Significance
Plasmodium falciparum reticulocyte binding-like homologous protein 5 (PfRH5) is a leading blood-stage malaria vaccine candidate that elicits potent strain-transcending invasion inhibitory antibodies. However, it lacks both transmembrane domains and a GPI-anchor and is thus anchored to the merozoite surface through an unknown mechanism. We have demonstrated that PfRH5 and its known partner, PfRH5-interacting protein (PfRipr), associates with a conserved GPI-anchored protein, Cysteine-rich protective antigen (CyRPA), to form a complex on the merozoite surface. CyRPA was shown to be GPI-linked, refractory to knockout, and like PfRH5, elicited potent strain-transcending invasion inhibitory antibodies. This discovery elucidates the formation of a previously unidentified PfRH5/PfRipr/CyRPA protein complex on the merozoite surface, which facilitates the PfRH5–Basigin interaction and offers another highly conserved, potent target (CyRPA) for novel antimalarial strategies that could abrogate formation of this crucial complex.
Keywords: malaria, erythrocyte invasion, protein–protein interactions, blood-stage vaccines, PfRH5
Abstract
Erythrocyte invasion by Plasmodium falciparum merozoites is a highly intricate process in which Plasmodium falciparum reticulocyte binding-like homologous protein 5 (PfRH5) is an indispensable parasite ligand that binds with its erythrocyte receptor, Basigin. PfRH5 is a leading blood-stage vaccine candidate because it exhibits limited polymorphisms and elicits potent strain-transcending parasite neutralizing antibodies. However, the mechanism by which it is anchored to the merozoite surface remains unknown because both PfRH5 and the PfRH5-interacting protein (PfRipr) lack transmembrane domains and GPI anchors. Here we have identified a conserved GPI-linked parasite protein, Cysteine-rich protective antigen (CyRPA) as an interacting partner of PfRH5-PfRipr that tethers the PfRH5/PfRipr/CyRPA multiprotein complex on the merozoite surface. CyRPA was demonstrated to be GPI-linked, localized in the micronemes, and essential for erythrocyte invasion. Specific antibodies against the three proteins successfully detected the intact complex in the parasite and coimmunoprecipitated the three interacting partners. Importantly, full-length CyRPA antibodies displayed potent strain-transcending invasion inhibition, as observed for PfRH5. CyRPA does not bind with erythrocytes, suggesting that its parasite neutralizing antibodies likely block its critical interaction with PfRH5-PfRipr, leading to a blockade of erythrocyte invasion. Further, CyRPA and PfRH5 antibody combinations produced synergistic invasion inhibition, suggesting that simultaneous blockade of the PfRH5–Basigin and PfRH5/PfRipr/CyRPA interactions produced an enhanced inhibitory effect. Our discovery of the critical interactions between PfRH5, PfRipr, and the GPI-anchored CyRPA clearly defines the components of the essential PfRH5 adhesion complex for P. falciparum erythrocyte invasion and offers it as a previously unidentified potent target for antimalarial strategies that could abrogate formation of the crucial multiprotein complex.
Erythrocyte invasion by Plasmodium falciparum merozoites is crucial for malaria pathogenesis, and thus the parasite has evolved an extensive molecular machinery to ensure invasion through multiple pathways (1–3). The quest to develop successful blood-stage malaria vaccines that efficiently block this process have focused on essential parasite proteins like merozoite surface protein 1 (MSP-1) and apical membrane antigen 1 (AMA-1); however, these are highly polymorphic, unable to elicit strain-transcending neutralizing antibodies, and have thus failed in field trials (4). Among the large repertoire of invasion-related proteins, the family of P. falciparum reticulocyte binding-like homologous (PfRH) proteins have emerged as key determinants of different invasion pathways (2, 3), of which PfRH5 is the only essential conserved parasite ligand (5–8) that elicits potent strain-transcending neutralizing antibodies (9–12). It is localized in the rhoptry and secreted to the merozoite surface during erythrocyte invasion (6). It does not seem to be under immune pressure (9, 13) and is favored to be a leading vaccine candidate. PfRH5 has been shown to interact with another parasite protein, PfRipr (P. falciparum RH5 interacting protein) (14). However, both these proteins lack transmembrane domains as well as a GPI anchor, and thus the mechanism through which PfRH5 is secured on the surface of an invading merozoite to facilitate its functional role during invasion still remains unknown. It is likely that PfRH5 might be attached to the merozoite surface as a complex with other essential proteins other than PfRipr, identification of which could open new therapeutic avenues against malaria.
Here we show that PfRH5 and PfRipr interact with a GPI-linked parasite protein, CyRPA (Cysteine-rich protective antigen) (15) to form an essential complex on the surface of an invading merozoite. Individual antibodies against each of the three proteins successfully coimmunoprecipitated all three proteins, confirming their presence as a multiprotein complex. Analysis of the native parasite protein complex by different chromatographic techniques further confirmed that all three protein components coeluted together and were present as a much higher molecular mass species than their individual molecular masses. We also demonstrated that the three proteins are colocalized on the apical surface of the invading merozoite, of which only CyRPA was shown to be GPI-linked. Importantly, antibodies against full-length CyRPA potently blocked erythrocyte invasion by multiple P. falciparum strains, as observed previously only for PfRH5 antibodies (9–12). Because CyRPA does not bind with the erythrocyte surface, it seems that the parasite-neutralizing CyRPA antibodies function by impeding its interaction with PfRH5 or PfRipr. Hence, we have identified and validated a GPI-linked parasite protein, CyRPA, as another essential interacting partner of PfRH5 that is responsible for tethering it to the merozoite surface. Further, we have shown that like PfRH5, CyRPA is a conserved target of potent antibody-mediated blockade of erythrocyte invasion and thus seems to be another highly promising blood-stage vaccine candidate.
Results
CyRPA Interacts with PfRH5 and PfRipr to Form a High Molecular Weight Multiprotein Complex.
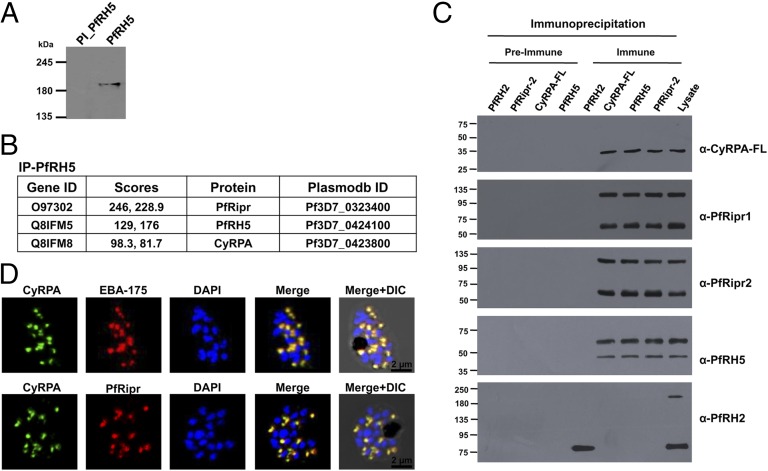
Consistent with a previous report (14), blue native gel electrophoresis (BN-PAGE) of the parasite lysate detected the 63-kDa full-length PfRH5 protein as a ∼200 kDa molecular mass species (Fig. 1A). PfRipr is 123 kDa, and considering these two secreted proteins without their signal peptides, the ∼200-kDa complex would comprise of an unknown protein component(s) of ∼30–40 kDa.
Fig. 1.
PfRH5 interacts with CyRPA and PfRipr to form a higher-order complex. (A) BN-PAGE showed PfRH5 migrating as a 200-kDa higher molecular mass species. PI, preimmune antibodies. (B) Mass spectrometric analysis of proteins coimmunoprecipitated by anti-PfRH5 antibodies to identify unique PfRH5 interacting partners consistently detected only PfRH5, PfRipr, and CyRPA, with significant high scores in two independent experiments. (C) Validation of PfRH5/PfRipr/CyRPA interaction by immunoblotting analysis of the coimmunoprecipitated proteins using their respective immune and preimmune antibodies. PfRH5, PfRipr, and CyRPA-FL antibodies only immunoprecipitated the PfRH5/PfRipr/CyRPA complex. The corresponding preimmune and another negative control PfRH2-Pro1 antibody did not immunoprecipitate the complex. PfRH2-Pro1 (amino acids 76–494) antibodies detected the processed forms (220 kDa; 80 kDa) of the native 360-kDa PfRH2 in the parasite lysate (16) but immunoprecipitated only the 80-kDa fragment (14). (D) Immunofluorescence microscopy detected CyRPA to colocalize with micronemal proteins (EBA-175, PfRipr). (Scale bar, 2 µm.)
To identify the unknown component(s) of the 200 kDa complex, we performed coimmunoprecipitation with schizont-stage lysates using functional PfRH5 antibodies described recently (12). Mass spectrometric analysis of the proteins coimmunoprecipitated by the PfRH5 immune antibodies in comparison with those immunoprecipitated by the preimmune control antibodies identified several parasite proteins, of which only three—PfRH5 itself, PfRipr, and a previously unidentified interacting parasite protein, CyRPA (15) (PF3D7_0423800)—were consistently detected with significant high scores between independent experiments (Fig. 1B and Dataset S1). The rest of the proteins had very low scores and were not reproducibly detected between independent experiments, suggesting that they were background and not significant (Dataset S1). These data suggested that PfRH5, PfRipr, and CyRPA form a multiprotein complex in the parasite.
Further to confirm this finding, we produced several recombinant protein constructs (full-length and/or partial) of CyRPA (Fig. S1A) and PfRipr (Fig. S1B), whose antibodies specifically detected the corresponding native proteins in the parasite lysate (Fig. S2A). As observed for PfRH5, antibodies raised against full-length CyRPA (CyRPA-FL) and PfRipr-2 in comparison with their respective preimmune control antibodies specifically coimmunoprecipitated all three components of the PfRH5/PfRipr/CyRPA complex, which were all detected with high scores by mass spectrometry (Fig. S2B and Dataset S1). However, antibodies against the partial constructs of CyRPA (CyRPA-1, CyRPA-2) and PfRipr (Ripr-1) failed to immunoprecipitate even their respective native parasite proteins. The coimmunoprecipitated proteins were also detected by immunoblotting using the respective antibodies (Fig. 1C). Antibodies against another PfRH homologous member, PfRH2 (16), did not coimmunoprecipitate any of the three PfRH5/PfRipr/CyRPA interacting proteins (Fig. 1C), underscoring the specificity of the PfRH5 antibodies and the interactions between the three components of the PfRH5/PfRipr/CyRPA complex.
Analysis of the Native PfRH5/PfRipr/CyRPA Parasite Protein Complex.
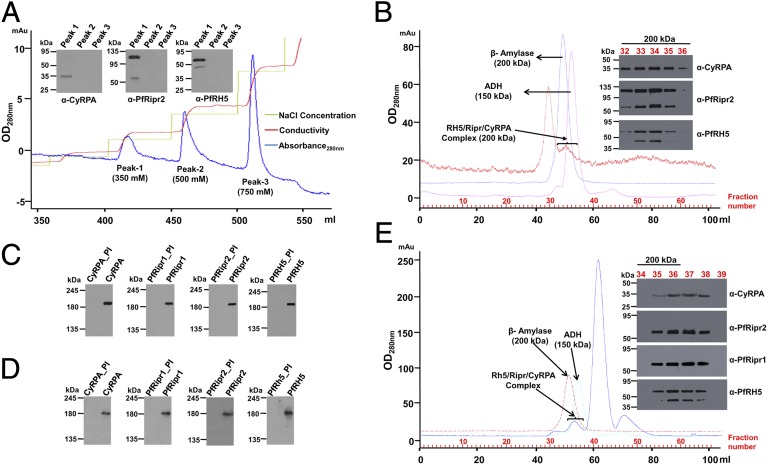
We analyzed the PfRH5/PfRipr/CyRPA complex from parasite lysate using anion-exchange chromatography. The predicted pIs of CyRPA, PfRipr, and PfRH5 are 5.31, 6.74, and 8.81, respectively. Elution with an NaCl gradient showed three distinct peaks, of which the three proteins were detected only in peak 1 (350 mM NaCl) (Fig. 2A). Because PfRH5, with a predicted pI of 8.81, is not likely to bind an anion-exchange resin, its coelution with CyRPA and PfRipr further supports that the three proteins exist as a complex. The peak 1 protein fraction was further analyzed by size exclusion chromatography (SEC) and BN-PAGE (Fig. 2 B and C). SEC confirmed that the three proteins eluted in fractions corresponding to a molecular mass of 200 kDa (Fig. 2B). Detection of PfRH5, PfRipr, and CyRPA at the same position on BN-PAGE (Fig. 2C) and SEC further supports the existence of an intact PfRH5/PfRipr/CyRPA complex. Merozoite surface proteins involved in erythrocyte invasion are shed in the culture medium (culture supernatant) during the invasion process. SEC and BN-PAGE analysis of the culture supernatant indicated that an intact PfRH5/PfRipr/CyRPA complex exists even in culture supernatants (Fig. 2 D and E). Whereas PfRH5 exhibits erythrocyte binding activity, we were unable to detect any such activity for either PfRipr or CyRPA (Fig. S3A), suggesting that although the PfRH5/PfRipr/CyRPA complex remains intact in the culture supernatant, it likely disintegrates upon PfRH5 binding with Basigin. Our data are consistent with the previous report for PfRipr, which identified it as an interacting partner of PfRH5 but with no erythrocyte binding activity (14).
Fig. 2.
Native PfRH5, PfRipr, and CyRPA interact to form a 200-kDa multiprotein complex in the parasite. (A) Schizont lysate analyzed by anion-exchange chromatography detected PfRH5, PfRipr, and CyRPA to coelute at 350 mM NaCl (peak 1). (B) SEC-200 analysis of peak 1 proteins detected PfRH5, PfRipr, and CyRPA in fractions (32–36) corresponding to 200 kDa. BN-PAGE of SEC fraction 34 (C) and culture supernatant (D) confirmed that PfRH5, PfRipr, and CyRPA coexisted as an intact 200-kDa native protein complex. *PI represents the corresponding preimmune sera. (E) SEC-200 analysis of culture supernatant also detected PfRH5, PfRipr, and CyRPA in fractions (35–38) corresponding to ∼200 kDa. β-Amylase and alcohol dehydrogenase (ADH) are SEC molecular weight standards.
CyRPA Is Present at the Apical Surface of an Invading Merozoite.
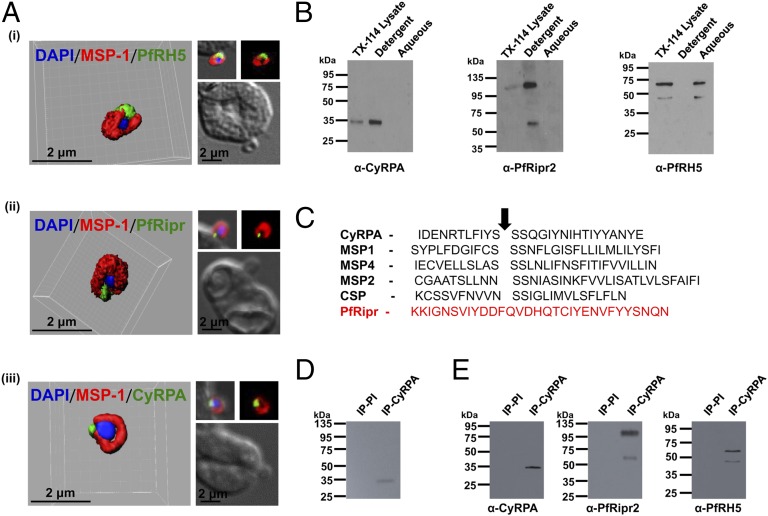
Confocal immunofluorescence microscopy using known micronemal proteins (EBA-175, PfRipr) localized CyRPA to the micronemes of schizont-stage parasites (Fig. 1D and Fig. S3B). No colocalization was observed with the rhoptry proteins PfRH2 and PfRH5 (Fig. S2C). Immunofluorescence staining of invading merozoites that were fixed using the nonpermeable fixative paraformaldehyde/glutaraldehyde showed that the three proteins were apically colocalized on the merozoite surface, which was stained by MSP-119 antibodies (Fig. 3A and Fig. S3C). As a control, a well-described cytosolic parasite protein, NapL (nucleosome assembly protein L) (17), was not detected under the same nonpermeable fixing conditions (Fig. S3D) and could only be visualized when the parasites were permeabilized using Triton X-100 (Fig. S3E), consistent with a previous report (18). Thus, the three proteins are secreted from their respective organelles to the surface of the invading merozoite, where they form the PfRH5/PfRipr/CyRPA complex.
Fig. 3.
PfRH5/PfRipr/CyRPA complex is GPI-anchored to the merozoite surface. (A) 3D reconstruction of IFA z-stacks during merozoite invasion, coimmunostained with MSP-1 detected PfRH5, PfRipr, and CyRPA at the apical end of the merozoite surface. In the 3D images, the γ settings were altered for visual representation only. (B) Triton X-114 separates PfRipr and CyRPA (detergent fraction) from PfRH5 (aqueous fraction). (C) CyRPA has the ω-site (SSS); weak hydrophobic tail as observed in known GPI-anchored parasite proteins, which PfRipr lacks. Arrow denotes the predicted GPI:protein-transamidase cleavage site, where GPI is attached. (D) Immunoprecipitation of tritium-radiolabeled CyRPA from lysates of schizont-stage parasites grown in the presence of tritium labeled GPI anchor precursors d-[6-3H(N)]glucosamine hydrochloride and d-[2-3H]mannose confirmed the presence of a GPI anchor. (E) Immunoblot analysis of anti-CyRPA coimmunoprecipitated proteins (same radiolabeled sample) confirmed the presence of PfRH5/PfRipr/CyRPA.
CyRPA Is Tethered to the Merozoite Surface by a GPI Moiety.
Because the three proteins lack transmembrane domains, the mechanism by which this complex is tethered to the surface had remained unknown until now. Triton X-114 phase partitioning (19, 20) of the schizont-stage lysate detected CyRPA and PfRipr only in the detergent-resistant fraction (Fig. 3B), suggesting that they are membrane bound, whereas PfRH5 being in the aqueous fraction was not directly membrane-associated. CyRPA possesses characteristic features of GPI-anchored proteins—a weak hydrophobic tail and an MSP-1 like ω-site (21, 22) (Fig. 3C), which is absent in PfRH5 and PfRipr. Antibodies against the full-length CyRPA immunoprecipitated radio-labeled native CyRPA from parasites grown in the presence of GPI anchor precursors d-[6-3H(N)]glucosamine hydrochloride and d-[2-3H]mannose, which are known to be incorporated in the glycan core of the GPI moiety (22, 23) (Fig. 3D), thus validating CyRPA to be a GPI-anchored protein. The radiolabeled signal was specifically observed only for native CyRPA (35 kDa), with no signal observed at the molecular sizes corresponding to either PfRH5 (63 kDa) or PfRipr (123 kDa or 60 kDa), although these unlabeled proteins were coimmunoprecipitated by the CyRPA antibodies (Fig. 3E). Thus, we have demonstrated CyRPA to be the only GPI-anchored protein from the PfRH5/PfRipr/CyRPA multiprotein complex that directly associates with the merozoite surface.
CyRPA Is an Essential Parasite Protein.
Using genetic manipulation, PfRH5 and PfRipr have been previously reported to be essential for erythrocyte invasion (6, 14). Similarly, our repeated attempts to disrupt the CyRPA gene failed, suggesting that CyRPA is also indispensable (Fig. S4A). This is consistent with its function of tethering the two essential parasite proteins to the merozoite surface, which are also refractory to genetic disruption. In addition, it has been previously reported that most genes encoding GPI-anchored merozoite proteins with the exception of MSP-5 are refractory to genetic deletion (24), and thus on similar lines, we have also not been able to knock out the CyRPA gene.
PfRH proteins are known to be differentially expressed among different P. falciparum strains (2, 3). However, coherent with their critical function, the three proteins, particularly PfRH5, were found to be consistently expressed in five different parasite strains that exhibit phenotypic variation in their erythrocyte invasion properties (Fig. S4B).
Antibodies Against CyRPA Display Potent, Strain-Transcending Invasion Inhibition Against Multiple P. falciparum Strains.
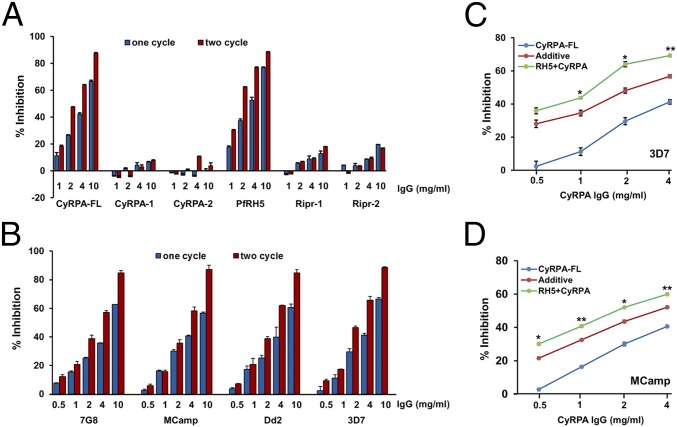
As demonstrated previously for PfRH5 (12), antibodies against CyRPA-FL exhibited highly potent invasion inhibition, with an EC50 of ∼3 mg/mL (total IgG) (Fig. 4 A and B). CyRPA-FL antibodies exhibited potent strain-transcending invasion inhibition by blocking invasion (∼85%, 10 mg/mL total IgG) of four P. falciparum strains known to have distinct invasion phenotypes. Importantly, only CyRPA-FL antibodies were able to successfully immunoprecipitate all three proteins of the native PfRH5/PfRipr/CyRPA complex. CyRPA-1/-2 antibodies that failed to immunoprecipitate the complex did not show any invasion inhibitory activity (Fig. 4A). Because CyRPA does not bind with the erythrocyte surface it seems that anti-full-length CyRPA antibodies abrogate the interaction of CyRPA with either PfRH5 or PfRipr or both. In comparison, however, Ripr-2 antibodies that immunoprecipitated the complex did not show any invasion inhibitory activity (Fig. 4A). This could be because these antibodies were raised only against a small region of PfRipr that may not represent the entire functional region that mediates complex formation. CyRPA-FL antibodies in combination with anti-PfRH5 produced synergistic invasion inhibition against both a sialic acid independent strain, 3D7 (Fig. 4C), and a sialic acid dependent strain, MCamp (Fig. 4D), as validated by the phenomenon of Bliss-additivity reported previously (25). The synergistic inhibition produced by the combination of CyRPA and PfRH5 antibodies suggests that blocking two essential molecular interactions (PfRH5-BSG; PfRH5/PfRipr/CyRPA complex formation) in combination neutralized parasite invasion significantly more than when targeting only a single interaction. As expected, combining anti-Ripr-1/-2 with anti-PfRH5 antibodies did not show any significant additive or synergistic invasion inhibition (Fig. S4C). On lines similar to that of PfRH5, our data have identified CyRPA to be a potent target of antibody-mediated blockade of erythrocyte invasion by multiple P. falciparum strains.
Fig. 4.
Invasion inhibitory efficacy of the CyRPA and PfRipr-1/-2 antibodies. (A) Total rat IgGs containing antibodies against the corresponding recombinant proteins were evaluated for their invasion inhibitory activity in one-cycle (40 h after invasion) and two-cycle (90 h after invasion) assays against the P. falciparum strain 3D7. Full-length CyRPA-FL IgGs exhibited potent dose dependent invasion inhibition. (B) Anti-CyRPA antibodies exhibited strain-transcending invasion inhibition. (C) CyRPA antibodies in presence of a fixed concentration of PfRH5 (1 mg/mL) antibodies exhibited synergistic inhibition in one cycle (40 h after invasion) against the sialic acid independent strain, 3D7, (D) as well as a sialic acid dependent strain, MCamp. Blue curve represents the invasion inhibitory activity of the individual CyRPA-FL antibodies. Green curve represents the invasion inhibition observed with varying concentration of CyRPA-FL antibodies in presence of a fixed concentration of anti-PfRH5 IgG (1 mg/mL). Red curve represents the theoretical invasion inhibitory activity predicted by Bliss additivity. Anti-PfRH5 IgGs used at 1 mg/mL gave an invasion inhibition of 26.27(±2.17) and 19.4 (±2.4) for 3D7 and MCamp, respectively. The error bars represent the SEM of three independent experiments performed in duplicate. *P ≤ 0.05; **P ≤ 0.01.
Discussion
PfRH5 has recently attracted immense attention in the malaria field (5–12), particularly with the discovery of Basigin as its erythrocyte receptor and the fact that the PfRH5–Basigin interaction was found to be essential for erythrocyte invasion by multiple P. falciparum strains (7). Several groups further demonstrated that specific antibodies raised against recombinant forms of PfRH5 potently blocked erythrocyte invasion by multiple worldwide P. falciparum strains that were known to exhibit diverse invasion phenotypes (9–12). This definitely was a great advancement, because previously such potent strain-transcending inhibition against multiple P. falciparum strains could not be demonstrated with leading essential blood-stage vaccine candidates (AMA-1, MSP-1). PfRH5 was also shown to be a major determinant of host cell tropism, which was first reported for P. falciparum invasion of Aotus nancymaae erythrocytes (5) and later for other species (8, 26). Thus, PfRH5 emerged as an essential erythrocyte-binding protein that played a crucial role in erythrocyte invasion.
However, a curious aspect of PfRH5 was that the native parasite protein lacked both a transmembrane domain and a GPI anchor signal, and thus it was not clearly understood how this leading blood-stage vaccine candidate was secured onto the surface of the invading merozoite such that it could perform its functional role of binding with Basigin and mediating erythrocyte invasion. Recently, it was reported that PfRH5 interacts with PfRipr that was localized to the micronemes (14). PfRipr is a 123-kDa protein with 10 EGF-like domains. However, PfRipr also does not contain a transmembrane or GPI-anchor signal sequence, and hence it has remained a puzzle until now as to how PfRH5 is attached on the invading merozoite surface.
We have recently raised specific antibodies against full-length recombinant PfRH5 generated in Escherichia coli that exhibited potent invasion inhibition (12), similar to that observed with PfRH5 antibodies raised against either the adenoviral vector (9) or mammalian PfRH5-CD4 fusion protein (11). Using these specific antibodies we performed coimmunoprecipitation experiments in which the PfRH5 antibodies, in comparison with its preimmune control antibodies, pulled down PfRipr as well as another previously unidentified interacting partner, CyRPA (15). CyRPA is a ∼35-kDa protein and thus we have identified the third component of the 200-kDa high molecular weight complex that comprised PfRH5 and PfRipr.
The formation of this three protein complex has been confirmed by several approaches. First, individual antibodies against each of the three proteins specifically coimmunoprecipitated only the three native parasite proteins (PfRH5, PfRipr, CyRPA). The coimmunoprecipitated proteins were confirmed by both mass spectrometry (LC-MS) as well as immunoblotting using the respective specific antibodies. None of these three proteins were coimmunoprecipitated by antibodies against another PfRH homolog protein, PfRH2, clearly corroborating that the three proteins were specifically interacting with each other to form a high-ordered complex. This was further confirmed by ion-exchange chromatography, whereby all three proteins were found to coelute with each other despite the fact that the PfRH5 protein, with a pI of 8.81, would not be expected to bind with an anion exchange resin. SEC (gel filtration) and native gel electrophoresis of the parasite proteins from both the lysate and culture supernatant showed that the three proteins existed as a ∼200-kDa species, which was higher than their individual molecular masses and undoubtedly confirmed that the three proteins were part of a higher-order multiprotein complex.
Consistent with the expression of PfRH5 on the merozoite surface and its binding with Basigin, all of the three proteins were localized on the apical surface of the invading merozoite. These imaging studies were performed using nonpermeable fixatives, thus confirming expression of the PfRH5/PfRipr/CyRPA complex on the surface of the invading merozoite. PfRH5 is localized in the rhoptry bulb, whereas PfRipr has been reported to be in the micronemes. CyRPA was also found to be localized in the micronemes, and thus all three proteins are secreted from their respective apical organelles to the merozoite surface, where they constitute the 200-kDa GPI-anchored multiprotein complex.
Importantly, we have experimentally demonstrated and validated the native CyRPA parasite protein to be GPI anchored by using radiolabeled precursors of the GPI moiety, as shown previously for other GPI-anchored Plasmodium proteins (22, 23). The resultant radioactive signal got incorporated only in CyRPA and not in PfRipr or PfRH5, confirming the presence of a GPI anchor in the native CyRPA parasite protein. Thus, the 200-kDa PfRH5/PfRipr/CyRPA protein complex is tethered to the merozoite surface through the GPI anchor of the CyRPA protein. We have therefore clearly elucidated the mechanism as to how PfRH5 is secured to the merozoite surface such that it is able to function and mediate erythrocyte invasion.
CyRPA is highly conserved (single polymorphism among 18 P. falciparum strains) (15) and like PfRH5 is not under immune pressure (9, 13). The CyRPA gene could not be genetically knocked out, and its indispensible nature suggests that its interaction with PfRH5 and PfRipr is imperative for erythrocyte invasion. CyRPA-FL antibodies potently blocked erythrocyte invasion of multiple P. falciparum strains, which is a characteristic that has been shown previously only for PfRH5 antibodies (9–12). Thus, like PfRH5, CyRPA poses to be a leading blood-stage vaccine candidate that elicits potent strain-transcending parasite-neutralizing antibodies. To the best of our knowledge, this is the only report apart from PfRH5 in which antibodies against an individual antigen are able to inhibit multiple P. falciparum strains with great efficacy, and hence our study has major implications on current efforts to develop a blood-stage malaria vaccine.
Our data substantiate a previous study that found CyRPA monoclonal antibodies raised against a cell surface expressed recombinant protein to inhibit in vivo P. falciparum growth in a passive immunoprotection animal model (15). Our work has proved CyRPA to be GPI anchored and has identified its physiological role to secure PfRH5, a key parasite ligand that lacks any membrane anchorage moiety, to the merozoite surface. Further, we have revealed the mechanism of action of the CyRPA invasion inhibitory antibodies. Unlike PfRH5, CyRPA does not exhibit erythrocyte binding activity, and thus its antibodies cannot impede the ligand–receptor interaction that mediates erythrocyte attachment. We have raised a panel of CyRPA antibodies against both the full-length protein (CyRPA-FL) as well as partial constructs (CyRPA-1/-2). Among these only the CyRPA-FL antibodies immunoprecipitated the PfRH5/PfRipr/CyRPA complex and exhibited potent strain-transcending invasion inhibition against multiple P. falciparum strains. On the other hand, antibodies raised against the CyRPA-1/-2 partial constructs failed to immunoprecipitate the complex and also exhibited poor invasion inhibitory activity. These data clearly imply that only the antibodies that could recognize the native conformation of the CyRPA parasite protein that initially allowed them to successfully pull down the complex in the first place were successful in inhibiting erythrocyte invasion by abrogating the formation of the PfRH5/PfRipr/CyRPA complex in the invading merozoite. The CyRPA antibodies were able to enhance the invasion inhibitory potential of PfRH5 antibodies because a significant synergistic inhibitory effect was produced by the combination of CyRPA and PfRH5 antibodies, which could be attributed to the blockade of two essential molecular interactions (PfRH5-BSG; PfRH5/PfRipr/CyRPA complex formation) simultaneously.
In summary, we have identified CyRPA as the third critical interacting partner of PfRH5 and PfRipr that tethers the complex to the merozoite surface through a GPI anchor. On the basis of this physiological role in the parasite, we believe that CyRPA should be termed as RRMAP (RH5-Ripr membrane anchoring protein), which is more reflective of its biological function. Both CyRPA and PfRipr have no direct role in erythrocyte attachment but secure PfRH5 by formation of a stable protein complex on the merozoite surface, facilitating its critical interaction with Basigin. We have thus identified a crucial protein–protein interaction among CyRPA, PfRH5, and PfRipr for erythrocyte invasion, which when targeted produced potent invasion inhibition among multiple P. falciparum strains. Currently, targeting protein–protein interactions for development of therapeutics is gaining significance (27, 28). Similar to the AMA1-RON2 studies (29, 30), structural analysis of the PfRH5/PfRipr/CyRPA complex may identify small molecules that could impede complex formation. Recently, immunization of the AMA1-RON2 peptide complex, but not AMA1 alone, elicited complete protection against Plasmodium yoelii challenge in mice, suggesting that administration of vaccine antigens as a complex may prove more efficacious than immunizing individual antigens or even their mixtures (31). In light of this report, our discovery of the PfRH5/PfRipr/CyRPA complex has major implications for the development of novel blood-stage malaria vaccines. The sole dependence across multiple P. falciparum strains on the formation of this essential protein complex for erythrocyte invasion could therefore be harnessed for development of novel therapeutics against malaria.
Experimental Procedures
Ethics Statement.
The animal studies described below were approved by the International Centre for Genetic Engineering and Biotechnology (ICGEB) Institutional Animal Ethics Committee (IAEC; reference no. MAL-51), constituted as per the guidelines of the Department of Biotechnology, Government of India.
Coimmunoprecipitation and Mass Spectrometric Analysis.
Immunoprecipitation of schizont lysates was performed with both immune and preimmune control antibodies (32) as prescribed by the Pierce Crosslink IP-kit (Thermo Scientific). Before immunoprecipitation the lysates were precleared using the control Protein G Sepharose beads. The coimmunoprecipitated samples were digested by trypsin and further analyzed by LC-MS. The proteins were identified by blasting the peptides over a P. falciparum database (Uniprot), using Proteome Discoverer (Thermo Scientific). The proteins immunoprecipitated by the immune antibodies were compared with those immunoprecipitated by the preimmune control antibodies to identify the unique interacting partners (32).
Recombinant Protein Production.
Three CyRPA constructs (CyRPA-1: 29–208 amino acids; CyRPA-2: 209–362 amino acids; CyRPA-FL: 29–362 amino acids) and two PfRipr constructs corresponding to EGF-like domains present at both amino (Ripr-1) and carboxyl-terminus (Ripr-2) were amplified from either genomic DNA (3D7) or cDNA prepared from 3D7 total RNA, and further cloned in the pET-24b expression vector (Novagen). All constructs were expressed in BL21 (DE3) E. coli cells. The recombinant proteins were purified to homogeneity as described in SI Experimental Procedures. The identities of the recombinant proteins were confirmed by LC-MS.
Analysis of the PfRH5/PfRipr/CyRPA Native Parasite Protein Complex.
The lysate obtained from schizonts was buffer exchanged in TBS (pH 7.5) and then loaded onto a Q-Sepharose ion-exchange column (GE Healthcare). Elution was done using a stepwise gradient of NaCl. The three peaks obtained at 350, 500, and 750 mM of NaCl were analyzed by immunoblotting. The peak 1 fraction containing the PfRH5, PfRipr, and CyRPA proteins was loaded onto a SEC-200 column (SEC, GE Healthcare). SEC-200 analysis of culture supernatant was done similarly.
P. falciparum culture (33), BN-PAGE (34), erythrocyte binding assay (35), isolation of viable merozoites (18), immunofluorescence microscopy (36), tritium labeling (22, 23), animal immunization, and invasion inhibition assay (37) experiments were done as described previously.
For detailed methods please refer to SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Dr. Louis Miller (NIH) for providing the P. falciparum clones; Dr. Amit Sharma [International Centre for Genetic Engineering and Biotechnology (ICGEB)] and Dr. Asif Mohmmed (ICGEB) for providing the NapL antibody and pCC1 vector, respectively; Dr. Alka Galav, Rakesh Kumar Singh, and Ashok Das from the ICGEB animal facility for their assistance; Dr. Inderjeet Kaur at the mass spectrometry facility of the Malaria group, ICGEB, for helping with the LC-MS study; and Ms. Surbhi Dabral at the malaria microscopy facility for technical help in the imaging study. D.G. is the recipient of the Ramalingaswami Fellowship from the Department of Biotechnology (DBT), Government of India. This work was supported by the following grants to D.G. from the Bill & Melinda Gates Foundation (Grand Challenges Explorations): DBT (Rapid Grants for Young Investigators and Program Support Grant). K.S.R. is the recipient of the Senior Research Fellowship of the Council of Scientific and Industrial Research, Government of India; E.A. is the recipient of the ICGEB International Ph.D. predoctoral fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415466112/-/DCSupplemental.
References
- 1.Gaur D, Mayer DCG, Miller LH. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int J Parasitol. 2004;34(13-14):1413–1429. doi: 10.1016/j.ijpara.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Gaur D, Chitnis CE. Molecular interactions and signaling mechanisms during erythrocyte invasion by malaria parasites. Curr Opin Microbiol. 2011;14(4):422–428. doi: 10.1016/j.mib.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Cowman AF, Berry D, Baum J. The cellular and molecular basis for malaria parasite invasion of the human red blood cell. J Cell Biol. 2012;198(6):961–971. doi: 10.1083/jcb.201206112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010;120(12):4168–4178. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayton K, et al. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe. 2008;4(1):40–51. doi: 10.1016/j.chom.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum J, et al. Reticulocyte-binding protein homologue 5—an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int J Parasitol. 2009;39(3):371–380. doi: 10.1016/j.ijpara.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Crosnier C, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480(7378):534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanaguru M, Liu W, Hahn BH, Rayner JC, Wright GJ. RH5-Basigin interaction plays a major role in the host tropism of Plasmodium falciparum. Proc Natl Acad Sci USA. 2013;110(51):20735–20740. doi: 10.1073/pnas.1320771110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas AD, et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun. 2011;2:601. doi: 10.1038/ncomms1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas AD, et al. Neutralization of Plasmodium falciparum merozoites by antibodies against PfRH5. J Immunol. 2014;192(1):245–258. doi: 10.4049/jimmunol.1302045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bustamante LY, et al. A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants. Vaccine. 2013;31(2):373–379. doi: 10.1016/j.vaccine.2012.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy KS, et al. Bacterially expressed full-length recombinant Plasmodium falciparum RH5 protein binds erythrocytes and elicits potent strain-transcending parasite-neutralizing antibodies. Infect Immun. 2014;82(1):152–164. doi: 10.1128/IAI.00970-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards JS, et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol. 2013;191(2):795–809. doi: 10.4049/jimmunol.1300778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, et al. An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS Pathog. 2011;7(9):e1002199. doi: 10.1371/journal.ppat.1002199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dreyer AM, et al. Passive immunoprotection of Plasmodium falciparum-infected mice designates the CyRPA as candidate malaria vaccine antigen. J Immunol. 2012;188(12):6225–6237. doi: 10.4049/jimmunol.1103177. [DOI] [PubMed] [Google Scholar]
- 16.Sahar T, et al. Plasmodium falciparum reticulocyte binding-like homologue protein 2 (PfRH2) is a key adhesive molecule involved in erythrocyte invasion. PLoS ONE. 2011;6(2):e17102. doi: 10.1371/journal.pone.0017102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra BR, Olivieri A, Silvestrini F, Alano P, Sharma A. Biochemical characterization of the two nucleosome assembly proteins from Plasmodium falciparum. Mol Biochem Parasitol. 2005;142(2):237–247. doi: 10.1016/j.molbiopara.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Alam MM, Pal-Bhowmick I, Brzostowski JA, Chitnis CE. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 2010;6(2):e1000746. doi: 10.1371/journal.ppat.1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko YG, Thompson GA., Jr Purification of glycosylphosphatidylinositol-anchored proteins by modified triton X-114 partitioning and preparative gel electrophoresis. Anal Biochem. 1995;224(1):166–172. doi: 10.1006/abio.1995.1024. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Coppel RL. Triton X-114 phase partitioning for antigen characterization. Methods Mol Med. 2002;72:581–585. doi: 10.1385/1-59259-271-6:581. [DOI] [PubMed] [Google Scholar]
- 21.Marshall VM, et al. A second merozoite surface protein (MSP-4) of Plasmodium falciparum that contains an epidermal growth factor-like domain. Infect Immun. 1997;65(11):4460–4467. doi: 10.1128/iai.65.11.4460-4467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naik RS, et al. Glycosylphosphatidylinositol anchors of Plasmodium falciparum: molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis. J Exp Med. 2000;192(11):1563–1576. doi: 10.1084/jem.192.11.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinds L, Green JL, Knuepfer E, Holder AA, Grainger M. Novel putative glycosylphosphatidylinositol-anchored micronemal antigen of Plasmodium falciparum that binds to erythrocytes. Eukaryot Cell. 2009;8(12):1869–1879. doi: 10.1128/EC.00218-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders PR, et al. A set of glycosylphosphatidyl inositol-anchored membrane proteins of Plasmodium falciparum is refractory to genetic deletion. Infect Immun. 2006;74(7):4330–4338. doi: 10.1128/IAI.00054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams AR, et al. Enhancing blockade of Plasmodium falciparum erythrocyte invasion: Assessing combinations of antibodies against PfRH5 and other merozoite antigens. PLoS Pathog. 2012;8(11):e1002991. doi: 10.1371/journal.ppat.1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayton K, et al. Various PfRH5 polymorphisms can support Plasmodium falciparum invasion into the erythrocytes of owl monkeys and rats. Mol Biochem Parasitol. 2013;187(2):103–110. doi: 10.1016/j.molbiopara.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullard A. Protein-protein interaction inhibitors get into the groove. Nat Rev Drug Discov. 2012;11(3):173–175. doi: 10.1038/nrd3680. [DOI] [PubMed] [Google Scholar]
- 28.Zinzalla G, Thurston DE. Targeting protein-protein interactions for therapeutic intervention: A challenge for the future. Future Med Chem. 2009;1(1):65–93. doi: 10.4155/fmc.09.12. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan P, et al. Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc Natl Acad Sci USA. 2011;108(32):13275–13280. doi: 10.1073/pnas.1110303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan P, et al. Disrupting malaria parasite AMA1-RON2 interaction with a small molecule prevents erythrocyte invasion. Nat Commun. 2013;4:2261. doi: 10.1038/ncomms3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srinivasan P, et al. Immunization with a functional protein complex required for erythrocyte invasion protects against lethal malaria. Proc Natl Acad Sci USA. 2014;111(28):10311–10316. doi: 10.1073/pnas.1409928111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang BX, Kim HY. Effective identification of Akt interacting proteins by two-step chemical crosslinking, co-immunoprecipitation and mass spectrometry. PLoS ONE. 2013;8(4):e61430. doi: 10.1371/journal.pone.0061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 34.Niepmann M, Zheng J. Discontinuous native protein gel electrophoresis. Electrophoresis. 2006;27(20):3949–3951. doi: 10.1002/elps.200600172. [DOI] [PubMed] [Google Scholar]
- 35.Gaur D, et al. Recombinant Plasmodium falciparum reticulocyte homology protein 4 binds to erythrocytes and blocks invasion. Proc Natl Acad Sci USA. 2007;104(45):17789–17794. doi: 10.1073/pnas.0708772104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riglar DT, et al. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011;9(1):9–20. doi: 10.1016/j.chom.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Pandey AK, et al. Identification of a potent combination of key Plasmodium falciparum merozoite antigens that elicit strain-transcending parasite-neutralizing antibodies. Infect Immun. 2013;81(2):441–451. doi: 10.1128/IAI.01107-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.