Significance
Subarachnoid hemorrhage (SAH) is a devastating stroke subtype associated with an early age at onset and significant morbidity and mortality. Cerebral vasospasm (CV) is a common complication of SAH and a key contributor to poor outcomes due to the resulting brain ischemia and/or infarction. Blood bioproducts have been implicated in the development of CV, and haptoglobin (Hp), the hemoglobin-binding protein, may aid in attenuating this cascade of toxic effects. Here, we demonstrate that Hp phenotype is an independent risk factor for focal CV, and importantly, for global CV. We also show that Hp phenotype predicts mortality and poor outcomes. Although this work focuses on SAH, we expect that these findings will also apply to other acute neurological conditions.
Keywords: aneurysm, hemoglobin, iron, ischemia, vasoconstriction
Abstract
Cerebral vasospasm (CV) and the resulting delayed cerebral ischemia (DCI) significantly contribute to poor outcomes following aneurysmal subarachnoid hemorrhage (aSAH). Free hemoglobin (Hb) within the subarachnoid space has been implicated in the pathogenesis of CV. Haptoglobin (Hp) binds free pro-oxidant Hb, thereby modulating its harmful effects. Humans can be of three Hp phenotypes: Hp1-1, Hp2-1, or Hp2-2. In several disease states, the Hp2-2 protein has been associated with reduced ability to protect against toxic free Hb. We hypothesized that individuals with the Hp2-2 phenotype would have more CV, DCI, mortality, and worse functional outcomes after aSAH. In a sample of 74 aSAH patients, Hp2-2 phenotype was significantly associated with increased focal moderate (P = 0.014) and severe (P = 0.008) CV and more global CV (P = 0.014) after controlling for covariates. Strong trends toward increased mortality (P = 0.079) and worse functional outcomes were seen for the Hp2-2 patients with modified Rankin scale at 6 wk (P = 0.076) and at 1 y (P = 0.051) and with Glasgow Outcome Scale Extended at discharge (P = 0.091) and at 1 y (P = 0.055). In conclusion, Hp2-2 phenotype is an independent risk factor for the development of both focal and global CV and also predicts poor functional outcomes and mortality after aSAH. Hp phenotyping may serve as a clinically useful tool in the critical care management of aSAH patients by allowing for early prediction of those patients who require increased vigilance due to their inherent genetic risk for the development of CV and resulting DCI and poor outcomes.
Aneurysmal subarachnoid hemorrhage (aSAH) affects ∼30,000 people per year in the United States, with 30-d mortality rates as high as 50% (1, 2). Only 20–25% of survivors regain their original functional capacity due to chronic cognitive impairments and physical disabilities (3). Cerebral vasospasm (CV) is a frequent complication, and this prolonged vasoconstriction may lead to delayed cerebral ischemia (DCI), a known contributor to poor functional outcomes following aSAH (2).
Although various hypotheses have been put forward to explain the development of aSAH-related CV, the presence of red blood cells, hemoglobin (Hb), and Hb breakdown products within close proximity to major cerebral vessels have been strongly implicated in the pathogenesis (4–7). Haptoglobin (Hp) is an acute-phase protein with a primary function of binding free Hb (8). Formation of this Hp–Hb complex directly detoxifies Hb and mediates its safe clearance (9–11). There are two Hp alleles in the human population, Hp1 and Hp2, which allow for three possible Hp genotypes: Hp1-1, Hp2-1, and Hp2-2. The Hp2-2 protein has been reported to have a reduced ability to bind and detoxify free Hb and impairs the safe clearance of the Hp–Hb complex (12). Therefore, we hypothesized that the Hp2-2 phenotype may negatively contribute to aSAH outcomes by mediating a greater degree of Hb-mediated oxidative and inflammatory brain injury.
Previous clinical studies aimed at correlating Hp phenotype with the incidence of CV and aSAH outcomes have demonstrated different results, likely resulting from methodological variations, diverse patient populations, and limited data (3, 13, 14). Despite this past research investigating whether Hp genotype is predictive of CV and aSAH outcomes, several issues remain unaddressed. The purpose of this study was to comprehensively evaluate whether Hp phenotype is an independent risk factor for CV, clinical deterioration as a result of CV-induced DCI, poor functional outcomes, and mortality after aSAH.
Results
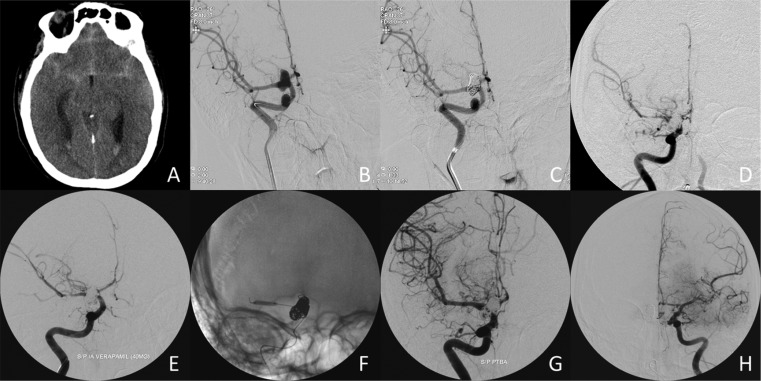
Of the 74 aSAH patients in this study, 11 were found to be Hp1-1 (14.9%), 39 Hp2-1 (52.7%), and 24 Hp2-2 (32.4%), which is in agreement with previously reported Hp allele frequencies in this geographic region (12). Fig. 1 shows an example of the Hp typing. Demographics, patient characteristics, and the severity of aSAH for each of the Hp phenotype groups are listed in Table 1. Overall, this cohort was predominately female (73.0%) and Caucasian (77.0%), with a mean age (±SD) of 54.7 ± 15.3 (range 20–88 y). The Hp phenotype groups did not show any significant differences between age, sex, race, Glasgow Coma Scale (GCS), World Federation of Neurological Surgeons (WFNS), Fisher grade, Hunt–Hess grade, or aneurysm size (Table 1). Hp2-2 patients did tend to receive clipping more often than Hp1-1/2-1 patients (70.8% vs. 48%, respectively, P = 0.083). Fig. 2 provides a typical case example of a 34-y-old female patient included in this study, who developed moderate and severe CV bilaterally in multiple vessels, DCI, and poor outcome.
Fig. 1.
Demonstration of the Hp typing methods used, showing examples of both the aSAH patients and longitudinal controls of known Hp type that were incorporated in all Hp typing of patients. (A) Hp genotyping of control DNA samples. Lanes 1 and 12 show DNA ladders; lanes 3, 5, 7, 8, and 11 show the Hp1-1 genotype; lanes 2, 6, and 9 show the Hp2-1 genotype; and lanes 4 and 10 show the Hp2-2 genotype. The bands corresponding to Hp1 and Hp2 were subsequently confirmed to be specific by restriction enzyme analysis. (B) Hp phenotyping of serum samples from controls and aSAH patients. Lane 1 shows a molecular weight marker; lanes 3, 5, 6, and 8 show the Hp 1–1 phenotype; lanes 2 and 9 show Hp 2–1 individuals; and lanes 4 and 7 show the Hp2-2 phenotype. The controls in lanes 2–4 correspond to the same controls in lanes 2–4 in A, demonstrating the matching Hp types between the two methods.
Table 1.
Demographics, patient characteristics, and subarachnoid hemorrhage severity stratified by Hp phenotype
| Variable | Overall (n = 74) | Hp1-1 (n = 11) | Hp2-1 (n = 39) | Hp2-2 (n = 24) | Hp1-1/2–1 (n = 50) | P value* |
| Age, mean ± SD | 54.7 ± 15.3 | 54.7 ± 18.0 | 56.6 ± 14.0 | 51.6 ± 16.3 | 56.2 ± 14.7 | 0.254 |
| Sex, n (%) | ||||||
| Female | 54 (73.0) | 9 (81.8) | 28 (71.8) | 17 (70.8) | 37 (74.0) | 0.785 |
| Male | 20 (27.0) | 2 (18.2) | 11 (28.2) | 7 (29.2) | 13 (26.0) | |
| Race, n (%) | ||||||
| Black | 14 (18.9) | 3 (27.3) | 9 (23.1) | 2 (8.3) | 12 (24.0) | 0.223 |
| White | 57 (77.0) | 7 (63.6) | 29 (74.4) | 21 (87.5) | 36 (72.0) | |
| Hispanic | 3 (4.1) | 1 (9.1) | 1 (2.6) | 1 (4.2) | 2 (4.0) | |
| GCS, mean ± SD | 12.0 ± 3.3 | 9.4 ± 4.1 | 12.3 ± 3.1 | 12.8 ± 2.7 | 11.7 ± 3.5 | 0.169 |
| WFNS, mean ± SD | 2.5 ± 1.3 | 3.5 ± 1.2 | 2.4 ± 1.2 | 2.3 ± 1.3 | 2.6 ± 1.3 | 0.363 |
| Fisher grade, n (%) | ||||||
| 2 | 7 (9.5) | 0 (0.0) | 3 (7.7) | 4 (16.7) | 3 (6.0) | 0.256 |
| 3 | 24 (32.4) | 4 (36.4) | 14 (35.9) | 6 (25.0) | 18 (36.0) | |
| 3+4 | 43 (58.1) | 7 (63.6) | 22 (56.4) | 14 (58.3) | 29 (58.0) | |
| Hunt–Hess grade, mean ± SD | 2.8 ± 0.9 | 3.5 ± 0.9 | 2.7 ± 0.9 | 2.6 ± 0.9 | 2.9 ± 1.0 | 0.197 |
| Aneurysm size (mm), mean ± SD | 6.3 ± 3.1 | 5.4 ± 2.6 | 6.4 ± 2.8 | 6.7 ± 3.9 | 6.2 ± 2.7 | 0.903 |
| Treatment, n (%) | ||||||
| Clipping | 41 (55.4) | 8 (72.7) | 16 (41.0) | 17 (70.8) | 24 (48.0) | 0.083 |
| Coiling | 33 (44.6) | 3 (27.3) | 23 (59.0) | 7 (29.2) | 26 (52.0) |
For comparison between Hp2-2 and Hp1-1/2-1.
Fig. 2.
A prototypical example of a 34-y-old female with aSAH. (A) Noncontrast head CT showing diffuse Fisher 3+4 SAH and layering intraventricular hemorrhage. (B) Initial cerebral angiography of the right internal carotid artery (ICA) demonstrates a right posterior communicating artery aneurysm. (C) Repeat cerebral angiography posttreatment by coiling. (D) At day 6 postcoiling, the patient developed progressive confusion and stupor with a left hemiparesis. Cerebral angiography demonstrates severe right M1 and moderate right M2 and A2 CV. (E) Following intra-arterial infusion of verapamil, there is minimal change in the degree of CV. (F) Angioplasty is performed. A balloon is inflated in the right M1. (G) There is significant improvement of the right M1 CV following angioplasty. (H) Angiography of the left ICA shows moderate left A1, A2, and M2 CV, which was treated with intra-arterial verapamil infusion only.
Radiographic Vasospasm.
For the 17 cerebral arteries evaluated, the mean number of vessels (±SD) with mild, moderate, and severe CV was 5.1 ± 3.3, 2.8 ± 2.4, and 1.5 ± 2.6, respectively. Hp2-2 phenotype was associated with 1.8 [confidence interval (CI) = (1.12, 2.98), P = 0.014] and 3.7 [CI = (1.33, 11.5), P = 0.008] times the number of vessels with moderate and severe CV, respectively (Table 2). We did not find a significant relationship between Hp2-2 phenotype and the number of vessels with mild CV [CI = (0.536, 1.38), P = 0.531; Table 2]. The overall global CV, corresponding to the sum of the individual CV values for each of the 17 arteries (±SD), was 15.2 ± 9.6. Hp2-2 phenotype was significantly associated with increased global CV, with a 6.5 higher total CV value [CI = (1.39, 11.9), P = 0.014; Table 2].
Table 2.
Multivariate analysis of radiographic vasospasm and delayed cerebral ischemia
| Outcome | Overall (n = 74) | Hp1-1 (n = 11) | Hp2-1 (n = 39) | Hp2-2 (n = 24) | Hp1-1/2–1 (n = 50) | 95% CI* | P value* |
| No. of vessels with mild CV, mean ± SD | 5.1 ± 3.3 | 5.6 ± 3.4 | 5.1 ± 3.6 | 4.9 ± 3.0 | 5.3 ± 3.5 | 0.536–1.38 | 0.531 |
| No. of vessels with moderate CV, mean ± SD | 2.8 ± 2.4 | 2.1 ± 1.7 | 1.9 ± 2.6 | 2.9 ± 2.4 | 2.4 ± 2.4 | 1.12–2.98 | 0.014 |
| No. of vessels with severe CV, mean ± SD | 1.5 ± 2.6 | 1.4 ± 3.2 | 1.1 ± 2.2 | 2.3 ± 3.0 | 1.2 ± 2.4 | 1.33–11.5 | 0.008 |
| Global CV, mean ± SD | 15.2 ± 9.6 | 15.9 ± 10.6 | 12.8 ± 9.0 | 19.1 ± 9.3 | 13.6 ± 9.4 | 1.39–11.9 | 0.014 |
| CV-induced DCI, n (%) | 22 (29.7) | 3 (27.3) | 9 (23.1) | 10 (41.7) | 12 (24.0) | 0.706–6.00† | 0.180† |
For comparison between Hp2-2 and Hp1-1/2-1 controlling for age, GCS, WFNS, Fisher grade, Hunt–Hess grade, aneurysm size, and treatment type.
OR = 2.1 for comparison between Hp2-2 and Hp1-1/2-1 controlling for age.
As part of these analyses, we also found that age, aneurysm size, and treatment type were associated with focal and global CV. It is estimated that the number of vessels with moderate or severe CV decreases by 2% [CI = (0.80%, 3.8%), P = 0.003] and 8% [CI = (4.6%, 11.3%), P < 0.0001], respectively, for each additional year of age (Table S1). Similarly, older age was also associated with less global CV, with a 0.41 lower total CV value for each additional year [CI = (−0.535, −0.215), P < 0.0001; Table S1]. Larger aneurysms tended to be correlated with less severe [CI = (1.10%, 24.6%), P = 0.062; Table S1] and global CV [CI = (−1.66, 0.033), P = 0.043; Table S1]. Likewise, patients who received coiling had more vessels with severe [CI = (2.13, 18.0), P = 0.001; Table S1] and global CV [CI = (0.953, 11.3); P = 0.047; Table S1]. We did not find a significant association between radiographic CV and aSAH severity identified by GCS, WFNS, Fisher grade, or Hunt–Hess grade (Table S1).
Delayed Cerebral Ischemia.
CV-induced DCI occurred in 22 of 74 (29.7%) aSAH patients in this study. We found that 10 of 24 (41.7%) Hp2-2 individuals developed DCI, compared with 12 of 50 (24.0%) Hp1-1/2-1 patients. Logistic regression controlling for age did not show a significant association between Hp2-2 phenotype and DCI [odds ratio (OR) = 2.1, CI = (0.706, 6.00), P = 0.180; Table 2]. Age was not associated with the incidence of DCI (Table S1).
Functional Outcomes.
The overall mean (±SD) modified Rankin Scale (mRS) scores at discharge, 6 wk, and 1 y after aSAH were 3.9 ± 1.3, 3.1 ± 1.7, and 2.5 ± 2.0, respectively. Likewise, the overall mean (±SD) Glasgow Outcome Scale Extended (GOSE) scores at discharge, 6 wk, and 1 y post-aSAH were 3.1 ± 1.5, 3.9 ± 2.0, and 5.2 ± 2.5, respectively. A multivariate analysis of Hp phenotype and functional outcomes (identified by the mRS and GOSE) controlling for age, GCS, WFNS, Fisher grade, Hunt–Hess grade, aneurysm size, and treatment type (clipping vs. coiling) demonstrated a strong trend toward Hp2-2 phenotype and worse functional outcomes (Table 3). Hp2-2 individuals had mRS scores 0.84 and 1.20 higher at 6 wk [CI = (−0.090, 1.78), P = 0.076] and 1 y [CI = (−0.006, 2.38), P = 0.051], respectively. Similarly, Hp2-2 individuals had GOSE scores 0.74 and 1.45 lower at discharge [CI = (−1.60, 0.121), P = 0.091] and 1 y [CI = (−2.92, 0.030), P = 0.055], respectively. For each unit increase on GCS, mRS and GOSE scores at 1 y were 0.25 lower [CI = (−0.538, 0.034), P = 0.082; Table S2] and 0.39 higher [CI = (0.033, 0.746), P = 0.033; Table S2], respectively. We did not find any other significant predictors of functional outcomes at discharge, 6 wk, or 1 y (Table S2).
Table 3.
Multivariate analysis of functional outcomes and mortality
| Outcome | Overall (n = 74) | Hp1-1 (n = 11) | Hp2-1 (n = 39) | Hp2-2 (n = 24) | Hp1-1/2-1 (n = 50) | 95% CI* | P value* |
| GOSE at discharge, mean ± SD | 3.1 ± 1.5 | 3.3 ± 1.3 | 3.4 ± 1.5 | 2.7 ± 1.5 | 3.4 ± 1.5 | −1.60–0.121 | 0.091 |
| GOSE at 6 wk, mean ± SD | 3.9 ± 2.0 | 4.5 ± 1.9 | 3.9 ± 1.8 | 3.6 ± 2.2 | 4.1 ± 1.8 | −1.86–0.507 | 0.256 |
| GOSE at 12 mo, mean ± SD | 5.2 ± 2.5 | 5.4 ± 2.7 | 5.5 ± 2.3 | 4.6 ± 2.8 | 5.5 ± 2.3 | −2.92–0.030 | 0.055 |
| mRS at discharge, mean ± SD | 3.9 ± 1.3 | 4.1 ± 1.2 | 3.7 ± 1.3 | 4.0 ± 1.4 | 3.8 ± 1.3 | −0.141–1.25 | 0.114 |
| mRS at 6 wk, mean ± SD | 3.1 ± 1.7 | 3.4 ± 1.8 | 2.8 ± 1.6 | 3.5 ± 1.8 | 2.9 ± 1.7 | −0.090–1.78 | 0.076 |
| mRS at 12 mo, mean ± SD | 2.5 ± 2.0 | 2.5 ± 2.3 | 2.2 ± 1.7 | 2.9 ± 2.3 | 2.2 ± 1.9 | −0.006–2.38 | 0.051 |
| Mortality, n (%) | 11 (14.9) | 1 (9.1) | 4 (10.3) | 6 (25.0) | 5 (10.0) | 0.871–13.3† | 0.079† |
For comparison between Hp2-2 and Hp1-1/2-1 controlling for age, GCS, WFNS, Fisher grade, Hunt–Hess grade, aneurysm size, and treatment type.
OR = 3.3, for comparison between Hp2-2 and Hp1-1/2-1 controlling for age.
Mortality.
The overall mortality rate was 11 of 74 (14.9%). We found that 6 of 24 (25.0%) Hp2-2 individuals died, compared with only 5 of 50 (10.0%) Hp1-1/2-1 patients. Logistic regression controlling for age showed a trend toward increased mortality for the Hp2-2 individuals [OR = 3.3, CI = (0.871, 13.3), P = 0.079; Table 3]. Age was not associated with mortality (Table S2).
Discussion
CV has long been regarded as a key contributor to poor outcomes after aSAH, mainly due to the resulting DCI and cerebral infarction that may occur (15). The purpose of this study was to determine whether individuals with the Hp2-2 phenotype had increased risk for CV, DCI, mortality, and poor outcomes following aSAH. We found that Hp2-2 individuals had significantly more vessels with moderate and severe focal radiographic CV, and given the design of this study, we are, to our knowledge, the first to show that Hp2-2 phenotype is predictive of global CV. We also observed strong trends toward Hp2-2 phenotype and poor outcomes as identified by both the mRS and GOSE at discharge, 6 wk, and 1 y postbleed. Additionally, we found a significant relationship between Hp2-2 phenotype and increased incidence of mortality.
After aSAH, hemolysis within the subarachnoid space releases massive amounts of Hb, a molecule that we believe has strongly pro-oxidative and proinflammatory properties when not confined within a red blood cell. We and others have speculated that free Hb would be the major instigator of CV through a multifactorial mechanism involving the generation of reactive intermediates that cause endothelial cell damage, depletion of the vasodilator nitric oxide, and proliferation of smooth muscle cells, a combination that ultimately leads to sustained vasoconstriction and DCI (5–7, 16). In a phenotype-dependent manner, the redox potential and clearance of Hb is directly reduced and improved by Hp, respectively, where the Hp2-2 protein has been suggested to have less overall protective abilities (9–11, 17–19).
Although there have been previous studies investigating the role of Hp phenotype in aSAH (3, 13, 14), there are a few critical differences between these studies, compared with the one described here, which could possibly explain the varied results: (i) subjects of different ethnicity, (ii) different clinical management approaches, and (iii) variations in study methodologies. This study forms a bridge between the previous ones, filling in some gaps and also providing previously unidentified findings aimed at a better understanding of the role of Hp phenotype in predicting CV, DCI, mortality, and poor outcomes after aSAH.
Indeed, previous studies have varied in the methods used for CV determination, including the type of imaging modality used, number of arteries evaluated, and criteria used for determination of CV. Here, we evaluated a large number of vessels for each patient and graded the CV as mild, moderate, or severe instead of dichotomizing to “yes” or “no.” In this way, we obtained a comprehensive view of CV in each of the patients, including an analysis of both the distribution and the severity. This approach also allowed us to evaluate CV from a global standpoint. In the minority of patients, these determinations were done using computed tomography angiography (CTA) imaging, rather than the gold standard cerebral angiography. However, previous studies have shown good correlation between CTA and angiography measurement of arterial diameters (20–22). If there was a discrepancy between CTA and angiography, CTA tended to overestimate the degree of CV. Here, the majority of patients with CTA imaging were Hp1-1 or Hp2-1, and thus overestimation in this case would lead to less observed differences although Hp2-2 individuals still had significantly more focal moderate (P = 0.017) and severe (P = 0.009) CV and more global CV (P = 0.014). Furthermore, these findings suggest that there is less of a clinical need to do invasive imaging studies in Hp1-1/2-1 patients, indirectly substantiating our findings of Hp2-2 phenotype and increased risk for CV. In the two previous studies that looked at Hp phenotype and CV after aSAH, Ohnishi et al. (13) found an association between Hp2-2 phenotype and increased risk for angiographical CV, whereas Borsody et al. (14) found no such link. The latter study included 32 patients with aSAH, and angiography data were not available for all subjects. However, with their more abundant transcranial Doppler ultrasonography data, Borsody et al. did find an association between the presence of the Hp2 allele and increased incidence of CV.
Our results showing that Hp2-2 phenotype is an independent risk factor for global CV is of particular interest because patients who develop CV and DCI requiring endovascular treatment may later develop CV and DCI in a different arterial distribution that was not affected or treated in the first episode (23, 24). As Tekle et al. (24) proposed, these events suggest that some patients may have a greater overall propensity for developing CV and DCI after aSAH. We have shown that Hp2-2 phenotype is predictive of global CV, although it is important to note that while a greater percentage of Hp2-2 patients experienced DCI (41.7% vs. 24%), this trend did not reach statistical significance (P = 0.180). This finding is likely due to our small sample size: if the difference between groups is truly 17.7 percentage points, our study had only 24% power to detect it. Ohnishi et al. (13) were essentially the only other group to analyze the incidence DCI in the context of Hp phenotype, and they similarly found the Hp2-2 group trending toward increased risk.
As part of our multivariate analysis, we also found that other factors were independent predictors of CV. Younger age was associated with more focal moderate (P = 0.003) and severe CV (P < 0.0001) and with more global CV (P < 0.0001), which correlates well with previous studies (25–27). Fisher grade is a classification of the amount and distribution of subarachnoid blood on admission CT scans after aneurysm rupture and is a well-known predictor of CV (28–30). Our current study was underpowered to evaluate Fisher grade as a risk factor for CV, as only two of the seven Fisher 2 patients had imaging available to evaluate vasospasm. Although we were not able to reliably draw statistical conclusions, two other factors suggest less CV risk for the Fisher 2 patients in this cohort: (i) none of the seven Fisher 2 patients had DCI and (ii) the lack of CV diagnostic imaging studies performed in these patients imply that there was no clinical need. With respect to aneurysm size and treatment modality, previous studies have shown conflicting evidence regarding the risk for CV (31–36). Here, we found a correlation between coiling and increased CV, which is in contradiction to larger studies that demonstrate no difference between groups or favor coiling for lower risk (31, 32, 35, 36). We are uncertain of why coiled patients in this study tended to have more CV, although we cannot exclude that those patients less prone to CV were more often surgically clipped.
Individuals with the Hp2-2 phenotype had increased mortality and poor aSAH outcomes, as identified by both the GOSE and mRS on a continuous basis at discharge, 6 wk, and 1 y. No previous studies have correlated Hp phenotype and long-term (1 y) functional outcomes after aSAH or provided outcome data on a continuum. Furthermore, the observed differences in outcomes between the Hp phenotype groups become more exaggerated with increased time postbleed, suggesting that Hp phenotype may be a valuable marker for predicting long-term neurologic disability. In a recent study by Kantor et al. (3), Hp2-2 genotype was associated with poor outcome identified by dichotomized mRS at 3 mo postbleed although no significant differences were seen with mortality or dichotomized Glasgow Outcome Scale. In contrast, Ohnishi et al. (13) found no association between Hp phenotype and the 3-mo dichotomized mRS score. As they pointed out, the results of their study are not directly applicable to other populations, given the racial differences and marked variation in Hp genotype frequencies depending on geographic location.
The combination of these previous studies and the current one suggests that racial background is not an important confounding variable when evaluating Hp phenotype and the risk for developing CV, although it may be important when evaluating its role in predicting outcomes. The former is likely indicative of the inherent biological roles of Hb and Hp, where Hb is a primary instigator of CV and Hp is important in mediating clearance of toxic-free Hb from the body, which is Hp phenotype-dependent. The latter point may suggest that other genetic factors may also be responsible for poor outcomes after aSAH, given that positive correlations between Hp phenotype and outcomes have been shown in Western populations and that no such association was found in an Asian population. In support of this hypothesis, it was recently suggested in a cross-sectional study of hospital discharges in the United States that individuals of Asian/Pacific Islander descent had worse outcomes after SAH compared with other racial/ethnic groups (37). Alternatively, different clinical management approaches could also explain the conflicting results of these studies regarding the predictive potential of Hp phenotype and poor aSAH outcomes.
Study Limitations.
A main limitation of this study is its retrospective nature, particularly with regard to determining DCI. Accurate determination of DCI, even in the prospective setting, remains difficult due to the complex clinical course of aSAH patients. It can be challenging to discern CV-induced DCI from other causes of neurologic change including metabolic derangements, fever, infection, hydrocephalus, seizure, and respiratory complications. In this study, we performed a thorough chart review to look for these other possible causes of clinical deterioration, and when possible, we confirmed true CV-induced DCI by brain imaging demonstrating ischemia/infarction and/or clinical improvement following CV treatment (balloon angioplasty, intra-arterial infusion of verapamil). In contrast to DCI, the retrospective nature of this study does not affect our evaluation of aSAH outcomes or CV because these determinations were performed prospectively or by review of imaging collected as part of routine care, respectively.
Other limitations of this study stem from our methodology used for evaluation of CV. A single expert reviewed imaging, and thus the interreader variability is unclear, although to reduce potential bias, the reader was blinded to Hp phenotype and aSAH clinical course. In addition, there is no validated methodology for assessing CV or a standard way of reporting these data. Many groups dichotomize this outcome to “yes” or “no” CV, which likely reduces the variability, but provides less information regarding the location and severity. Other groups present the data with a grading of CV although these methods also vary in terms of the grading system (number of groups) and criteria for each grouping (% reduction in vessel diameter). Here, we have used two methods to evaluate CV: an individual comparison of the number of vessels with a particular degree of CV and an approach that we termed “global CV.” Although such techniques have not been extensively validated, we have used these methods to obtain a comprehensive view of CV. A study using similar methods for determining the degree of CV in each vessel has shown good interobserver variability (20).
Conclusions.
These results demonstrate that Hp2-2 phenotype is an independent risk factor for the development of focal and global CV and may also predict mortality and poor outcomes after aSAH. We are actively collaborating with several groups to continue enrolling patients in the sample biorepository to confirm and extend the results presented here, because some of these analyses were limited by the sample size. The availability of such a genetic marker to predict CV, DCI, mortality, and poor outcomes would aid in the critical care management of aSAH patients, which continues to pose a considerable challenge to clinicians. Genotyping for the various Hp polymorphisms could be performed upon admission and has the potential for use in risk stratification by allowing for the identification of those patients requiring increased vigilance due to their inherent genetic risk for developing CV, DCI, and poor outcomes.
Methods
Following approval by the University of Florida institutional review board and informed consent, 74 patients with aSAH were enrolled at the University of Florida between November 2006 and December 2013. Patients over the age of 18 with a ruptured intracranial aneurysm were included. Patients with nonaneurysmal SAH were excluded.
Clinical Data and Biospecimen Collection.
Biospecimens were collected as part of two separate protocols: 62 patients were from a previous prospective study for identifying biomarkers in SAH, and an additional 12 patients were from our ongoing sample biorepository for studying brain injuries. Blood was obtained from an arterial line or by i.v. puncture and processed for storage of serum. Patient demographics, including age, sex, and race, were collected as part of enrollment for both protocols. For the prospective study, treatment type (clipping, coiling), aneurysm size and location, and inpatient notes were collected as part of the study. For the biorepository patients, these data were abstracted through a retrospective chart review.
The initial clinical presentation and severity of aSAH were determined by the following scales: WFNS, GCS, Fisher grade, and Hunt–Hess grade. For the majority of patients, WFNS, GCS, and Hunt–Hess grade were collected prospectively by the treating physician. An endovascular neurosurgeon, blinded to Hp phenotyping results, reviewed charts and imaging to fill in any missing data and to determine Fisher grades (28).
Outcomes were assessed using the mRS and GOSE. For the prospective study, these data were collected at discharge, 6 wk, and 1 y. For the biorepository, mRS scores were recorded at discharge and last clinic follow-up.
Radiographic Vasospasm.
To assess for radiographic CV, CTA and cerebral angiography imaging were reviewed by an endovascular neurosurgeon blinded to Hp phenotype and aSAH clinical course. Cerebral angiography was used to grade the degree of CV when available, and CTA was used when angiography was not performed. Imaging performed closest to postbleed day 7 was used to grade CV as mild (<33% narrowing), moderate (33–66% narrowing), or severe (>66% narrowing). A CV grade was assigned bilaterally to the supraclinoid carotid artery, proximal middle cerebral artery (MCA) (M1), distal MCA (M2), proximal anterior cerebral artery (ACA) (A1), distal ACA (A2), vertebral artery, proximal posterior cerebral artery (PCA) (P1), distal PCA (P2), and for the basilar artery. Each of these arteries were assigned a score of 0, 1, 2, or 3 corresponding to absent, mild, moderate, or severe CV, respectively. We defined the term “global CV” as corresponding to the sum of the CV values for the 17 cerebral arteries evaluated for each patient.
Clinical Deterioration from Delayed Cerebral Ischemia.
Clinical deterioration as a result of CV-induced DCI was defined on the basis of acute mental status changes after excluding for other causes (metabolic, hydrocephalus, fever, infection, seizure). Clinical improvement after initiation of hypertensive therapy, intra-arterial treatment of CV with verapamil, balloon angioplasty, or brain imaging demonstrating ischemia was used for confirmation of DCI.
Hp Typing.
The Hp type of aSAH patients was determined using a modified previously described method based on detecting the α1- and α2-chain size differences of the denatured Hp protein (38). A serum sample from each patient was diluted 75-fold, mixed with an equal volume of 2× sample buffer (Bio-Rad), and boiled at 95 °C for 5 min. Ten microliters was loaded onto a 12% polyacrylamide gel and electrophoresed at 100 V for 10 min, followed by 150 V for 50 min. Samples were transferred to polyvinylidene fluoride membranes, which were blocked for 1 h at room temperature with 0.5% casein in Tris-buffered saline containing 0.01% Tween-20 (TBST). Membranes were incubated overnight at 4 °C with polyclonal rabbit anti-human Hp (Dako) diluted 1:7,500 in blocking buffer supplemented with 0.2% Tween-20. After four washes in TBST, the membranes were incubated for 1 h at room temperature with peroxidase-labeled goat anti-rabbit IgG (Vector Labs) diluted 1:10,000 in blocking buffer supplemented with 0.2% Tween-20 and 0.01% SDS. Following four washes in TBST, chemiluminescence was visualized using SuperSignal West Pico substrate (Thermo Scientific) with a FluorChem E detection system (ProteinSimple). Hp phenotyping was performed without knowledge of aSAH clinical course. Serum samples from controls of known Hp type were incorporated in all analyses. The type for these controls was determined by two separate methods, including the one described above, with 100% match. Fig. 1 provides a representative example of the Hp typing methods developed here, showing both these controls and examples of aSAH patients of all Hp types.
Statistical Analyses.
All statistical analyses were performed by a biostatistician using the R statistical software package (V.3.0.2). To compare outcomes across the Hp phenotype groups, logistic regression was used for the dichotomous outcomes (DCI and mortality), linear regression for the continuous scale outcomes (global CV, GOSE, and mRS), and negative binomial regression for the count outcomes (number of vessels with a specific CV value). The logistic regression models included only age as a covariate due to a limited number of patients with DCI and mortality in each of the Hp phenotype groups. For the linear regression and negative binomial regression multivariate models, age, GCS, WFNS, Fisher grade, Hunt–Hess grade, aneurysm size, and treatment type were included as covariates. The Hp2-2 group was compared with the Hp1-1/2-1 group because of a relatively small number of patients in the Hp1-1 group (n = 11) and a similar functional profile for Hp1-1 and Hp2-1 (12). A two-sided P value less than 0.05 was considered significant for all analyses.
Supplementary Material
Acknowledgments
We thank the study participants who made this work possible by providing biological samples and clinical data. We also thank Dr. Stephen B. Lewis for providing the prospectively collected samples and Sonisha Warren and Adrienne Royster for helping to maintain and collect the prospectively collected and biorepository samples. This work was supported by a grant from the McKnight Brain Research Foundation, Brain and Spinal Cord Injury Research Trust Fund (to S.B. and S.D.), American Heart Association Fellowship 18940001 (to J.L.L.), and National Institutes of Health Grants T32 HL83810 (to J.L.L.), F31 NS086441 (to J.L.L.), and R01 NS046400 (to S.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412833112/-/DCSupplemental.
References
- 1.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369(9558):306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 2.Bederson JB, et al. American Heart Association Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 3.Kantor E, et al. Haptoglobin genotype and functional outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2014;120(2):386–390. doi: 10.3171/2013.10.JNS13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galea J, et al. The intrathecal CD163-haptoglobin-hemoglobin scavenging system in subarachnoid hemorrhage. J Neurochem. 2012;121(5):785–792. doi: 10.1111/j.1471-4159.2012.07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr KR, Zuckerman SL, Mocco J. Inflammation, cerebral vasospasm, and evolving theories of delayed cerebral ischemia. Neurol Res Int. 2013;2013:506–584. doi: 10.1155/2013/506584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macdonald RL, Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke. 1991;22(8):971–982. doi: 10.1161/01.str.22.8.971. [DOI] [PubMed] [Google Scholar]
- 7.Mendez NV, et al. Clinical implications of bilirubin-associated neuroprotection and neurotoxicity. Int J Clin Anesthesiol. 2013;1(2):1013. [PMC free article] [PubMed] [Google Scholar]
- 8.Levy AP, et al. Haptoglobin: Basic and clinical aspects. Antioxid Redox Signal. 2010;12(2):293–304. doi: 10.1089/ars.2009.2793. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S, et al. Haptoglobin alters oxygenation and oxidation of hemoglobin and decreases propagation of peroxide-induced oxidative reactions. Free Radic Biol Med. 2012;53(6):1317–1326. doi: 10.1016/j.freeradbiomed.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Buehler PW, et al. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood. 2009;113(11):2578–2586. doi: 10.1182/blood-2008-08-174466. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen JH, Etzerodt A, Svendsen P, Moestrup SK. The haptoglobin-CD163-heme oxygenase-1 pathway for hemoglobin scavenging. Oxid Med Cell Longev. 2013;2013:523652. doi: 10.1155/2013/523652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42(10):1589–1600. [PubMed] [Google Scholar]
- 13.Ohnishi H, et al. Haptoglobin phenotype predicts cerebral vasospasm and clinical deterioration after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2013;22(4):520–526. doi: 10.1016/j.jstrokecerebrovasdis.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Borsody M, Burke A, Coplin W, Miller-Lotan R, Levy A. Haptoglobin and the development of cerebral artery vasospasm after subarachnoid hemorrhage. Neurology. 2006;66(5):634–640. doi: 10.1212/01.wnl.0000200781.62172.1d. [DOI] [PubMed] [Google Scholar]
- 15.Budohoski KP, et al. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2014;85(12):1343–1353. doi: 10.1136/jnnp-2014-307711. [DOI] [PubMed] [Google Scholar]
- 16.Ayer RE, Zhang JH. Oxidative stress in subarachnoid haemorrhage: Significance in acute brain injury and vasospasm. Acta Neurochir Suppl (Wien) 2008;104(Suppl):33–41. doi: 10.1007/978-3-211-75718-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldenstein H, Levy NS, Levy AP. Haptoglobin genotype and its role in determining heme-iron mediated vascular disease. Pharmacol Res. 2012;66(1):1–6. doi: 10.1016/j.phrs.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalet-Litman S, Moreno PR, Levy AP. The haptoglobin 2-2 genotype is associated with increased redox active hemoglobin derived iron in the atherosclerotic plaque. Atherosclerosis. 2010;209(1):28–31. doi: 10.1016/j.atherosclerosis.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asleh R, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003;92(11):1193–1200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson SD, Rosen DS, Bardo D, Macdonald RL. 2010. Arterial diameters on catheter and computed tomographic angiography. World Neurosurg 73(3):165–173; discussion e125. [DOI] [PubMed]
- 21.Yoon DY, Choi CS, Kim KH, Cho BM. Multidetector-row CT angiography of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: Comparison of volume-rendered images and digital subtraction angiography. AJNR Am J Neuroradiol. 2006;27(2):370–377. [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhary SR, et al. Prospective evaluation of multidetector-row CT angiography for the diagnosis of vasospasm following subarachnoid hemorrhage: A comparison with digital subtraction angiography. Cerebrovasc Dis. 2008;25(1-2):144–150. doi: 10.1159/000112325. [DOI] [PubMed] [Google Scholar]
- 23.Dhar R, et al. Relationship between angiographic vasospasm and regional hypoperfusion in aneurysmal subarachnoid hemorrhage. Stroke. 2012;43(7):1788–1794. doi: 10.1161/STROKEAHA.111.646836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tekle WG, et al. High risk of new episode of symptomatic vasospasm in unaffected arteries in subarachnoid hemorrhage patients receiving targeted endovascular treatment for symptomatic focal vasospasm. Neurocrit Care. 2014;20(3):399–405. doi: 10.1007/s12028-013-9825-2. [DOI] [PubMed] [Google Scholar]
- 25.Magge SN, et al. Association of a younger age with an increased risk of angiographic and symptomatic vasospasms following subarachnoid hemorrhage. J Neurosurg. 2010;112(6):1208–1215. doi: 10.3171/2009.9.JNS081670. [DOI] [PubMed] [Google Scholar]
- 26.Charpentier C, et al. Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke. 1999;30(7):1402–1408. doi: 10.1161/01.str.30.7.1402. [DOI] [PubMed] [Google Scholar]
- 27.Kale SP, et al. Age-associated vasospasm in aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2013;22(1):22–27. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y, Zuckerman SL, Mocco J. Current controversies in the prediction, diagnosis, and management of cerebral vasospasm: Where do we stand? Neurol Res Int. 2013;2013:373–458. doi: 10.1155/2013/373458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kistler JP, et al. The relation of cerebral vasospasm to the extent and location of subarachnoid blood visualized by CT scan: A prospective study. Neurology. 1983;33(4):424–436. doi: 10.1212/wnl.33.4.424. [DOI] [PubMed] [Google Scholar]
- 31.Dumont AS, et al. Endovascular treatment or neurosurgical clipping of ruptured intracranial aneurysms: Effect on angiographic vasospasm, delayed ischemic neurological deficit, cerebral infarction, and clinical outcome. Stroke. 2010;41(11):2519–2524. doi: 10.1161/STROKEAHA.110.579383. [DOI] [PubMed] [Google Scholar]
- 32.Gross BA, Rosalind Lai PM, Frerichs KU, Du R. Treatment modality and vasospasm after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2013;82(6):e725–e730. doi: 10.1016/j.wneu.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Macdonald RL, Rosengart A, Huo D, Karrison T. Factors associated with the development of vasospasm after planned surgical treatment of aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;99(4):644–652. doi: 10.3171/jns.2003.99.4.0644. [DOI] [PubMed] [Google Scholar]
- 34.Holling M, et al. Prognostic value of histopathological findings in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2009;110(3):487–491. doi: 10.3171/2008.8.JNS08789. [DOI] [PubMed] [Google Scholar]
- 35.de Oliveira JG, et al. 2007. Comparison between clipping and coiling on the incidence of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Neurosurg Rev 30(1):22–30; discussion 30–31.
- 36.Jones J, et al. 2014. Cerebral vasospasm patterns following aneurysmal subarachnoid hemorrhage: An angiographic study comparing coils with clips. J Neurointerv Surg, 10.1136/neurintsurg-2014-011374.
- 37.Jaja BN, et al. Racial/ethnic differences in inpatient mortality and use of institutional postacute care following subarachnoid hemorrhage. J Neurosurg. 2013;119(6):1627–1632. doi: 10.3171/2013.7.JNS13544. [DOI] [PubMed] [Google Scholar]
- 38.Beutler E, Gelbart T, Lee P. Haptoglobin polymorphism and iron homeostasis. Clin Chem. 2002;48(12):2232–2235. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.