represents a delay in the circadian rhythm of REM sleep propensity, which is temporally coincident with the sleep propensity rhythm (25).
The spectral composition of the light emitted by the LE-eBook may explain why the magnitude of the melatonin-suppressing and phase-shifting response observed was greater than would be predicted for this moderately low level of light (3). In humans, exposure to short-wavelength monochromatic light in the evening has been shown to induce greater circadian and alerting responses than exposure to the same number of photons of longer-wavelength monochromatic light (17–19, 28–34), even though the shorter-wavelength light may have a much lower illuminance level when measured in photopic lux (35). For this reason, it has recently been proposed that lux is an inappropriate measure for estimation of the impact of light on melatonin suppression, circadian-phase shifting, and other non–image-forming effects of retinal light exposure (35).
This study had a number of limitations. First, melatonin suppression was assessed on the fifth and final evening of each reading condition. Although it is likely that the phase shift in the LE-eBook condition had already occurred by this time, melatonin suppression was calculated by using the shifted area under the curve (AUC) from the following evening and thus should control for any phase shift. Therefore, the greater suppression seen was not due to an effect of a delayed phase in the LE-eBook condition. Second, the duration of the evening reading sessions were 4 h long. However, given that the average teenager in the United States spends 7.5 h per day engaged in recreational media plus time spent on homework—which both occur in the late afternoon/evening, including the hour before bedtime (36), and which both involve exposure to light-emitting screens (e.g., LE eReaders, computers, televisions, tablets, smartphones, video game consoles, etc.)—the 4-h exposure interval used in this study is likely in the range of screen time exposure experienced by millions of Americans each evening. Third, in the present study, the LE-eBook was set to maximum brightness throughout the 4-h reading session, whereas, by comparison, the print-book condition consisted of reflected exposure to very dim light. However, a number of newer models of light-emitting devices are even brighter than those used in this study. Moreover, in this study, the LE-eBook reader was held at a fixed distance (30–45 cm) from the eye, further from the eye than many people might have chosen (therefore reducing retinal light exposure), particularly for users of smaller devices who may hold the smaller screens closer to the eyes. Lastly, although the short-wavelength light from the LE-eBook may have been responsible for the effects reported here, this study did not include a light-emitting device with longer wavelength for comparison, so our findings may be due to the difference in irradiance level rather than spectral composition.
This study demonstrates that use of a light-emitting electronic device in the hours before bedtime can have significant impact on sleep, alertness, and the circadian clock. The 10-min-shorter sleep latency after the print-book condition compared with sleep latency after the LE-eBook condition is similar to the effect size of eszopiclone treatment on sleep latency in patients with primary insomnia (37). Our findings provide evidence that the electric light to which we are exposed between dusk and bedtime has profound biological effects. Because technology use in the hours before bedtime is most prevalent in children and adolescents (36), physiological studies on the impact of such light exposure on both learning and development are needed. Further investigation of the physiological and medical effects of electronic devices is warranted, because the acute responses to the short-wavelength–enriched light emitted by them may have longer-term health consequences than previously considered.
Materials and Methods
Informed written consent was obtained from study participants before enrollment in the study. The protocol was approved by the Partners Human Research Committee, and all procedures were conducted according to the Declaration of Helsinki. Study participants were compensated for their participation.
Study Participants and Screening Procedures.
Twelve young healthy adults completed the 14-d in-patient study protocol (six females and six males; mean age ± SD: 24.92 ± 2.87). Potential participants were extensively screened for physical and psychological health, which included questionnaires, laboratory tests, physical examination, EKG, eye examination, and psychological interview to determine suitability for participation in the study. Participants with chronic medical or psychological conditions or sleep disorders and those taking prescription medications were excluded from study. History of night work or shift work in the prior 3 y and travel across more than one time zone in the previous 3 mo was also exclusionary. The presence of any eye or vision abnormality or the inability to read in dim light without the use of corrective lenses was exclusionary. Participants were instructed to refrain from use of medications, drugs, alcohol, nicotine, or caffeinated products for 3 wk before admission, which was verified by toxicological testing upon admission to the laboratory. Participants were also required to maintain a fixed 8-h sleep schedule (10:00 PM to 6:00 AM), to complete a daily sleep/wake log, and to call in their bedtimes and wake times every day during this 3-wk interval. This sleep schedule was verified by wrist actigraphy (Actiwatch-L; Philips/Respironics) during the week before admission.
Study Protocol.
Participants lived in a private room in the Intensive Physiological Monitoring Unit of the Center for Clinical Investigation of Brigham and Women’s Hospital during the 14-d protocol. They were scheduled to sleep on the identical fixed 8-h sleep schedule (10:00 PM to 6:00 AM) they maintained for 3 wk before admission. The randomized, crossover protocol design consisted of two conditions: (i) reading an LE-eBook (iPad; Apple Inc., Cupertino, CA) in otherwise very dim room light for ∼4 h before bedtime for five consecutive evenings, and (ii) reading printed books in the same very dim room light for ∼4 h before bedtime for five consecutive evenings (Fig. 1). Primary outcome measures included sleep latency, timing and amount of melatonin secretion, and self-reported ratings and EEG measures of sleepiness/alertness. On three occasions (days 1, 7, and 13) participants completed constant posture (CP) procedures for 4 h before and 4 h after the 8-h sleep episode.
Reading Sessions and Lighting Conditions.
A total of 10 reading sessions—5 in the LE-eBook condition and 5 in the printed book condition—were scheduled during the 14-d study. Participants were randomized to the order of reading condition. Reading sessions began at 6:00 PM and ended at 10:00 PM just before bedtime, with a 15-min “break” scheduled at 8:45–9:00 PM. For the first ∼3 h portion of the reading session (6:00–8:45 PM) participants read while seated in a fixed location in the room. During the break, they were allowed to stop reading and do other activities (walk around the room, prepare for bed, etc.) until 9:00 PM, when they resumed the reading session. For this last hour, participants read while seated in bed. During LE-eBook reading sessions, the light-emitting device was set to maximum brightness and placed in a stand that held it at a fixed angle. This stand was placed on a table directly in front of the individual at a 30- to 45-cm distance from their eyes. Participants were allowed to turn pages on the LE-eBook, but were asked not to hold it while reading or make any adjustments to the settings. During the printed book reading sessions, participants were allowed to hold the book at any desired distance from their eyes. Participants selected their own reading materials and supplied their own printed books. There were two requirements regarding reading material in either electronic or printed form: (i) it had to consist of printed text on the page (no pictures, illustrations, graphic novels, magazines, puzzles, etc.); and (ii) it had to be considered “pleasure” or “leisure” reading (no textbooks, reference books, or coursework). A technician was present for all reading sessions to coordinate and administer the reading session, ensure compliance of the participants, and collect and record the light measurements.
Light readings were recorded during all reading sessions at multiple times: at the beginning, at the end, and at 1 h intervals during the reading sessions. Illuminance was measured by using an IL1400 radiometer/powermeter with a SEL-033/Y/W detector (International Light, Inc., Peabody, MA) with the sensor placed next to the participant’s eye and pointed at the LE-eBook or printed book. For the LE-eBook reading condition, the distance between the participant and the LE-eBook in the stand was adjusted (e.g., moved closer or farther) if the light reading measured outside of the range of 30–50 photopic lux in the angle of gaze so that the light measurement was maintained within range.
Ambient room lighting during the study was from ceiling-mounted 4100K fluorescent lamps (Philips Lighting, Eindhoven, The Netherlands). During reading sessions, CP, and upon waking, the room light was ∼0.0048 W/m2 at 137 cm from the floor in the vertical plane with a maximum <0.025 W/m2 at 187 cm from the floor in the horizontal plane anywhere in the room. During the rest of the waking episodes, participants were in typical indoor room lighting of ∼0.23 W/m2 at 137 cm from the floor in the vertical plane, with a maximum of 0.48 W/m2 at 187 cm from the floor in the horizontal plane anywhere in the room. During all scheduled sleep episodes, participants were in darkness.
Radiometric light measurements of electronic devices were conducted in the same light conditions and at the same distance (30–45 cm) as during the reading sessions (described above). The irradiance output in the range of 380–780 nm at 4-nm intervals was converted to 1-nm intervals for calculation of the human retinal photopigment illuminance measures (cyanopic, melanopic, rhodopic, chloropic, and erythropic lux) (35).
CP Procedures.
CP procedures occurred on day 1 (baseline) and on days 7 and 13, after the five consecutive nights of each reading condition for the assessment of circadian phase of the melatonin rhythm. Participants remained in bed in a semirecumbent posture with minimal activity for 4 h before and 4 h after the 8-h sleep episode. Room temperature and dim light conditions remained constant during the CP; participants were in darkness fully recumbent during the sleep episode.
Plasma Melatonin.
Hourly blood samples were collected via an indwelling forearm IV catheter during portions of the protocol for measurement of melatonin levels. Samples were collected and then frozen (−80 °C) for subsequent assay. Plasma melatonin samples were assayed (SolidPhase Inc., Portland, ME) using the BÜHLMANN Melatonin Radio-immunoassay (ALPCO Diagnostics, Salem, NH), which has a functional sensitivity of 0.9 pg/mL and an analytical sensitivity of 0.3 pg/mL, an intraassay precision of 7.9–8.2%, and interassay precision of 11.7%.
Melatonin suppression was determined by using the AUC (by trapezoidal method) during the 4-h reading session on the fifth night of each reading condition and comparing it to the AUC during the corresponding 4-h time window during the CP 24 h following the reading session. Circadian phase of the dim light melatonin onset (DLMO) was calculated as the time at which levels of melatonin rose above 25% of the peak-to-trough amplitude of a three-harmonic waveform fitted to the 24-h melatonin data from the CP (38, 39). Phase shifts were calculated as the difference between the DLMO from the CP after the five-night reading condition and the DLMO from the CP before the reading condition (i.e., shift = DLMO from day 13 – DLMO from day 7).
Because of missing blood samples during the fifth night of one reading session, one study participant was excluded from analysis of melatonin suppression. Another participant was excluded from analysis of DLMO timing due to missing blood samples during one of the CPs. Therefore, melatonin suppression and phase were each determined in 11 participants.
Sleep and Wake Recordings.
PSG was recorded during the final two sleep episodes and for several hours before and after the sleep episode of each reading condition for a total of four PSG recordings per study participant. Surface electrodes were applied to specific locations on the face and scalp to record the EEG (F3/M2, F4/M1, C3/M2, C4/M1, O1/M2, O2/M1), and the left and right electrooculogram, and the submental electromyogram. The data were collected by using the Vitaport-3 system (TEMEC Instruments B.V., Kerkrade, The Netherlands). Signals were sampled at 256 Hz, low-pass filtered, and stored at 128 Hz.
For sleep recordings, data were scored in 30-s epochs according to standard criteria (40). Sleep measures included latency to sleep onset (time from lights off until the first occurrence of stage N2), TST, sleep efficiency (ratio of TST/the time spent in bed), wake after sleep onset, and time spent in each stage of sleep (N1, N2, N3, and REM). Wake recordings were scored in 30-s epochs to verify wakefulness and identify any unintentional episodes of sleep. Waking EEG recordings collected during the 3-min KDT were also quantified by spectral analysis. They were first inspected visually to identify and remove 2-s epochs contaminated by artifacts such as eye blinks and eye or body movements. The data were then subjected to a Fast Fourier Transform procedure, and power spectra were calculated for 2-s epochs over the frequency range of 0.5–20.0 Hz in 0.5-Hz bins.
Subjective and Objective Measures of Sleepiness/Alertness.
Subjective sleepiness was measured with a computer-administered KSS. The KSS is a 9-point Likert scale with all numbers having valid point values, but only the odd numbers have descriptions: 1 representing “extremely alert,” 3 representing “alert,” 5 representing “neither alert nor sleepy,” and 9 representing “extremely sleepy” (9, 41). Study participants completed the KSS each evening ∼1 h before bedtime and several times each morning: within 1–5 min after scheduled wake time and then every 4–10 min for 1 h after wake time. Participants typically completed the KSS in <30 s, and the computer screen was set to a dim light level of <8 lx (0.025 W/m2) from the participants’ eye in the angle of gaze.
Participants also completed the Karolinska Drowsiness Test (KDT; ref. 9) (3 min eyes open) during which they were instructed to relax, keep eyes open, and maintain a fixed gaze on a black dot in front of them while avoiding any movements or frequent blinking. The KDT allowed for waking EEG recording under standardized and reproducible conditions where artifacts from movement were minimized.
Statistical Analysis.
Statistical analyses were performed by using SAS (Version 9.2; SAS Institute, Cary, NC). We compared sleep and circadian measures between reading conditions using a mixed model analysis with factors Condition (LE-eBook or printed book), Order, and their Interaction (Condition X Order). Mixed model was also used for comparing KSS score and EEG power with factors Condition, Order, Time (repeated measures in the first hour after awakening), and the Interaction (Condition X Time). Post hoc paired Student t tests were used for comparisons between conditions for subjective and objective measures of sleepiness. P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank the study participants, the staff of the Brigham and Women’s Hospital Center for Clinical Investigation [part of the Harvard Catalyst Clinical and Translational Science Center (CTSC)], the staffs of the Division of Sleep Medicine Sleep and EEG Core and Chronobiology Core, Dayna Bradstreet for significant contributions to recruitment and conduct of the study, and Michael Herf and Robert Lucas, Ph.D. for assistance with the spectral light analysis. We also thank Wei Wang, Ph.D. for assistance with statistical analysis supported by the Harvard Catalyst CTSC [National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) Award UL1 RR025758 and financial contributions from the Brigham and Women’s Hospital (BWH), Harvard University, and its other affiliated academic health care centers]. This work was supported by NIH Grant R01HL077453. The inpatient studies were conducted in the Harvard CTSC, supported by NCRR Grant UL1 RR025758. A-M.C. was supported in part by NIH Grant K01HL115458. D.A. was supported in part by the German Aerospace Center. J.F.D. was supported in part by NIH Grant R01HL094654. C.A.C. was supported in part by NASA NNX10AF47G and the National Space Biomedical Research Institute through NASA NCC 9-58.
Footnotes
Conflict of interest statement: Dr. Czeisler has received consulting fees from or served as a paid member of scientific advisory boards for: Boston Celtics; Boston Red Sox; Citgo Inc.; Cleveland Browns; Merck; Novartis; Purdue Pharma LP; Quest Diagnostics, Inc.; Teva Pharmaceuticals Industries Ltd.; Valero Inc.; Vanda Pharmaceuticals, Inc. Dr. Czeisler currently owns an equity interest in Lifetrac, Inc.; Somnus Therapeutics, Inc.; Vanda Pharmaceuticals, Inc., and between October 2012 and October 2013, Apple, Inc. and Microsoft, Inc. Dr. Czeisler received royalties from McGraw Hill, Penguin Press/Houghton Mifflin Harcourt, and Philips Respironics, Inc. and has received grants and research support from Cephalon Inc., National Football League Charities, Philips Respironics, ResMed Foundation, San Francisco Bar Pilots and Sysco. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms, including matters involving Bombardier, Inc.; Delta Airlines; FedEx; Greyhound; Michael Jackson's mother and children; Purdue Pharma, L.P.; United Parcel Service and the United States of America.
This article is a PNAS Direct Submission.
See Commentary on page 946.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418490112/-/DCSupplemental.
References
- 1.Czeisler CA. Perspective: Casting light on sleep deficiency. Nature. 2013;497(7450):S13. doi: 10.1038/497S13a. [DOI] [PubMed] [Google Scholar]
- 2.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15(5 Pt 1):3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Human melatonin suppression by light is intensity dependent. J Pineal Res. 1989;6(2):149–156. doi: 10.1111/j.1600-079x.1989.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 5.Brainard GC, et al. Dose-response relationship between light irradiance and the suppression of plasma melatonin in human volunteers. Brain Res. 1988;454(1-2):212–218. doi: 10.1016/0006-8993(88)90820-7. [DOI] [PubMed] [Google Scholar]
- 6.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115(1):75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 8.Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11(6):453–464. doi: 10.1016/j.smrv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1–2):29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 10.Costa G, Haus E, Stevens R. Shift work and cancer - considerations on rationale, mechanisms, and epidemiology. Scand J Work Environ Health. 2010;36(2):163–179. doi: 10.5271/sjweh.2899. [DOI] [PubMed] [Google Scholar]
- 11.Straif K, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 12.Erren TC. Shift work, cancer and “white-box” epidemiology: Association and causation. Epidemiol Perspect Innov. 2010;7:11. doi: 10.1186/1742-5573-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dijk DJ. Why do we sleep so late? J Sleep Res. 2013;22(6):605–606. doi: 10.1111/jsr.12111. [DOI] [PubMed] [Google Scholar]
- 14.Perrin F, et al. Nonvisual responses to light exposure in the human brain during the circadian night. Curr Biol. 2004;14(20):1842–1846. doi: 10.1016/j.cub.2004.09.082. [DOI] [PubMed] [Google Scholar]
- 15.Figueiro MG, Bullough JD, Bierman A, Fay CR, Rea MS. On light as an alerting stimulus at night. Acta Neurobiol Exp (Wars) 2007;67(2):171–178. doi: 10.55782/ane-2007-1645. [DOI] [PubMed] [Google Scholar]
- 16.Revell VL, Barrett DC, Schlangen LJ, Skene DJ. Predicting human nocturnal nonvisual responses to monochromatic and polychromatic light with a melanopsin photosensitivity function. Chronobiol Int. 2010;27(9–10):1762–1777. doi: 10.3109/07420528.2010.516048. [DOI] [PubMed] [Google Scholar]
- 17.Chellappa SL, et al. Non-visual effects of light on melatonin, alertness and cognitive performance: Can blue-enriched light keep us alert? PLoS ONE. 2011;6(1):e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cajochen C, et al. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol (1985) 2011;110(5):1432–1438. doi: 10.1152/japplphysiol.00165.2011. [DOI] [PubMed] [Google Scholar]
- 19.Cajochen C, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90(3):1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 20.Dollins AB, Lynch HJ, Wurtman RJ, Deng MH, Lieberman HR. Effects of illumination on human nocturnal serum melatonin levels and performance. Physiol Behav. 1993;53(1):153–160. doi: 10.1016/0031-9384(93)90024-a. [DOI] [PubMed] [Google Scholar]
- 21.Chang AM, Scheer FA, Czeisler CA, Aeschbach D. Direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans depend on prior light history. Sleep. 2013;36(8):1239–1246. doi: 10.5665/sleep.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SM. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 2003;26(6):695–700. doi: 10.1093/sleep/26.6.695. [DOI] [PubMed] [Google Scholar]
- 23.Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: Comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1413–R1420. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- 24.Vandewalle G, et al. Daytime light exposure dynamically enhances brain responses. Curr Biol. 2006;16(16):1616–1621. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Czeisler CA, Zimmerman JC, Ronda JM, Moore-Ede MC, Weitzman ED. Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep. 1980;2(3):329–346. [PubMed] [Google Scholar]
- 26.Czeisler CA, Weitzman Ed, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: Its duration and organization depend on its circadian phase. Science. 1980;210(4475):1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 27.Weitzman ED, Czeisler CA, Zimmerman JC, Ronda JM. The timing of REM sleep and its relation to spontaneous awakening during temporal isolation in man. Sleep Res. 1980;9:280. [PubMed] [Google Scholar]
- 28.Wood B, Rea MS, Plitnick B, Figueiro MG. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl Ergon. 2013;44(2):237–240. doi: 10.1016/j.apergo.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Brainard GC, et al. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535(Pt 1):261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88(9):4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 32.Lockley SW, et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29(2):161–168. [PubMed] [Google Scholar]
- 33.Münch M, et al. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1421–R1428. doi: 10.1152/ajpregu.00478.2005. [DOI] [PubMed] [Google Scholar]
- 34.Santhi N, et al. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 2012;53(1):47–59. doi: 10.1111/j.1600-079X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 35.Lucas RJ, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37(1):1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gradisar M, et al. The sleep and technology use of Americans: Findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9(12):1291–1299. doi: 10.5664/jcsm.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krystal AD, et al. Eszopiclone treatment for insomnia: Effect size comparisons in patients with primary insomnia and insomnia with medical and psychiatric comorbidity. Prim Care Companion CNS Disord. 2012;14(4) doi: 10.4088/PCC.11m01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int. 2001;18(5):801–808. doi: 10.1081/cbi-100107515. [DOI] [PubMed] [Google Scholar]
- 39.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17(2):181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 40.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- 41.Gillberg M, Kecklund G, Åkerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17(3):236–241. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.