Abstract
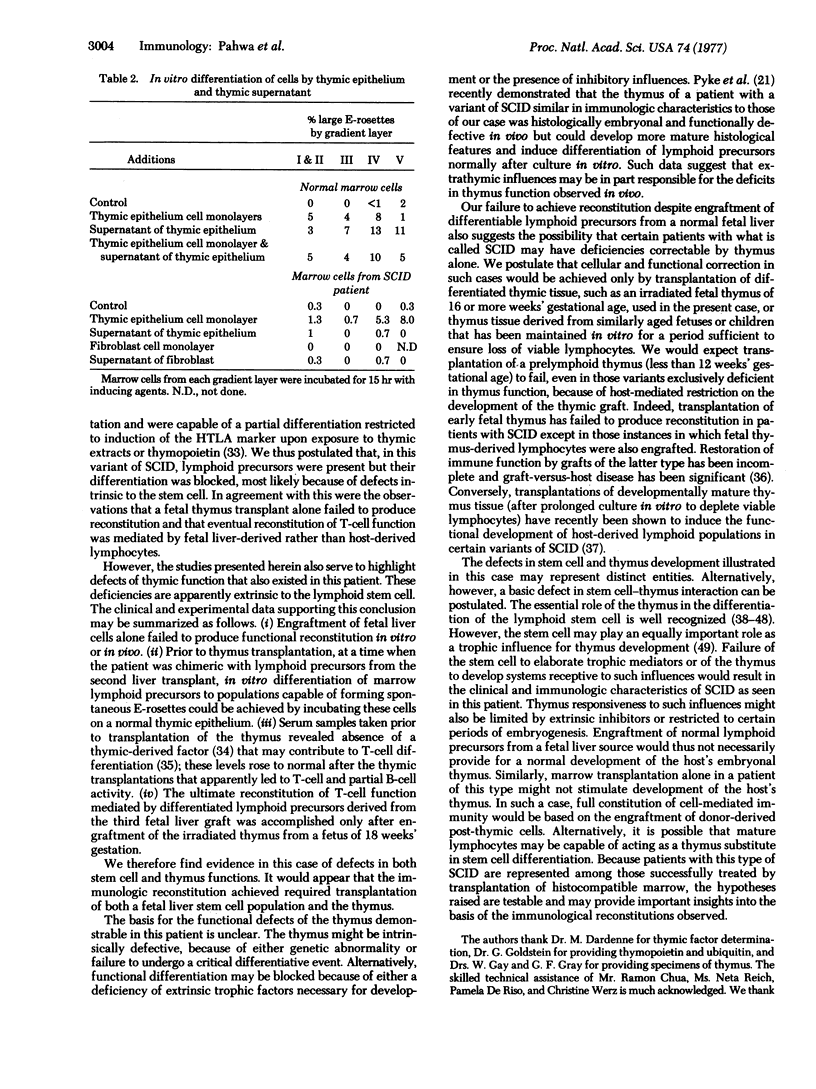
Bone marrow cells from a patient with severe combined immunodeficiency were studied in vitro for thymus-dependent lymphocyte (T cell) differentiation by using, at varying times, thymic epithelial monolayers and culture supernatants, thymopoietin, ubiquitin, and thymic extract as inducing agents. On initial evaluation, with thymopoietin or human thymic extract, only a partial differentiation of marrow cells was achieved into cells bearing the human T cell antigenicity without the capacity to form rosettes with sheep erythrocytes, suggesting that the stem cells were defective. Two fetal liver transplantations aimed at reconstitution were unsuccessful, despite evidence of chimerism. Induction studies at that time demonstrated rosetting capacity (with sheep erythrocytes) of the patient's bone marrow cells after coculture with thymic epithelial monolayers but not with their supernatants. An 18-week fetal thymus (irradiated) was then transplanted, but the transplantation was unsuccessful and no clear evidence of chimerism was demonstrated. Subsequently, transplantation of another fetal liver resulted in chimerism and immunologic reconstitution. Serum thymic factor activity rose from 1:2 before transplantation to 1:16 after reconstitution. The combined use of fetal thymus and liver may provide effective immunological reconstitution in some variants of severe combined immunodeficiency.
Keywords: lymphoid differentiation, embryology, transplantation, thymic epithelium, chimerism
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackeret C., Llüss H. J., Hitzig W. H. Hereditary sever combined immunodeficiency and adenosine deaminase deficiency. Pediatr Res. 1976 Jan;10(1):67–70. doi: 10.1203/00006450-197601000-00013. [DOI] [PubMed] [Google Scholar]
- Ammann A. J., Wara D. W., Salmon S., Perkins H. Thymus transplantation. Permanent reconstitution of cellular immunity in a patient with sex-linked combined immunodeficiency. N Engl J Med. 1973 Jul 5;289(1):5–9. doi: 10.1056/NEJM197307052890102. [DOI] [PubMed] [Google Scholar]
- Bentwich Z., Douglas S. D., Siegal F. P., Kunkel H. G. Human lymphocyte-sheep erythrocyte rosette formation: some characteristics of the interaction. Clin Immunol Immunopathol. 1973 Jul;1(4):511–522. doi: 10.1016/0090-1229(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Buckley R. H., Whisnant K. J., Schiff R. I., Gilbertsen R. B., Huang A. T., Platt M. S. Correction of severe combined immunodeficiency by fetal liver cells. N Engl J Med. 1976 May 13;294(20):1076–1081. doi: 10.1056/NEJM197605132942002. [DOI] [PubMed] [Google Scholar]
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Fudenberg H., Good R. A., Goodman H. C., Hitzig W., Kunkel H. G., Roitt I. M., Rosen F. S., Rowe D. S., Seligmann M., Soothill J. R. Primary immunodeficiencies. Report of a World Health Organization Committee. Pediatrics. 1971 May;47(5):927–946. [PubMed] [Google Scholar]
- GOOD R. A., DALMASSO A. P., MARTINEZ C., ARCHER O. K., PIERCE J. C., PAPERMASTER B. W. The role of the thymus in development of immunologic capacity in rabbits and mice. J Exp Med. 1962 Nov 1;116:773–796. doi: 10.1084/jem.116.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOOD R. A., PAPERMASTER B. W. ONTOGENY AND PHYLOGENY OF ADAPTIVE IMMUNITY. Adv Immunol. 1964;27:1–115. doi: 10.1016/s0065-2776(08)60706-3. [DOI] [PubMed] [Google Scholar]
- Githens J. H., Fulginiti V. A., Suvatte V., Schroter G., Hathaway W. E., Pearlman D. S., Kay H. E., Terasaki P. I., Hill G. J., Kempe C. H. Grafting of fetal thymus and hematopoietic tissue in infants with immune deficiency syndromes. Transplantation. 1973 May;15(5):427–434. [PubMed] [Google Scholar]
- Goldstein G. Isolation of bovine thymin: a polypeptide hormone of the thymus. Nature. 1974 Jan 4;247(5435):11–14. doi: 10.1038/247011a0. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Scheid M., Hammerling U., Schlesinger D. H., Niall H. D., Boyse E. A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good R. A. Immunodeficiency in developmental perspective. Harvey Lect. 1973;67:1–107. [PubMed] [Google Scholar]
- Górski A. J., Dupont B., Hansen J. A., Good R. A. Leukocyte migration inhibitory factor (LMIF) induced by concanavalin A: standardized microassay for production in vitro. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3197–3200. doi: 10.1073/pnas.72.8.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górski A. J., Dupont B., Hansen J. A., O'Reilly R., Smithwick E., Górska R., Good R. A. Leukocyte migration inhibitory factor (LMIF) profile in primary and secondary immunodeficiency disease. Clin Exp Immunol. 1976 Dec;26(3):505–510. [PMC free article] [PubMed] [Google Scholar]
- Hong R., Cooper M. D., Allan M. J., Kay H. E., Meuwissen H., Good R. A. Immunological restitution in lymphopenic immunological deficiency syndrome. Lancet. 1968 Mar 9;1(7541):503–506. doi: 10.1016/s0140-6736(68)91468-2. [DOI] [PubMed] [Google Scholar]
- Hong R., Santosham M., Schulte-Wissermann H., Horowitz S., Hsu S. H., Winkelstein J. A. Reconstitution of B and T lymphocyte function in severe combined immunodeficiency disease after transplantation with thymic epithelium. Lancet. 1976 Dec 11;2(7998):1270–1272. doi: 10.1016/s0140-6736(76)92031-6. [DOI] [PubMed] [Google Scholar]
- Hooper J. A., McDaniel M. C., Thurman G. B., Cohen G. H., Schulof R. S., Goldstein A. L. Purification and properties of bovine thymosin. Ann N Y Acad Sci. 1975 Feb 28;249:125–144. doi: 10.1111/j.1749-6632.1975.tb29063.x. [DOI] [PubMed] [Google Scholar]
- Incefy G. S., Boumsell L., Touraine J. L., Espérance P. L., Smithwick E., O'Reilly R., Good R. A. Enhancement of T-lymphocyte differentiation in vitro by thymic extracts after bone marrow transplantation in severe combined immunodeficiencies. Clin Immunol Immunopathol. 1975 Jul;4(2):258–268. doi: 10.1016/0090-1229(75)90061-6. [DOI] [PubMed] [Google Scholar]
- Incefy G. S., Dardenne M., Pahwa S., Grimes E., Pahwa R. N., Smithwick E., O'Reilly R., Good R. A. Thymic activity in severe combined immunodeficiency diseases. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1250–1253. doi: 10.1073/pnas.74.3.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incefy G. S., Grimes E., Kagan W. A., Goldstein G., Smithwick E., O'Reilly R., Good R. A. Heterogeneity of stem cells in severe combined immunodeficiency. Clin Exp Immunol. 1976 Sep;25(3):462–471. [PMC free article] [PubMed] [Google Scholar]
- Incefy G. S., L'Esperance P., Good R. A. In vitro differentiation of human marrow cells into T lymphocytes by thymic extracts using the rosette technique. Clin Exp Immunol. 1975 Mar;19(3):475–483. [PMC free article] [PubMed] [Google Scholar]
- JANKOVIC B. D., WAKSMAN B. H., ARNASON B. G. Role of the thymus in immune ractions in rats. I. The immunologic response to bovine serum albumin (antibody formation, Arthus reactivity, and delayed hypersensitivity) in rats thymectomized or splenectomized at various times after birth. J Exp Med. 1962 Aug 1;116:159–176. doi: 10.1084/jem.116.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES H. B. Estimation of radiation effects at small exposures. Fed Proc. 1961 Jul;20(Suppl 8):26–28. [PubMed] [Google Scholar]
- Keightley R. G., Lawton A. R., Cooper M. D., Yunis E. J. Successful fetal liver transplantation in a child with severe combined immunodeficiency. Lancet. 1975 Nov 1;2(7940):850–853. doi: 10.1016/s0140-6736(75)90238-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER J. F. Immunological function of the thymus. Lancet. 1961 Sep 30;2(7205):748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- Meuwissen H. J., Rodey G., McArthur J., Pabst H., Gatti R., Chilgren R., Hong R., Frommel D., Coifman R., Good R. A. Bone marrow transplantation. Therapeutic usefulness and complications. Am J Med. 1971 Oct;51(4):513–532. doi: 10.1016/0002-9343(71)90257-9. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Owen J. J. Experimental studies on the development of the thymus. J Exp Med. 1967 Oct 1;126(4):715–726. doi: 10.1084/jem.126.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSOBA D., MILLER J. F. THE LYMPHOD TISSUES AND IMMUNE RESPONSES OF NEONATALLY THYMECTOMIZED MICE BEARING THYMUS TISSUE IN MILLIPORE DIFFUSION CHAMBERS. J Exp Med. 1964 Jan 1;119:177–194. doi: 10.1084/jem.119.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARROTT D. M. Strain variation in mortality and runt disease in mice thymectomized at birth. Transplant Bull. 1962 Apr;29:102–104. [PubMed] [Google Scholar]
- Pyke K. W., Dosch H., Ipp M. M., Gelfand E. W. Demonstration of an intrathymic defect in a case of severe combined immunodeficiency disease. N Engl J Med. 1975 Aug 28;293(9):424–428. doi: 10.1056/NEJM197508282930904. [DOI] [PubMed] [Google Scholar]
- Pyke K. W., Gelfand E. W. Morphological and functional maturation of human thymic epithelium in culture. Nature. 1974 Oct 4;251(5474):421–423. doi: 10.1038/251421a0. [DOI] [PubMed] [Google Scholar]
- Stutman O., Yunis E. J., Good R. A. Thymus: an essential factor in lymphoid repopulation. Transplant Proc. 1969 Mar;1(1):614–615. [PubMed] [Google Scholar]
- Tulunay O., Good R. A., Yunis E. J. Protection of lethally irradiated mice with allogeneic fetal liver cells: influence of irradiation dose on immunologic reconstitution. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4100–4104. doi: 10.1073/pnas.72.10.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UPHOFF D. E. Perclusion of secondary phase of irradiation syndrome by inoculation of fetal hematopoietic tissue following lethal total-body x-irradiation. J Natl Cancer Inst. 1958 Mar;20(3):625–632. [PubMed] [Google Scholar]
- Yunis E. J., Fernandes G., Smith J., Good R. A. Long survival and immunologic reconstitution following transplantation with syngeneic or allogeneic fetal liver and neonatal spleen cells. Transplant Proc. 1976 Dec;8(4):521–525. [PubMed] [Google Scholar]



