Significance
In molecular clouds out of which stars and planetary systems form, simple solid-state molecules made in large part of H2O, CO, CO2, CH3OH, and NH3 are abundantly present. In these environments, energetic and thermal processes on these ices, which can be simulated in the laboratory, lead to complex organic matter. Possibly at the origin of the organic matter in our Solar System and incorporated into planetesimals, this material may be considered as a potential source for prebiotic chemistry on telluric planets, following a process that may be quite universal. The composition of these laboratory-evolved ices includes potentially prebiotic species such as amino acids and, as presented in this paper, aldehydes and sugars.
Keywords: glyceraldehyde, glycolaldehyde, astrochemistry, precometary ices, prebiotic evolution
Abstract
Evolved interstellar ices observed in dense protostellar molecular clouds may arguably be considered as part of precometary materials that will later fall on primitive telluric planets, bringing a wealth of complex organic compounds. In our laboratory, experiments reproducing the photo/thermochemical evolution of these ices are routinely performed. Following previous amino acid identifications in the resulting room temperature organic residues, we have searched for a different family of molecules of potential prebiotic interest. Using multidimensional gas chromatography coupled to time-of-flight mass spectrometry, we have detected 10 aldehydes, including the sugar-related glycolaldehyde and glyceraldehyde—two species considered as key prebiotic intermediates in the first steps toward the synthesis of ribonucleotides in a planetary environment. The presence of ammonia in water and methanol ice mixtures appears essential for the recovery of these aldehydes in the refractory organic residue at room temperature, although these products are free of nitrogen. We finally point out the importance of detecting aldehydes and sugars in extraterrestrial environments, in the gas phase of hot molecular clouds, and, more importantly, in comets and in primitive meteorites that have most probably seeded the Earth with organic material as early as 4.2 billion years ago.
In dense molecular clouds, interstellar ices are by far the most abundant molecular fraction in the solid state compared with the gas-phase molecules. They are observed in the midinfrared range around protostars, from which planets, but also much debris, such as comets and asteroids, eventually form. Their composition is dominated by H2O, followed by CO, CO2, CH3OH, NH3, CH4, and some less abundant molecules, although their respective abundances can vary from one (type of) source to another (1). Ices are subjected to various energetic processes such as cosmic rays and UV irradiation (2) as well as thermal reactions, which initiate a rich solid-state chemistry from diffusion within the bulk of reactive species such as ions and radicals, which ultimately recombine to form new and more complex molecules. This molecular complexity is difficult to trace in astrophysical settings and to characterize in the solid state by in situ infrared spectroscopy alone because of the presence of the dominant simple ice species as well as the deep and broad silicate feature along the line of sight that hide minor features expected from complex molecules. However, in warmer regions such as hot molecular cores, icy mantles can sublimate, and complex molecules become more easily detectable and identifiable by radio astronomy due to their rotational spectra (3). Detected gas-phase molecules are thus more complex than those observed in the solid phase, but models show that an important initial solid-state (ice) chemistry is usually required to explain the measured abundances, which cannot result from gas-phase reactions alone (4, 5).
Laboratory experiments that simulate the photochemical evolution of simple interstellar ices (including C, H, O, and N elements) lead to the production of a complex semirefractory organic residue after warming up to room temperature (6, 7), which is often considered as an analog of precometary matter. Such residues display a macromolecular structure, including a large variety of chemical functions such as alcohols, amines, amides, esters, carboxylic acids, etc. (8, 9). Among the numerous individual molecules detected in these residues, hexamethylenetetramine (HMT) is a dominant species (10–13), although it seems not to have any direct prebiotic implication (14). On the other hand, several molecules of potential prebiotic interest have been found, such as amino and diamino acids (14–18), hydantoin (19), urea and glycolic acid (6, 20), lipid precursors (21), quinones when polycyclic aromatic hydrocarbons are irradiated in water ice (22), and even nucleobases—however, only when pyrimidine is added to the initial ice mixture (23–25). The presence of sugars, in particular those included in nucleotides that are the building blocks of nucleic acids, has never been reported in these residues.
In this study, we report the first to our knowledge detection of aldehydes in room temperature organic residues, including glycolaldehyde and the chiral glyceraldehyde. We discuss their potential implications for prebiotic chemistry within an astrophysical scenario that emphasizes the central role of extraterrestrial ice photo/thermochemistry as an ubiquitous phenomenon in protostellar media and protoplanetary disk environments (26).
Results
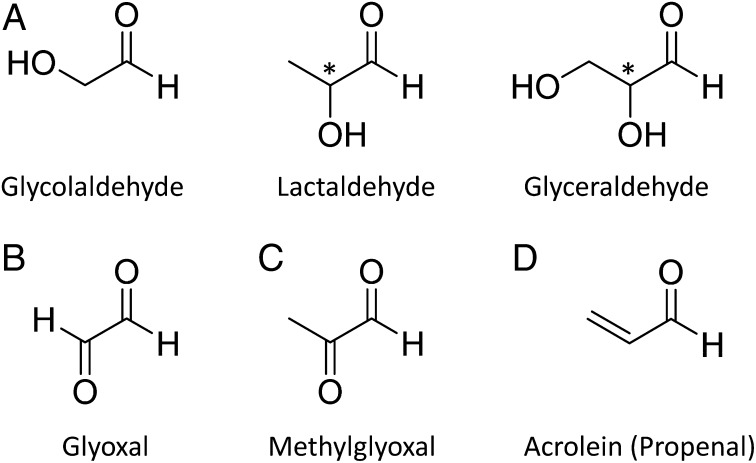
Ten different aldehydes, ranging from one to four carbon atoms (Table 1), were detected in the organic residue resulting from the processing of an initial H2O:13CH3OH:NH3 (12:3.5:1) ice mixture: formaldehyde (H2CO), glycolaldehyde (CH2OHCHO), acetaldehyde (CH3CHO), propanal (CH3CH2CHO), propenal (CH2CHCHO), lactaldehyde (CH3CHOHCHO), glyceraldehyde (CH2OHCHOHCHO), glyoxal (OCHCHO), methylglyoxal [CH3C(O)CHO], and butyraldehyde [CH3(CH2)2CHO]. Our analysis of aldehydes in the organic residues uses a definitive means of detection, 2D gas chromatography, coupled to time-of-flight mass spectrometry (GC×GC–TOFMS). All of these aldehydes—some of them exhibiting additional functional groups (Fig. 1)—were identified as their O-pentafluorobenzyl oxime (PFBO) and O-pentafluorobenzyl oxime-trimethylsilyl ether (PFBO-TMS) derivatives, respectively, using aqueous-phase derivatization with O-(pentafluorobenzyl)hydroxylamine (PFBHA). Nine out of ten aldehydes were identified by their mass fragmentation patterns, along with the chromatographic elution time in both dimensions of individual PFBHA-TMS–reacted authentic standard injections. Due to the lack of a lactaldehyde reference standard, the structural elucidation of lactaldehyde is solely based on the interpretation of its mass spectrum and its predicted retention time.
Table 1.
Aldehydes and sugar-related molecules identified in simulated precometary organic residues
| MS fragmentation/13C sample | MS fragmentation/12C standard | |||||||
| #Ca | Compound | Rt1b [min] | Rt2c [sec] | [M+•] | Other important ions, m/z | [M+•] | Other important ions, m/z | |
| 1 | Formaldehyde | 17.08 | 1.80 | 226d | 196, 181 | 225 | 195, 181 | |
| 2 | (Z)-Acetaldehyde | 20.35 | 1.94 | 241d | 211, 181 | 239 | 209, 181 | |
| (E)-Acetaldehyde | 21.20 | 1.92 | 241d | 211, 181 | 239 | 209, 181 | ||
| (Z)-Glycolaldehyde | 41.81 | 2.24 | 329e | 314, 181, 132, 104, 73 | 327 | 312, 181, 130, 103, 73 | ||
| (E)-Glycolaldehyde | 42.14 | 2.32 | 329e | 314, 181, 132, 104, 73 | 327 | 312, 181, 130, 103, 73 | ||
| (Z)-Glyoxal | 72.12 | 5.21 | 450f | 420, 255, 253, 237, 181 | 448 | 418, 253, 251, 235, 181 | ||
| (E)-Glyoxal | 74.54 | 5.14 | 450f | 420, 255, 253, 237, 181 | 448 | 418, 253, 251, 235, 181 | ||
| 3 | (Z)-Propanal | 25.49 | 1.94 | 256d | 239, 226, 239, 181 | 253 | 236, 223, 181 | |
| (E)-Propanal | 25.99 | 1.94 | 256d | 239, 226, 181 | 253 | 236, 223, 181 | ||
| (E,Z)-Propenal | 25.98 | 2.20 | 254d | 237, 224, 181 | 251 | 234, 221, 181 | ||
| (E,Z)-Propenal | 26.66 | 2.33 | 254d | 237, 224, 181 | 251 | 234, 221, 181 | ||
| (Z) Lactaldehyde | 46.39 | 2.54 | 344e | 329, 181, 73 | ||||
| (E) Lactaldehyde | 46.81 | 2.54 | 344e | 329, 181, 73 | ||||
| (Z) Glyceraldehyde | 51.47 | 2.55 | 431g | 417, 328, 251, 220, 181, 147, 104, 73 | 429 | 414, 326, 248, 218, 181, 147, 103, 73 | ||
| (E) Glyceraldehyde | 52.89 | 2.44 | 431g | 417, 328, 251, 220, 181, 147, 104, 73 | 429 | 414, 326, 248, 218, 181, 147, 103, 73 | ||
| (Z)-Methylglyoxal | 71.12 | 3.84 | 465f | 435, 284, 268, 181 | 463 | 432, 281, 265, 181 | ||
| (E)-Methylglyoxal | 74.54 | 4.14 | 465f | 435, 284, 268, 181 | 462 | 432, 281, 265, 181 | ||
| 4 | (Z) Butyraldehyde | 31.65 | 1.99 | 271d | 241h, 181 | 267 | 239f, 181 | |
| (E) Butyraldehyde | 31.74 | 2.04 | 271d | 241h, 181 | 267 | 239f, 181 | ||
Data were obtained from a VUV-irradiated ice mixture at 78 K containing water, 13C-labeled methanol, and ammonia, H2O:13CH3OH:NH3, in molar composition of 12:3.5:1. After water extraction of the residue at room temperature, the aldehydes were derivatized to form 1-(O-pentafluorobenzyl) oxime derivatives and identified by enantioselective GC×GC–TOFMS analysis.
Quantity of carbon atoms. bGC×GC retention time, first dimension. cGC×GC retention time, second dimension. dMolecular ion m/z value of 1-(O-pentafluorobenzyl) oxime (PFBO) derivatives. eMolecular ion m/z value of PFBO trimethylsilyl ether derivatives. fMolecular ion m/z value of di-PFBO derivatives. gMolecular ion m/z value of PFBO-bis(trimethylsilyl) ether derivatives. hMcLafferty rearrangement.
Fig. 1.
Selected aldehydes identified at room temperature in simulated precometary organic residues: (A) hydroxyaldehydes, (B) dialdehyde, (C) ketoaldehyde, and (D) an unsaturated aldehyde.
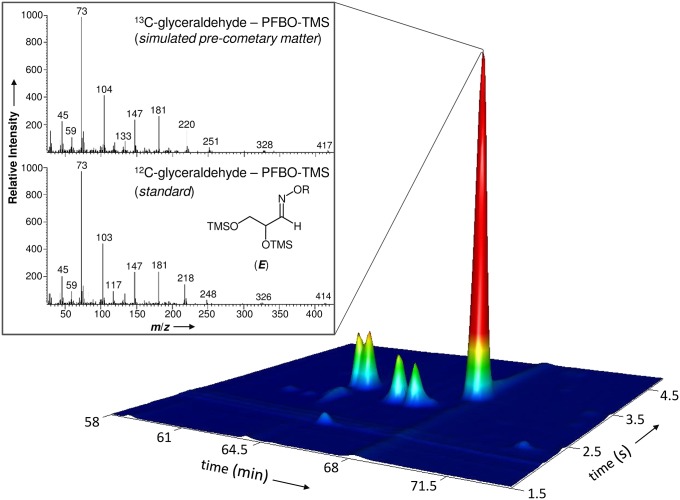
For glyceraldehyde, the mass spectrum of the detected molecule is displayed just above the mass spectrum of the corresponding standard (Fig. 2). We remind the reader here that organic residues were made with 13C only, whereas standards are composed of 12C. Thus molecular ions and fragments containing one or more carbon atom(s) are shifted accordingly and lead to unambiguous detections.
Fig. 2.
Glyceraldehyde detected in simulated precometary organic residues. Identification of glyceraldehyde as O-pentafluorobenzyl (R) oxime bis(trimethylsilyl) ether (PFBO-TMS) in laboratory organic residues using multidimensional gas chromatography. The corresponding external glyceraldehyde standard shows identical retention times and 12C isotopic signatures in its mass spectra. The mass fragmentation reveals that glyceraldehyde formed in the residue is entirely composed of 13C-isotopes provided by the 13CH3OH reactant present in the original ice mixture. Note that all aldehyde derivatives, except formaldehyde PFBO, can form syn (Z) and anti (E) stereoisomers of the oximes because the carbon nitrogen double bond (imine) formed during derivatization prevents rotation.
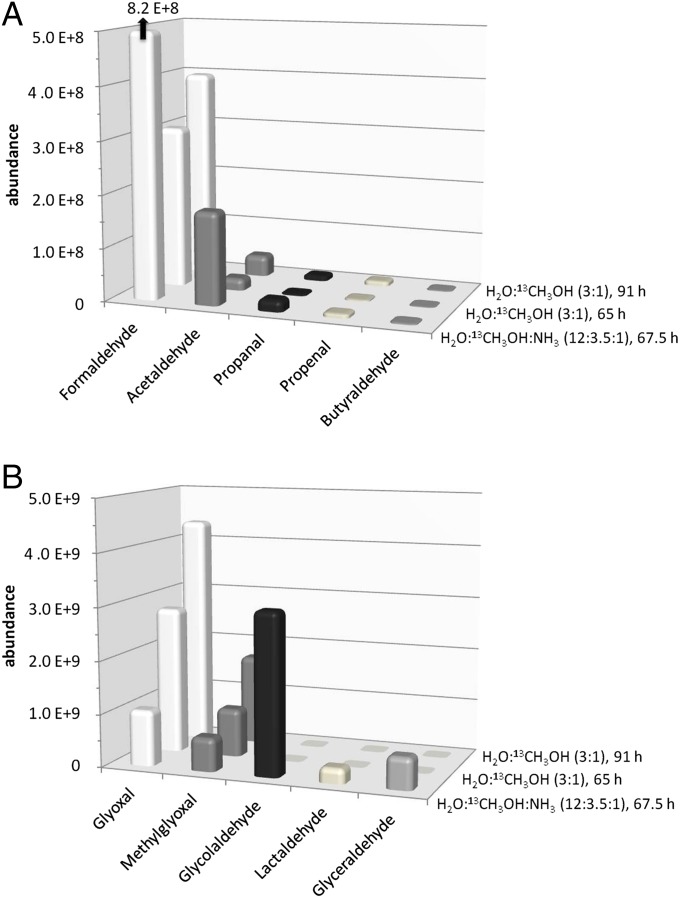
In the homologous series of aldehydes detected in the organic residues, the lower-molecular-weight members are usually more abundant (Fig. 3)—the exception here of formaldehyde may be due to its higher volatility and its gradual loss while heating the vacuum chamber up to room temperature after forming in the ices. The abundances of aldehydes (Fig. 3A) and hydroxyaldehydes (Fig. 3B) are decreasing with increasing carbon number, as previously observed for amino acids in such ice simulation experiments (13, 17). Three different samples were analyzed in this study: one containing ammonia (H2O:13CH3OH:NH3 = 12:3.5:1, with a simultaneous deposition and irradiation time of 67.5 h) and two without ammonia (H2O:13CH3OH = 3:1, one made during 65 h and one during 91 h, resulting in a higher quantity of organic residue for the second one). Relative aldehyde abundances for the two samples without ammonia follow the trend of the total experiment duration (the global amount of organic residue), although not strictly proportional to it (Fig. 3). Interestingly, the abundances of the simple aldehydes (formaldehyde, acetaldehyde, propanal, and butyraldehyde) are lower in the absence of ammonia in the initial ice mixture. Even more striking, the hydroxyaldehydes, including glycolaldehyde, lactaldehyde, and glyceraldehyde, are completely absent in the samples without ammonia (Figs. 3 and 4). In contrast, the abundances of the dialdehydes, glyoxal and methylglyoxal, are reduced in the presence of ammonia, an effect that we do not explain at this stage.
Fig. 3.
Abundances of (A) aldehydes and (B) dialdehydes, keto- and hydroxyaldehydes in the room temperature samples with and without ammonia in the initial ice mixtures.
Fig. 4.
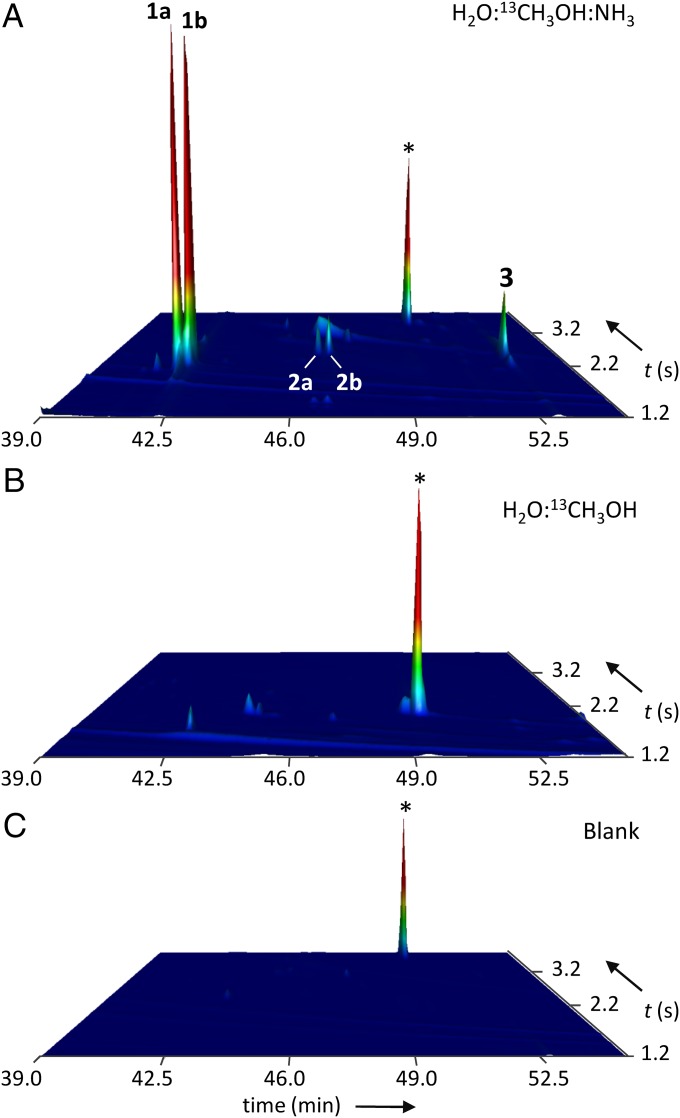
Nitrogen allows aldehyde retention at room temperature in simulated precometary organic residues. (A) Identification of glycolaldehyde (1a, b), lactaldehyde (2a, b), and (E)-glyceraldehyde (3) as O-pentafluorobenzyl oxime-trimethylsilylether derivatives from a VUV-irradiated ice mixture containing ammonia. Double peaks occur because two isomers are formed. (B) Complex aldehydes are absent in samples without ammonia in the initial ice mixture. Peaks marked with an asterisk are interferences caused by the derivatization protocol revealed by running (C) a blank sample of the derivatization reagents.
Astrophysical Discussion
Six out of the ten molecules in Table 1 have been detected in extraterrestrial environments, which include protostellar media but also Solar System objects such as comets and asteroids, including meteorites on the Earth’s surface. Formaldehyde is actually ubiquitous, observed in many places of the interstellar medium (3, 27), but also in the gas originating from comets (28) and in meteorites (29). Acetaldehyde, propanal (propionaldehyde), and propenal (acraldehyde) are detected in the hot molecular core Sagittarius B2 (30, 31), the well-known “Large Molecular Heimat” (LMH) of radio astronomers, as well as, for the first two, in meteorites (29). Butyraldehyde has been reported in the Murchison meteorite as well (29). Additionally, Cooper et al. (32) identified several sugars, sugar alcohols, and sugar acids, some of them with up to six carbon atoms, in the Murchison and Murray meteorites. Because of the prebiotic importance of sugar-related molecules, the following discussion will focus on glycolaldehyde and glyceraldehyde.
Glycolaldehyde has been detected in emission and/or in absorption by radio astronomy toward the galactic center source, Sagittarius B2(N) (33–37), in the hot molecular core, G31.41+0.31 (38), and in the solar-type protostellar binary, IRAS 16293–2422 (39). However, glycolaldehyde has never been detected in Solar System objects, for which only an upper limit could be determined in the Hale–Bopp comet (40, 41), as well as in the C/2012 F6 and C/2013 R1 comets (42). Glyceraldehyde has been searched for in the interstellar medium by Hollis et al. (31) toward Sgr B2 (N-LMH), but no lines were detected. Note, however, that aldehydes of higher molecular weights are more difficult and uncertain to search for because of the intrinsic complexity of their rotational spectra associated with the likely presence of several conformers/tautomers.
In the laboratory, only glycolaldehyde has been detected, in the ice phase. An infrared study of glycolaldehyde and its tentative identification in a proton-irradiated CO:CH3OH 100:1 ice mixture was reported in 2005 by Hudson et al. (43). In 2009, Öberg et al. (44) reported the formation of acetaldehyde and minor contributions of glycolaldehyde in UV-irradiated CH3OH-rich ice analogs. Bennett et al. (45) provided an upper limit for the formation rate of glycolaldehyde during ion bombardment of pure CH3OH ice. IR spectroscopy and mass spectrometry allowed Bennett and Kaiser (46) to establish the formation of glycolaldehyde in CH3OH:CO ices irradiated with energetic electrons.
The first formation mechanism speculated for glycolaldehyde was an interstellar equivalent of the formose reaction in solid or gas phase based on the polymerization of formaldehyde (35). Recently, Woods et al. (47, 48) considered several different reaction mechanisms in solid or gas phase, most of them previously suggested in the literature (33, 38, 46, 49, 50), and, from a theoretical point of view, studied their effectiveness at 10 K. To our knowledge, no formation mechanism in extraterrestrial conditions has been proposed for glyceraldehyde or higher-mass aldehydes, although the speculative “interstellar formose reaction” may again apply.
The favored mechanisms, involving CH3OH, H2CO, and HCO in the solid state, are applicable to our experimental protocol. Indeed, CH3OH is a component of the initial ice mixture and a major component of interstellar ices, and H2CO and HCO are some of the UV photoproducts, detected by in situ IR spectroscopy during the experiment. H2CO is observed in interstellar ices but not HCO, probably because of its instability as a radical coupled to its absorption range (1,850 cm−1), inaccessible from the ground and with very poor infrared spectroscopic data from the Infrared Space Observatory and Spitzer satellites. Moreover, observations support the idea that glycolaldehyde is sublimated into the gas phase from the grains' ice mantles (35, and references therein).
An important point to underscore here is that all of these experimental and theoretical studies are made at 10–20 K, mainly in the ice phase. Moreover, Hudson et al. (43) reported that glycolaldehyde sublimates completely after 10 min at 195 K. In our case, we have searched for the aldehydes, in the organic residues only, at 300 K. Remarkably, the samples still contain aldehydes, including glycolaldehyde, in a free form because our samples were not acid-hydrolyzed before analysis, which is the usual protocol for extracting amino acids. Indeed, the aldehydes should have been evaporated from the substrate well before reaching room temperature.
However, we did not detect the 10 aldehydes in each sample. As underlined above, organic residues made by irradiation of H2O:13CH3OH:NH3 contain all of the aldehydes. On the other hand, when NH3 was not present in the initial ice mixture (H2O:13CH3OH = 3:1), the hydroxyaldehydes glycolaldehyde, lactaldehyde, and glyceraldehyde were not detected, suggesting an essential role of NH3 for their presence in the residues. This importance of NH3 was already mentioned in the literature in similar experiments involving the photochemistry of interstellar ice analogs, for the formation of amphiphilic compounds (21) or polyoxymethylene (51, 52) within the organic residue. Although the underlying mechanism(s) in these experiments (including ours) may not be the same, they all point to the crucial role of NH3 for the formation and the final composition of the organic residues.
Finally, and independently of the exact understanding of the mechanism maintaining the aldehydes up to 300 K, one should remember that ammonia is actually present in protostellar ices (53), and aldehydes may indeed be formed and preserved on the grains' surface, as observed in our laboratory simulations, to later become incorporated in planetesimals and various parent bodies of meteorites.
Implications for Prebiotic Chemistry
Sugars are carbohydrates, components of nucleic acids (DNA, RNA). d-2-Deoxyribofuranose is the sugar subunit present in DNA, but it is currently thought that DNA was not actually the original genetic material and that our present DNA genomic composition evolved from a primordial RNA World state, with d-ribofuranose sugar subunits dictating genetic stereochemistry (54–56). However, the ultimate origin of the ribose sugar subunit essential to the RNA structure still remains unknown. Recently, experimental evidence was given, revealing that the origin of ribonucleotides could have bypassed the classical chemical synthesis and proceeded from the starting materials, glycolaldehyde and glyceraldehyde, via pentose aminooxazolines (57). These data suggest that glyceraldehyde and its derivatives were the fundamental asymmetric building blocks, from which enantiomerically enriched oligonucleotide intermediates were synthesized in a prebiotic RNA World.
The detection of glycolaldehyde and glyceraldehyde in our laboratory analogs of extraterrestrial organic residues may thus be of importance from a prebiotic point of view when considering the scenario of an exogenous delivery of organics to the early Earth’s surface by small Solar System bodies (58, 59). Furthermore, as for some meteoritic amino acids (60–62), glyceraldehyde may have been enantiomerically enriched by the same process, but with an opposite excess (d instead of l, as is the case in biological sugars and amino acids, respectively). These excesses could have originated from UV circularly polarized light irradiation, as previously suggested (for a review, see ref. 63) and recently experimentally shown by our group in the case of amino acids formed from precometary ices (64, 65), experiments in which a similar procedure to the one reported in this paper was used.
We emphasize here that such laboratory organic residues, as long as their initial ice mixture is composed of the simple molecules, H2O, CH3OH, and NH3, contain, in a single residue, a wide variety of organic molecules, including the important molecular bricks linked to the origin of life: amino acids, sugars, lipid precursors, urea, hydantoin, etc. All of these molecules could have thus simultaneously seeded individual comets and asteroids and have then been delivered at the same moment and location on the early Earth.
Finally, the search for glyceraldehyde, for which spectroscopic data exist in the millimeter range (66), should be attempted with the new Atacama Large Millimeter Array (ALMA) in the various astronomical gas-phase environments where glycolaldehyde is already detected. However, the lack of positive detection of the simplest amino acid, glycine, in the gas phase (67) underscores the lack of stability against cosmic or UV irradiation of such complex molecules in this gas phase. This was shown in laboratory experiments (68) for various amino acids in rare gas matrices, proxy for gas-phase media, and glyceraldehyde may well be similarly unstable in the gas phase of molecular clouds. Molecular complexity does need the protection offered by the solid-state environment (69). In fact, much more significant for prebiotic inference, glycolaldehyde and glyceraldehyde should be searched for in comets and in the Soluble Organic Matter (SOM) of the less possibly aqueously altered carbonaceous chondrites, such as the “Paris” meteorite (70). These proposed detections must be seen as the next step for supporting a scenario in which chemically evolved cosmic ices played a major role in the feeding of organic materials to the primitive solar nebula.
Conclusions
In this paper, we show that the UV irradiation of astrophysically relevant ice mixtures leads to the detection of a new family of molecules, the aldehydes, within the room temperature organic residues. Our results show that the formation of a large range of aldehydes in the solid phase of dense molecular clouds is likely. Among them, two are of particular importance for prebiotic processes: glycolaldehyde and glyceraldehyde are indeed chemical intermediates in ribonucleotide synthesis.
By changing the initial ice composition we observe the apparently essential role of NH3 in stabilizing light aldehydes within the refractory organic residues. This phenomenon could have been of a fundamental importance to allow these molecules to remain trapped in grains and later be incorporated into small Solar System bodies, from which meteorites found on the Earth originate. The recovery of these aldehydes in comets and in primitive chondrites may constitute a test, bridging our experimental simulations to the SOM of meteorites, linking molecular clouds’ ice chemistry to primitive materials in the solar nebula and further playing a significant role in prebiotic chemistry at the surface of telluric planets.
Finally, the reported data will be of importance for the Rosetta mission that landed on a cometary nucleus in November 2014 (71) to perform in situ measurements of cometary ices, particularly with the Cometary Sampling and Composition (COSAC) instrumentation, which contains a GC-MS device specifically designed for the characterization of organic molecules.
Materials and Methods
The experimental setup has already been described in detail elsewhere (e.g., ref. 72). In brief, it consists of a high-vacuum chamber (10−7 mbar), in which an infrared (IR) transparent MgF2 window is cooled down to 78 K. A gas mixture, previously prepared in an independent stainless steel gas line pumped down to about 5.10−6 mbar, is then injected into the chamber where it condenses on the cold MgF2 substrate to form a thin film of “ices.” These ices are, simultaneously to their deposition, irradiated by UV photons using a H2 microwave-discharge lamp (73). In our experiments the ratio of UV photons to deposited molecules is around 1. The entire experiment (deposition rate, ice mixture, and effect of UV photolysis) is monitored by IR spectroscopy.
For this study, we prepared one mixture composed of H2O, 13CH3OH, and NH3 in relative proportions 12:3.5:1, qualitatively representative of interstellar/precometary ices. We also made mixtures without ammonia (H2O:13CH3OH = 3:1), to examine the effect of a different ice mixture on the chemical composition of the final residues. Methanol, our unique source of carbon, was labeled with 13C to avoid any confusion with potential biological contamination in the handling and analysis processes of the samples. H2O (water, liquid) was purified by using a Millipore Direct Q5 system, 13CH3OH (methanol, liquid) was purchased from Aldrich (99.9% purity), and NH3 (ammonia, gas) was purchased from Messer (99.98% purity). The ratios between the components were determined by their partial pressures in the gas line, measured by an absolute pressure gauge (Baratron).
Note that the physicochemical state of the initial sample (temperature and ice composition) is used as a template of preaccretionary ices and not fully representative of interstellar conditions. At 78 K, enhanced diffusion of reactants allows for a faster chemical evolution than at 10 K without significantly affecting the nature of the organic residue, as noted by Muñoz Caro and Schutte (8). For the initial ice composition, we observe that CO and CO2 do appear during the photochemical process and are thus part of the icy molecular chemical reservoir.
After a total of 65–91 h of deposition and simultaneous irradiation, the samples were slowly warmed up to room temperature (about 0.15 K min−1), then removed from the vacuum chamber and kept under argon atmosphere in special sample holders for storage and transportation until their analysis at the Université de Nice-Sophia Antipolis, Institut de Chimie de Nice, Nice, France.
Samples were water-extracted from their substrate, derivatized, and then analyzed by 2D gas chromatography−time-of-flight mass spectrometry (GC×GC–TOFMS) using a LECO Pegasus IV D system. Supporting Information provides the detailed procedure.
Supplementary Material
Acknowledgments
We thank the Agence Nationale de la Recherche for Grant ANR-12-IS07-0006 and Consejo Nacional de Ciencia y Tecnología for Grant C001-CONACYT-ANR-188689. L.L.S.H. thanks the Centre National d'Etudes Spatiales for the continuous support of the experiments at Institut d'Astrophysique Spatiale (IAS).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418602112/-/DCSupplemental.
References
- 1.Öberg KI, et al. The Spitzer ice legacy: Ice evolution from cores to protostars. Astrophys J. 2011;740(2):109. [Google Scholar]
- 2.Shen CJ, Greenberg JM, Schutte WA, van Dishoeck EF. Cosmic ray induced explosive chemical desorption in dense clouds. Astron Astrophys. 2004;415(1):203–215. [Google Scholar]
- 3.Belloche A, Müller HSP, Menten KM, Schilke P, Comito C. Complex organic molecules in the interstellar medium: IRAM 30 m line survey of Sagittarius B2(N) and (M) Astron Astrophys. 2013;559:A47. [Google Scholar]
- 4.Garrod RT, Widicus Weaver SL, Herbst E. Complex chemistry in star-forming regions: An expanded gas-grain warm-up chemical model. Astrophys J. 2008;682(1):283–302. [Google Scholar]
- 5.Belloche A, Garrod RT, Müller HSP, Menten KM. Detection of a branched alkyl molecule in the interstellar medium: Iso-propyl cyanide. Science. 2014;345(6204):1584–1587. doi: 10.1126/science.1256678. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal VK, et al. Photochemical reactions in interstellar grains photolysis of CO, NH3, and H2O. Orig Life Evol Biosph. 1985;16(1):21–40. doi: 10.1007/BF01808047. [DOI] [PubMed] [Google Scholar]
- 7.Briggs R, et al. Comet Halley as an aggregate of interstellar dust and further evidence for the photochemical formation of organics in the interstellar medium. Orig Life Evol Biosph. 1992;22(5):287–307. doi: 10.1007/BF01810858. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz Caro GM, Schutte WA. UV-photoprocessing of interstellar ice analogs: New infrared spectroscopic results. Astron Astrophys. 2003;412:121–132. [Google Scholar]
- 9.Danger G, et al. Characterization of laboratory analogs of interstellar/cometary organic residues using very high resolution mass spectrometry. Geochim Cosmochim Acta. 2013;118:184–201. [Google Scholar]
- 10.Bernstein MP, Sandford SA, Allamandola LJ, Chang S, Scharberg MA. Organic compounds produced by photolysis of realistic interstellar and cometary ice analogs containing methanol. Astrophys J. 1995;454:327–344. [Google Scholar]
- 11.Muñoz Caro GM, Meierhenrich U, Schutte WA, Thiemann WH-P, Greenberg JM. UV-photoprocessing of interstellar ice analogs: Detection of hexamethylenetetramine-based species. Astron Astrophys. 2004;413:209–216. [Google Scholar]
- 12.Vinogradoff V, Duvernay F, Danger G, Theulé P, Chiavassa T. New insight into the formation of hexamethylenetetramine (HMT) in interstellar and cometary ice analogs. Astron Astrophys. 2011;530:A128. [Google Scholar]
- 13.Vinogradoff V, et al. Importance of thermal reactivity for hexamethylenetetramine formation from simulated interstellar ices. Astron Astrophys. 2013;551:A128. [Google Scholar]
- 14.Muñoz Caro GM, et al. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature. 2002;416(6879):403–406. doi: 10.1038/416403a. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein MP, Dworkin JP, Sandford SA, Cooper GW, Allamandola LJ. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature. 2002;416(6879):401–403. doi: 10.1038/416401a. [DOI] [PubMed] [Google Scholar]
- 16.Elsila JE, Dworkin JP, Bernstein MP, Martin MP, Sandford SA. Mechanisms of amino acid formation in interstellar ice analogs. Astrophys J. 2007;660(1):911–918. [Google Scholar]
- 17.Nuevo M, Auger G, Blanot D, d’Hendecourt L. A detailed study of the amino acids produced from the vacuum UV irradiation of interstellar ice analogs. Orig Life Evol Biosph. 2008;38(1):37–56. doi: 10.1007/s11084-007-9117-y. [DOI] [PubMed] [Google Scholar]
- 18.Meinert C, Filippi J-J, de Marcellus P, Le Sergeant d’Hendecourt L, Meierhenrich UJ. N-(2-aminoethyl)glycine and amino acids from interstellar ice analogues. Chempluschem. 2012;77(3):186–191. [Google Scholar]
- 19.de Marcellus P, Bertrand M, Nuevo M, Westall F, Le Sergeant D’Hendecourt L. Prebiotic significance of extraterrestrial ice photochemistry: Detection of hydantoin in organic residues. Astrobiology. 2011;11(9):847–854. doi: 10.1089/ast.2011.0677. [DOI] [PubMed] [Google Scholar]
- 20.Nuevo M, Bredehöft JH, Meierhenrich UJ, D’Hendecourt L, Thiemann WH-P. Urea, glycolic acid, and glycerol in an organic residue produced by ultraviolet irradiation of interstellar/pre-cometary ice analogs. Astrobiology. 2010;10(2):245–256. doi: 10.1089/ast.2009.0358. [DOI] [PubMed] [Google Scholar]
- 21.Dworkin JP, Deamer DW, Sandford SA, Allamandola LJ. Special feature— Self-assembling amphiphilic molecules: Synthesis in simulated interstellar/precometary ices. Proc Natl Acad Sci USA. 2001;98(3):815–819. doi: 10.1073/pnas.98.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein MP, et al. UV irradiation of polycyclic aromatic hydrocarbons in ices: Production of alcohols, quinones, and ethers. Science. 1999;283(5405):1135–1138. doi: 10.1126/science.283.5405.1135. [DOI] [PubMed] [Google Scholar]
- 23.Nuevo M, Milam SN, Sandford SA. Nucleobases and prebiotic molecules in organic residues produced from the ultraviolet photo-irradiation of pyrimidine in NH3 and H2O+NH3 ices. Astrobiology. 2012;12(4):295–314. doi: 10.1089/ast.2011.0726. [DOI] [PubMed] [Google Scholar]
- 24.Nuevo M, Milam SN, Sandford SA, Elsila JE, Dworkin JP. Formation of uracil from the ultraviolet photo-irradiation of pyrimidine in pure H2O ices. Astrobiology. 2009;9(7):683–695. doi: 10.1089/ast.2008.0324. [DOI] [PubMed] [Google Scholar]
- 25.Nuevo M, Materese CK, Sandford SA. The photochemistry of pyrimidine in realistic astrophysical ices and the production of nucleobases. Astrophys J. 2014;793(2):125. [Google Scholar]
- 26.Ciesla FJ, Sandford SA. Organic synthesis via irradiation and warming of ice grains in the solar nebula. Science. 2012;336(6080):452–454. doi: 10.1126/science.1217291. [DOI] [PubMed] [Google Scholar]
- 27.Snyder LE, Buhl D, Zuckerman B, Palmer P. Microwave detection of interstellar formaldehyde. Phys Rev Lett. 1969;22(13):679–681. [Google Scholar]
- 28.Snyder LE, Palmer P, de Pater I. Radio detection of formaldehyde emission from comet Halley. Astron J. 1989;97(1):246–253. [Google Scholar]
- 29.Jungclaus GA, Yuen GU, Moore CB, Lawless JG. Evidence for the presence of low molecular weight alcohols and carbonyl compounds in the Murchison meteorite. Meteoritics. 1976;11(3):231–237. [Google Scholar]
- 30.Gottlieb CA. In: Molecules in the Galactic Environment. Gordon MA, Snyder LE, editors. Wiley-Interscience; New York: 1973. pp. 181–186. [Google Scholar]
- 31.Hollis JM, Jewell PR, Lovas FJ, Remijan A, Møllendal H. Green Bank telescope detection of new interstellar aldehydes: Propenal and propanal. Astrophys J. 2004;610(1):L21–L24. [Google Scholar]
- 32.Cooper G, et al. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature. 2001;414(6866):879–883. doi: 10.1038/414879a. [DOI] [PubMed] [Google Scholar]
- 33.Halfen DT, Apponi AJ, Woolf N, Polt R, Ziurys LM. A systematic study of glycolaldehyde in Sagittarius B2(N) at 2 and 3 mm: Criteria for detecting large interstellar molecules. Astrophys J. 2006;639(1):237–245. [Google Scholar]
- 34.Hollis JM, Jewell PR, Lovas FJ, Remijan A. Green Bank telescope observations of interstellar glycolaldehyde: Low-temperature sugar. Astrophys J. 2004;613(1):L45–L48. [Google Scholar]
- 35.Hollis JM, Lovas FJ, Jewell PR. Interstellar glycolaldehyde: The first sugar. Astrophys J. 2000;540(2):L107–L110. [Google Scholar]
- 36.Hollis JM, Vogel SN, Snyder LE, Jewell PR, Lovas FJ. The spatial scale of glycolaldehyde in the galactic center. Astrophys J. 2001;554(1):L81–L85. [Google Scholar]
- 37.Requena-Torres MA, Martín-Pintado J, Martín S, Morris MR. The galactic center: The largest oxygen-bearing organic molecule repository. Astrophys J. 2008;672(1):352–360. [Google Scholar]
- 38.Beltrán MT, Codella C, Viti S, Neri R, Cesaroni R. First detection of glycolaldehyde outside the galactic center. Astrophys J. 2009;690(2):L93–L96. [Google Scholar]
- 39.Jørgensen JK, et al. Detection of the simplest sugar, glycolaldehyde, in a solar-type protostar with ALMA. Astrophys J. 2012;757(1):L4. [Google Scholar]
- 40.Crovisier J, et al. The composition of ices in comet C/1995 O1 (Hale–Bopp) from radio spectroscopy. Further results and upper limits on undetected species. Astron Astrophys. 2004;418:1141–1157. [Google Scholar]
- 41.Despois D, Biver N, Bockelée-Morvan D, Crovisier J. Observations of molecules in comets. In: Lis DC, Blake GA, Herbst E, editors. Astrochemistry: Recent Successes and Current Challenges, IAU Symposium. Cambridge Univ Press; Cambridge: 2005. pp. 469–478. [Google Scholar]
- 42.Biver N, et al. Complex organic molecules in comets C/2012 F6 (Lemmon) and C/2013 R1 (Lovejoy): Detection of ethylene glycol and formamide. Astron Astrophys. 2014;566:L5. [Google Scholar]
- 43.Hudson RL, Moore MH, Cook AM. IR characterization and radiation chemistry of glycolaldehyde and ethylene glycol ices. Adv Space Res. 2005;36(2):184–189. [Google Scholar]
- 44.Öberg KI, Garrod RT, van Dishoeck EF, Linnartz H. Formation rates of complex organics in UV irradiated CH3OH-rich ices. I. Experiments. Astron Astrophys. 2009;504(3):891–913. [Google Scholar]
- 45.Bennett CJ, Chen S-H, Sun B-J, Chang AHH, Kaiser RI. Mechanistical studies on the irradiation of methanol in extraterrestrial ices. Astrophys J. 2007;660(2):1588–1608. [Google Scholar]
- 46.Bennett CJ, Kaiser RI. On the formation of glycolaldehyde (HCOCH2OH) and methyl formate (HCOOCH3) in interstellar ice analogs. Astrophys J. 2007;661(2):899–909. [Google Scholar]
- 47.Woods PM, et al. On the formation of glycolaldehyde in dense molecular cores. Astrophys J. 2012;750(1):19. [Google Scholar]
- 48.Woods PM, et al. Glycolaldehyde formation via the dimerization of the formyl radical. Astrophys J. 2013;777(2):90. [Google Scholar]
- 49.Sorrell WH. Origin of amino acids and organic sugars in interstellar clouds. Astrophys J. 2001;555(2):L129–L132. [Google Scholar]
- 50.Charnley SB, Rodgers SD. 2005. Pathways to molecular complexity. Astrochemistry: Recent Successes and Current Challenges, IAU Symposium, eds Lis DC, Blake GA, Herbst E (Cambridge Univ Press, Cambridge), pp 237–246.
- 51.Schutte WA, Allamandola LJ, Sandford SA. An experimental study of the organic molecules produced in cometary and interstellar ice analogs by thermal formaldehyde reactions. Icarus. 1993;104(1):118–137. doi: 10.1006/icar.1993.1087. [DOI] [PubMed] [Google Scholar]
- 52.Duvernay F, Danger G, Theulé P, Chiavassa T. Formaldehyde chemistry in cometary ices: On the prospective detection of NH2CH2OH, HOCH2OH, and POM by the on-board ROSINA instrument of the Rosetta mission. Astrophys J. 2014;791(2):75. [Google Scholar]
- 53.Bottinelli S, et al. The c2d Spitzer spectroscopic survey of ices around low-mass young stellar objects. IV. NH3 and CH3OH. Astrophys J. 2010;718(3):1100–1117. [Google Scholar]
- 54.Woese C. In: The Genetic Code: the Molecular basis for Genetic Expression. Harper R, editor. Harper & Row; New York: 1967. pp. 179–195. [Google Scholar]
- 55.Crick FH. The origin of the genetic code. J Mol Biol. 1968;38(3):367–379. doi: 10.1016/0022-2836(68)90392-6. [DOI] [PubMed] [Google Scholar]
- 56.Orgel LA. Evolution of the genetic apparatus. J Mol Biol. 1968;38(3):381–393. doi: 10.1016/0022-2836(68)90393-8. [DOI] [PubMed] [Google Scholar]
- 57.Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459(7244):239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- 58.Oró J. Comets and the formation of biochemical compounds on the primitive Earth. Nature. 1961;190(4774):389–390. doi: 10.1007/BF01808302. [DOI] [PubMed] [Google Scholar]
- 59.Chyba C, Sagan C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature. 1992;355(6356):125–132. doi: 10.1038/355125a0. [DOI] [PubMed] [Google Scholar]
- 60.Cronin JR, Pizzarello S. Enantiomeric excesses in meteoritic amino acids. Science. 1997;275(5302):951–955. doi: 10.1126/science.275.5302.951. [DOI] [PubMed] [Google Scholar]
- 61.Glavin DP, Dworkin JP. Enrichment in L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc Natl Acad Sci USA. 2009;106(14):5487–5492. doi: 10.1073/pnas.0811618106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glavin DP, et al. Unusual nonterrestrial L-proteinogenic amino acid excesses in the Tagish Lake meteorite. Meteorit Planet Sci. 2012;47(8):1347–1364. [Google Scholar]
- 63.Meinert C, et al. Photochirogenesis: Photochemical models on the absolute asymmetric formation of amino acids in interstellar space. Phys Life Rev. 2011;8(3):307–330. doi: 10.1016/j.plrev.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 64.de Marcellus P, et al. Non-racemic amino acid production by ultraviolet irradiation of achiral interstellar ice analogs with circularly polarized light. Astrophys J Letters. 2011;727(2):L27. doi: 10.1016/j.plrev.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Modica P, et al. Enantiomeric excesses induced in amino acids by ultraviolet circularly polarized light irradiation of extraterrestrial ice analogs: A possible source of asymmetry for prebiotic chemistry. Astrophys J. 2014;788(1):79–89. [Google Scholar]
- 66.Lovas FJ, Suenram RD, Plusquellic DF, Møllendal H. The microwave spectrum of the C 3 sugars: Glyceraldehyde and 1,3-dihydroxy-2-propanone and the dehydration product 2-hydroxy-2-propen-1-al. J Mol Spectrosc. 2003;222(2):263–272. [Google Scholar]
- 67.Snyder LE, et al. A rigorous attempt to verify interstellar glycine. Astrophys J. 2005;619(2):914–930. [Google Scholar]
- 68.Ehrenfreund P, Bernstein MP, Dworkin JP, Sandford SA, Allamandola LJ. The photostability of amino acids in space. Astrophys J. 2001;550(1):L95–L99. [Google Scholar]
- 69.Le Sergeant d’Hendecourt L. Molecular complexity in astrophysical environments: From astrochemistry to “astrobiology“? Chemistry in Astrophysical Media, AstrOHP 2010, EPJ Web Conf. 2011;18 6001. [Google Scholar]
- 70.Hewins RH, et al. The Paris meteorite, the least altered CM chondrite so far. Geochim Cosmochim Acta. 2014;124:190–222. [Google Scholar]
- 71.Meierhenrich UJ. Comets and their Origin. Wiley-VCH; Weinheim, Germany: 2015. [Google Scholar]
- 72.Nuevo M, et al. Enantiomeric separation of complex organic molecules produced from irradiation of interstellar/circumstellar ice analogs. Adv Space Res. 2007;39(3):400–404. [Google Scholar]
- 73.Chen Y-J, et al. Vacuum ultraviolet emission spectrum measurement of a microwave-discharge hydrogen-flow lamp in several configurations: Application to photodesorption of CO ice. Astrophys J. 2014;781(1):15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.