Significance
Patients suffering from hematologic malignancies might have no alternative to eliminating their morbid immune system. Although lethal irradiation or chemotherapy largely eradicates tumor cells, it also destroys the hematopoietic system, which necessitates transplantation of bone marrow stem cells from a healthy donor. T cells within the graft attack remaining tumor cells. This graft-versus-leukemia (GvL) effect is highly appreciated, because it prevents tumor relapses. Coevally, however, T cells attack nonmalignant tissue of the host with life-threating consequences called graft-versus-host disease (GvHD). We found that absence of the transcription factors NFAT (nuclear factor of activated T cells) in T cells prevents harmful GvHD, but preserves the valuable GvL. Therefore, instead of broad immune suppression, we propose to target NFAT specifically during allogenic stem cell transplantation.
Keywords: hematopoietic stem cell transplantation, graft-versus-host disease, NFAT, graft-versus-leukemia effect, regulatory T cell
Abstract
Graft-versus-host disease (GvHD) is a life-threatening immunological complication after allogenic hematopoietic stem cell transplantation (allo-HCT). The intrinsic graft-versus-leukemia (GvL) effect, however, is the desirable curative benefit. Patients with acute GvHD are treated with cyclosporine A (CsA) or tacrolimus (FK506), which not only often causes severe adverse effects, but also interferes with the anticipated GvL. Both drugs inhibit calcineurin, thus at first suppressing activation of the nuclear factor of activated T cells (NFAT). Therefore, we explored the specific contribution of individual NFAT factors in donor T cells in animal models of GvHD and GvL. Ablation of NFAT1, NFAT2, or a combination of both resulted in ameliorated GvHD, due to reduced proliferation, target tissue homing, and impaired effector function of allogenic donor T cells. In contrast, the frequency of Foxp3+ regulatory T (Treg) cells was increased and NFAT-deficient Tregs were fully protective in GvHD. CD8+ T-cell recall response and, importantly, the beneficial antitumor activity were largely preserved in NFAT-deficient effector T cells. Thus, specific inhibition of NFAT opens an avenue for an advanced therapy of GvHD maintaining protective GvL.
Allogenic hematopoietic stem cell transplantation (allo-HCT) is an established therapy for the treatment of malignant diseases such as leukemia or lymphoma if patients are at high risk of relapses or refractory to conventional chemotherapy. However, its efficacy is curtailed by graft-versus-host disease (GvHD) (1, 2). Immunological incompatibility, so called MHC or HLA mismatch, between donor T cells and host tissue, triggers activation and expansion of allogenic T lymphocytes subsequently attacking the recipient’s body. The same donor T cells, however, protect against tumor relapse by mediating the desirable graft-versus-leukemia (GvL) effect (3–6). Therefore, reducing GvHD immunopathology while promoting beneficial GvL activity has become a major challenge in treating patients following allo-HCT (6).
Patients with acute GvHD are treated with cyclosporine A (CsA) or tacrolimus (FK506), although often causing severe adverse effects and interfering with GvL (7). Both drugs inhibit the calcium/calcineurin pathway and suppress activation of the nuclear factor of activated T cells (NFAT) transcription factors (8). NFAT proteins compose a unique family of calcium/calcineurin-regulated transcription factors with four genuine members: NFAT1 (NFATc2), NFAT2 (NFATc1), NFAT3 (NFATc4), and NFAT4 (NFATc3) (9). They were first described as transcription factors regulating T-cell response upon antigen receptor engagement (10). However, expression and activation of NFAT varies among different cell types and its function depends on cellular context and the cooperation with other transcription factors. For instance, whereas conventional T cells (Tcon) depend largely on NFAT (mainly NFAT1 and NFAT2) to achieve effector functions, Foxp3+ regulatory T cells (Treg) do not require high levels of NFAT for immune suppression (11, 12).
Numerous experimental animal models as well as preliminary clinical studies support the promising concept that Tregs mitigate GvHD, coevally maintaining GvL activity (13–16). Regrettably, methodologies for isolation of pure and sufficient numbers of donor Tregs or for their expansion in vitro are costly and inefficient, whereas in vitro expanded Tregs might even perturb their suppressive capacities (17–19). Because of the essential role of NFAT for Tcons, but not Tregs (11, 12, 20), we studied the specific contribution of individual NFAT family members to the immune pathogenesis of GvHD and, vice versa, their impact on antitumor activity of donor T cells. Allogenic donor T cells deficient in NFAT showed not only reduced proliferation, target tissue homing, and impaired effector function, but in contrast, increased Foxp3+ Treg frequencies following allo-HCT. Importantly, NFAT-deficient donor-derived Tregs were fully suppressive and protected from lethal acute GvHD. Nevertheless, antitumor activity of NFAT-deficient lymphocytes was well maintained after allo-HCT. Hence, these results highlighted NFAT factors as superior targets to prevent lethal GvHD while maintaining GvL activity.
Results
NFAT-Deficient T Cells Show Impaired Expansion and Target Organ Infiltration After Allo-HCT.
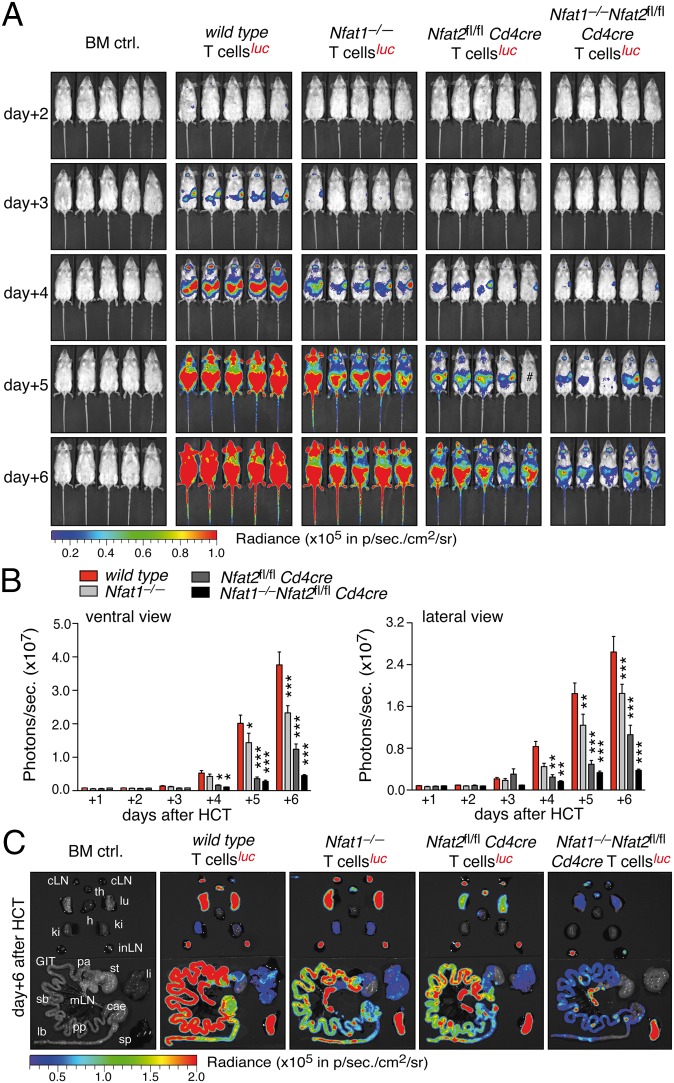
To investigate the role of individual NFAT factors in donor T cells in GvHD, we used an established murine disease model of (major mismatch) allo-HCT (C57BL/6, H-2b into BALB/c, H-2d) (21) (Fig. S1A). Proliferation and homing of donor T cells was monitored using noninvasive bioluminescence imaging (BLI). Transfer of allogenic bone marrow (BM) completely rescued lethally irradiated mice, whereas additional transfusion of allogenic T cells caused fatal GvHD within 30 d after allo-HCT (Fig. S1B). Analyzing luciferase-transgenic (luc+) CD90.1+ donor T cells from WT, Nfat1−/−, Nfat2fl/fl Cd4cre, and Nfat1−/−Nfat2fl/fl Cd4cre (DKO) mice revealed NFAT to be required for alloantigen-driven T-cell expansion (Fig. 1 A and B and Fig. S1C). BLI signals from mice transplanted with NFAT1- or NFAT2-deficient T cells mirrored significantly reduced proliferation of luc+ T cells, whereas T cells deficient for both showed almost no expansion. Analysis of carboxyfluorescein succinimidyl ester (CFSE)-labeled CD4+ and CD8+ T cells of CD90.1+ WT or NFAT-deficient donors isolated from spleen and lymph nodes (LNs) confirmed the BLI data, i.e., less proliferation of NFAT single-deficient and almost no proliferation of double-deficient alloreactive T cells (Fig. S2). Furthermore, monitoring BLI signals in vivo or ex vivo by analyzing isolated organs revealed impaired homing of NFAT-deficient donor T cells to GvHD target tissues (Fig. 1C and Fig. S1D). Consistent with these findings, cell surface expression of α4β7 integrin and inflammation-associated adhesion and homing molecules, e.g., ligands of P- and E-selectin, were impaired (Fig. S3). These data suggested that alloreactive donor T cells functionally require NFAT for pathogenic expansion and target tissue infiltration after allo-HCT.
Fig. 1.
NFAT-deficient T cells show impaired expansion and target organ infiltration after allo-HCT. (A) Representative BLI of BALB/c mice transplanted with 1.2 × 106 luciferase+ allogenic WT, Nfat1−/−, Nfat2fl/fl Cd4cre, and DKO T cells together with 5 × 106 BM cells during the first 6 d after allo-HCT. (B) Quantification of ventral (Left) and lateral (Right) view BLI data from at least three independent experiments as shown in A; each experiment with five mice per group. (C) Representative ex vivo BLI analyses of internal organs 6 d after the transfer of 1.2 × 106 luciferase+ WT, Nfat1−/−, Nfat2fl/fl Cd4cre, and DKO T cells plus 5 × 106 BM cells. Statistical significance was calculated using two-way ANOVA (B) and Student’s t test (C); *P < 0.05, **P < 0.01, ***P < 0.001.
NFAT-Deficiency Hinders T Cells to Cause Fatal GvHD.
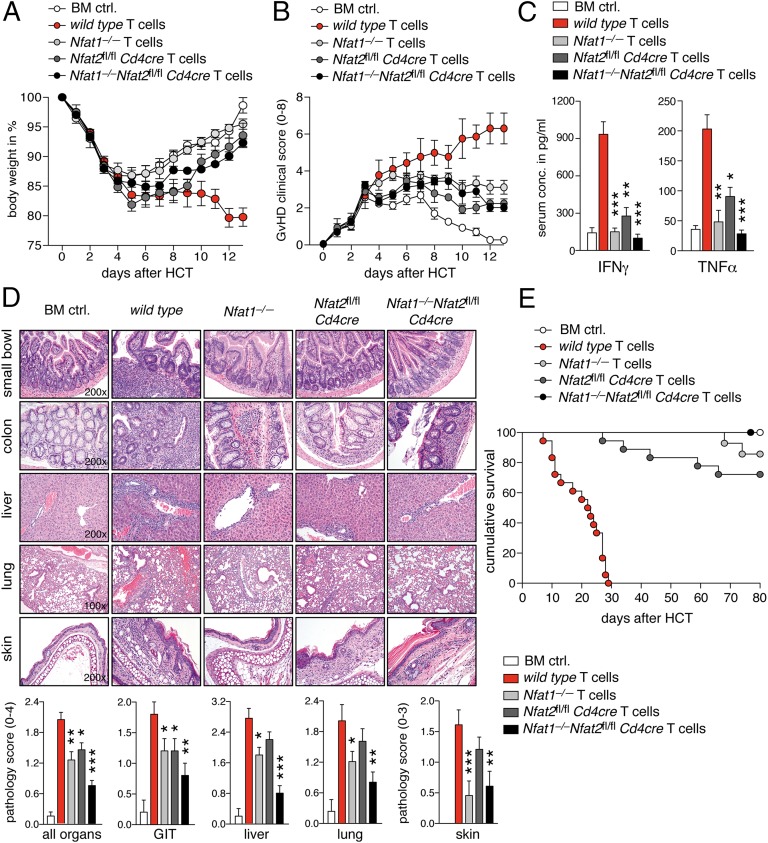
Mice suffering from acute GvHD lost weight rapidly. Conversely, mice transplanted with NFAT-deficient allogenic T cells experienced only moderate weight loss and overall milder clinical symptoms (Fig. 2 A and B). Delayed T-cell infiltration of target organs could be excluded, because long-term observation did not reveal a later onset of GvHD, but rather a consistent protection by NFAT-deficient T cells (Fig. S4 A–C). Levels of interferon (IFN)γ and tumor necrosis factor (TNF)α, cytokines critically involved in GvHD (immuno-) pathology, were significantly reduced in the sera of mice transplanted with NFAT-deficient T cells (Fig. 2C). Intracellular cytokine staining of CD90.1+CD4+ T cells confirmed that both NFAT1 and NFAT2 are involved in interleukin (IL)-2, IFNγ, and TNFα expression of allogenic donor cells during acute GvHD (Fig. S4 D–F). Histopathological scores of GvHD, assessed 12 d after transplantation, such as inflammation, apoptosis, bile duct, or vascular injury, and pneumonitis, reinforced the observation that NFAT-deficient donor T cells are less pathogenic compared with WT donor T cells after allo-HCT (Fig. 2D). Most importantly, mice transplanted with either NFAT1- or NFAT2-deficient donor T cells survived significantly longer and mice transplanted with DKO T cells were completely protected from fatal GvHD after allo-HCT (Fig. 2E). In conclusion, T cells devoid of either NFAT1 or NFAT2 cause significantly less GvHD pathology, and double deficiency even safeguards from GvHD completely.
Fig. 2.
NFAT-deficient T cells do not induce lethal GvHD. (A) Body weight changes of host BALB/c mice (n = 5) transplanted with 1.2 × 106 allogenic WT, Nfat1−/−, Nfat2fl/fl Cd4cre, or DKO T cells plus 5 × 106 BM cells. (B) Clinical GvHD scoring (score 0–8) of mice (n = 5) transplanted with allogenic WT, Nfat1−/−, Nfat2fl/fl Cd4cre, and DKO T cells and BM cells. (C) Analysis of serum IFNγ and TNFα serum 6 d after allo-HCT using cytometric bead array. Data are compiled from three independent experiments each with five mice per group. (D) Representative H&E-stained micrographs from small bowel, colon, liver, lung, and skin of mice transplanted with allogenic WT, Nfat1−/−, Nfat2fl/fl Cd4cre, and DKO T cells and BM cells 12 d after allo-HCT (Upper). Target organ pathology was assessed by an experienced hematopathologist blinded to the experimental setting (Lower); organs from 10 mice per group from two independent experiments were evaluated. (E) Survival of mice transplanted with 1.2 × 106 allogenic WT (n = 18), Nfat1−/− (n = 14), Nfat2fl/fl Cd4cre (n = 18), and DKO (n = 15) T cells plus 5 × 106 BM cells or BM cells alone (BM ctrl, n = 15). Median survival: WT: 23.5 d; BM ctrl, Nfat1−/−, Nfat2fl/fl Cd4cre, and DKO: all >80 d. Statistical significance was calculated using Student’s t test (C) and two-way ANOVA (D); *P < 0.05, **P < 0.01, ***P < 0.001.
Expansion of NFAT-Deficient Foxp3+ Tregs Ameliorates GvHD.
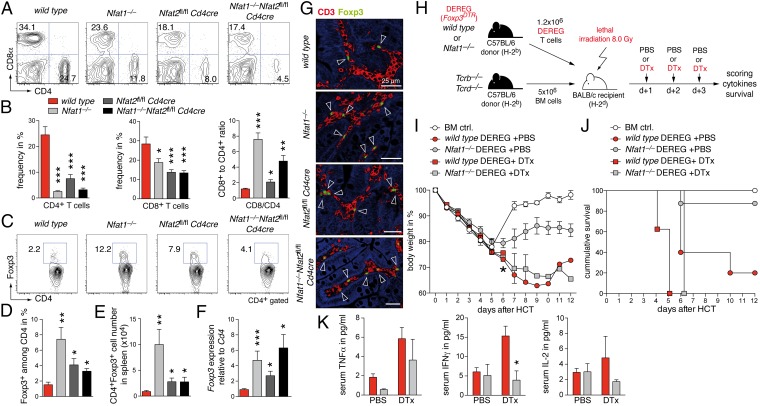
Despite reduced homing to target organs, transplanted NFAT-deficient T cells replenished cellularity of secondary lymphoid organs (Fig. S5 A and B). Spleens showed an even significantly better repopulation in the presence of NFAT-deficient T cells. Whereas both CD4+ and CD8+ T-cell numbers were decreased in mice transplanted with Nfat1−/−, Nfat2fl/fl Cd4cre, or DKO T cells (Fig. 3 A and B), the CD8-to-CD4 ratio tilted toward a CD8+ T-cell dominance (Fig. 3B). Tregs are known to protect or delay the onset of GvHD (18, 22). Accordingly, frequency of Foxp3+ Tregs among donor CD90.1+CD4+ lymphocytes was significantly increased in spleen and mesenteric lymph nodes (mLNs) (Fig. S5C). This resulted in augmented frequency, absolute number, and relative Foxp3 RNA expression within the splenic CD4+ T-cell population after transplantations with NFAT-deficient T cells (Fig. 3 C–F). Similar results were obtained for GvHD target tissues, such as small bowel and colon, when we analyzed the distribution of Foxp3+ Tregs by immunohistochemistry (Fig. 3G). Because we and others reported previously that NFAT-deficient Foxp3+ Tregs are fully suppressive in vitro and in vivo (11, 12, 20), we assessed the specific protective function of NFAT-deficient thymus-derived naturally occurring regulatory T cells (nTregs) in our GvHD model. First, we assessed the necessity of Tregs by depleting them during the course of GvHD using WT and Nfat1−/− mice backcrossed to depletion of regulatory T cell (DEREG) mice expressing a diphtheria toxin (DTx) receptor-enhanced green fluorescent protein fusion protein under the control of Foxp3 (Fig. 3H). Mice with either genotype of transplanted T cells experienced displayed augmented GvHD pathology with reduced body weight, shortened survival, and enhanced TNFα production (Fig. 3 I–K). Even so, the mice with only Nfat1−/− Tcons (after DTx treatment) were more protected from acute GvHD compared with WT cells, likewise illustrated in residual levels of IFNγ and IL-2 (Fig. 3K). An intrinsic inability to activate effector functions, however, is mostly evident in DKO Tcons (Fig. S5 D and E) (11, 12, 20). Because few Tregs can be sufficient to confer protection from GvHD (23), we cotransferred a suboptimal number (ratio Tcon:Treg = 2:1) of luc– CD90.2+CD4+CD25+ C57BL/6 nTregs (H-2b) along with C57BL/6 (H-2b) BM cells into lethally irradiated BALB/c mice (H-2d). Twenty-four hours later, C57BL/6 CD90.1+CD25– luc+ T cells (H-2b) were injected and the expansion of these Tcons was monitored using BLI (Fig. S6A). NFAT1- and NFAT2-deficient Foxp3+ nTregs were fully suppressive and restrained the expansion of luc+ WT responder T cells as effectively as WT nTregs (Fig. S6 C and D). Importantly, similarly to WT nTregs, Nfat1−/− and Nfat2fl/fl Cd4cre nTregs were able to ameliorate the clinical symptoms and lethality of GvHD (Fig. S6 B and E). Therefore, our data imply that the selective inhibition of either NFAT1 or NFAT2 is sufficient to inhibit the function of alloreactive effector cells, whereas nTregs remain operational, thereby suppressing GvHD.
Fig. 3.
Presence of NFAT-deficient Foxp3+ Tregs ameliorates GvHD. (A–G) Transfer of 1.2 × 106 allogenic WT, Nfat1−/−, Nfat2fl/fl Cd4cre, and DKO T cells analyzed 12 d after allo-HCT; quantification of data from two independent experiments each with five mice per group. (A) Frequency of splenic CD4+ and CD8+ T cells analyzed by flow cytometry. (B) Quantification of data as shown in A and calculation of the CD8-to-CD4 T-cell ratio. (C) Analysis of splenic CD4+Foxp3+ Treg frequency. (D) Quantification of splenic CD4+Foxp3+ Treg frequency as shown in C. (E) Absolute splenic CD4+Foxp3+ Treg cell number. (F) Foxp3 expression relative to Cd4 expression in spleen measured by qRT-PCR. (G) Two-color confocal microscopy of small bowel; CD3 (red), Foxp3 (green), autofluorescence (blue). (H) BALB/c mice were transplanted with 1.2 × 106 allogenic WT × DEREG (Foxp3DTR-tg) or Nfat1−/− × DEREG T cells together with 5 × 106 Tcrb−/−Tcrd−/− BM cells. Foxp3+ Treg cells were depleted by injecting 1 µg diphtheria toxin (DTx) on days 1, 2, and 3. (I) Body weight changes of host BALB/c mice transplanted with allogenic WT × DEREG (n = 9) and Nfat1−/− × DEREG T cells (n = 11) injected with PBS or DTx. Asterisk: all mice of the WT + DTx group died. (J) Survival of mice transplanted with Tcrb−/−Tcrd−/− BM cells together with allogenic WT × DEREG (n = 5) or Nfat1−/− × DEREG T cells (n = 8) injected with PBS or DTx after allo-HCT. Control mice were transplanted with Tcrb−/−Tcrd−/− BM cells alone (BM ctrl, n = 5). Median survival: WT × DEREG + PBS: 6.0 d; WT × DEREG + DTx: 5.1 d; Nfat1−/− × DEREG + DTx: 6.1 d; BM ctrl and Nfat1−/− × DEREG + PBS: >12 d. (K) Analysis of serum TNFα, IFNγ, and IL-2 6 d after allo-HCT described in H. Data are compiled from three mice per group. Statistical significance by Student’s t test (B and D–F); *P < 0.05, **P < 0.01, ***P < 0.001.
GvL Activity and Memory Response of NFAT-Deficient T Cells Are Largely Preserved.
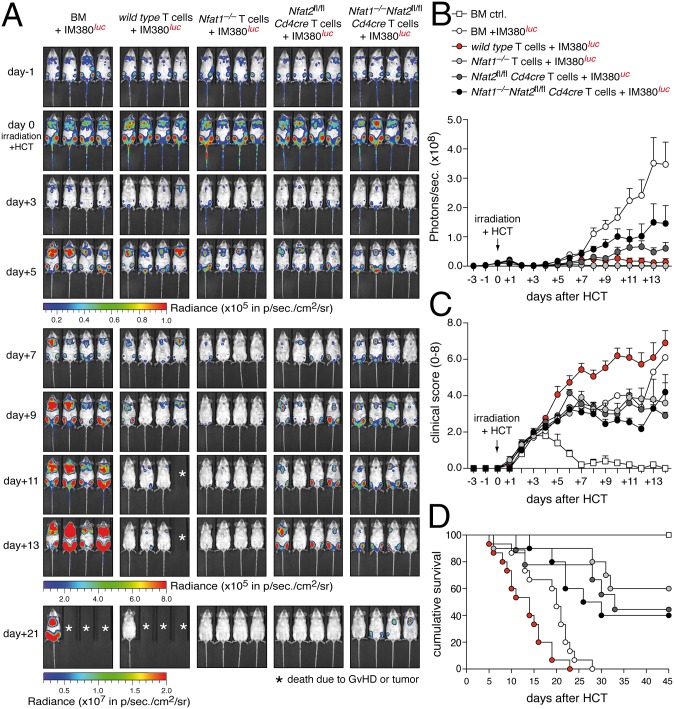
To explore the importance of NFAT factors for the GvL activity of donor T cells, we used two different models of malignant B-cell lymphoma. Aggressive IgH-myc-driven B-cell lymphomas were induced by injecting luc+ IM380 tumor cells (H-2d) i.v. into syngenic BALB/c host mice 6 d before allo-HCT (24). Mice with established tumors were lethally irradiated and transplanted with allogenic (H-2b) BM cells and luc– T cells (Fig. S7 A and B). In the absence of T-cell transfer, we observed a strong reduction in tumor burden within 2–3 d after irradiation, but massive relapse 5 d later (Fig. 4A), resulting in death of mice within 30 d after allo-HCT due to acute lymphoma (Fig. 4D). Therefore, this model mimics closely tumor relapse in patients with leukemia. Transplantation of allogenic WT T cells eradicated the majority of tumor cells (Fig. 4 A and B); however, these mice suffered from fatal GvHD and died even earlier than mice with relapsing tumors (Fig. 4 C and D). Strikingly, transfer of allogenic NFAT1-deficient T cells completely eradicated the tumor, but did not cause lethal GvHD. Similarly, although less efficient, transplantation of Nfat2fl/fl Cd4cre or DKO T cells improved the overall survival of tumor-bearing mice after allo-HCT by restraining tumor growth without inducing fatal GvHD. This was reflected in reduced weight loss (Fig. S7C) and decreased clinical indications of neoplasia and/or GvHD of mice transplanted with NFAT-deficient T cells (Fig. 4C). In a second tumor model, the transplantation of luc+ A20 leukemic B cells (H-2d) 24 h before allo-HCT revealed comparable results (Fig. S8), overall demonstrating that NFAT-deficient T cells cause less or no GvHD, but are potent facilitators of GvL. Therefore, we assessed if preservation of antileukemia responses by NFAT-deficient T cells correlated with maintained functionality of CD8+ T cells. NFAT1 was less expressed in naive CD8+ and NFAT2 less up-regulated in effector CD8+ T cells (Fig. S9A). In line, effector CD8+ T cells were less dependent than CD4+ T cells on NFAT for target gene expression (Fig. S9 B and C), and donor-derived CD90.1+CD8+ NFAT-deficient T cells expressed similar levels of granzyme B and perforin as WT control cells 6 d after allo-HCT (Fig. S10A). Only upon combined NFAT1–NFAT2 ablation, perforin expression and degranulation were somewhat reduced (Fig. S10 A and B).
Fig. 4.
NFAT-deficient T cells confer GvL effects. (A) Representative in vivo BLI of IM380 B-cell lymphoma-bearing mice with and without allo-HCT. The 2.5 × 105 IM380 luciferase+ tumor cells were injected 6 d before the transfer of 1.2 × 106 allogenic luciferase– WT, Nfat1−/−, Nfat2fl/fl Cd4cre, and DKO T cells plus 5 × 106 BM cells. Asterisk: death due to acute GvHD or tumor. (B) Quantification of ventral view BLI data from three independent experiments as shown in A with five mice per group and experiment. (C) Clinical scoring for tumor or GvHD (score 0–8) in mice inoculated with IM380 luciferase+ tumor cells and transplanted with allogenic WT, Nfat1−/−, Nfat2fl/fl Cd4cre, and DKO T cells. Data are representative of two independent experiments each with five mice per group. (D) Survival of mice inoculated with IM380 luciferase+ tumor cells and transplanted with allogenic WT (n = 15), Nfat1−/− (n = 10), Nfat2fl/fl Cd4cre (n = 10), and DKO (n = 10) T cells; combined data from two independent experiments. As a control, mice were transplanted with BM cells only (BM ctrl; n = 15). Median survival: BM + IM380: 20 d; WT + IM380: 14 d; DKO + IM380: 28 d; Nfat1−/− + IM380: 33 d; and Nfat2fl/fl Cd4cre + IM380 and BM ctrl: >45 d.
In addition to GvL activity, control of opportunistic infections (OIs) is an important issue after allo-HTC. To test Ag recall responses under allogenic inflammation conditions, we adapted our transplantation model (Fig. S10C). First, we immunized WT and NFAT-deficient C57/BL6 donor mice with the ovalbumin (OVA) peptide SIINFEKL. T cells from these mice contained comparable frequencies of OVA-reactive CD8+ T-cell clones before transplantation (Fig. S10D). Because those cells cannot recognize peptides presented by a different (mismatched) MHC, we used semiallogenic (i.e., haploidentical) CB6F1 mice as hosts. When host mice were boosted with the same Ag after HCT, comparable numbers of OVA-specific splenic CD8+ T cells of all genotypes could be detected 5 d later (Fig. S10D). Although IFNγ secretion was reduced, NFAT-deficient CD8+ T cells still produced high amounts of TNFα (Fig. S10E) and, importantly, could be reactivated when cocultured with OVA-expressing (syngenic to the T cells) tumor cells (Fig. S10 E and F). These data suggest that established effector and/or memory T cells against pathogens could be maintained when NFAT is absent.
Discussion
Prophylaxis of GvHD, although maintaining the GvL effect and preventing severe infectious diseases, remains the main challenge following allo-HCT (6). GvHD is commonly treated with CsA or FK506, effectively inhibiting the phosphatase calcineurin and subsequently NFAT activation central for T-cell stimulation (10). Unfortunately, such treatments have some severe side effects and constrain GvL. Therefore, current research attempts to dissect GvHD and GvL functionally build the base for future therapies. To this end, we examined the specific and individual roles of NFAT1 and NFAT2 transcription factors within animal models of GvHD and GvL. Whereas the ablation of one or two NFAT members protected from GvHD, GvL and Ag-specific memory responses were still achievable. GvHD was limited due to reduced GvHD-related effector functions and due to increased frequency of intact Treg cells. Nevertheless, NFAT-deficient T cells exerted effective GvL activity in the context of such a tipped balance toward Tregs.
Recently, NFAT activation during allo-HCT was visualized with the help of a lentiviral bioluminescent reporter (25). The spatial–temporal activation pattern of NFAT during acute GvHD uncovered strongest NFAT activation in the gastrointestinal tract, one of the primary GvHD target organs, within a few days after allo-HCT. This is in line with our data revealing that NFAT is functionally essential for target tissue homing via up-regulation of the gut-homing receptor α4β7-integrin.
Although the strongest effect was observed with DKO T cells, single ablation already strikingly attenuated GvHD immunopathology. Thus, NFAT2—in particular the short isoform NFAT2/αA directed by the Nfat2 P1 promoter—is directly involved in T-cell activation, proliferation, and expression of proinflammatory cytokines (12, 26). However, in vitro Nfat1−/− T cells show elevated proliferation, hyperactivation, and a bias toward a Th2 immune response (9). Interestingly, this was not relevant in this MHC-mismatched and lymphopenic environment, dominantly favoring a Th1 response. Also, in vitro Th1-skewing conditions for Nfat1−/− (or even Nfat1−/−Nfat4−/−) CD4+ T cells did not give rise to excessive IL-4, IL-5, and IL-13 expression. Further support is substantiated by the clinical observation of decreased GvHD pathology in patients transplanted with umbilical cord blood T cells (27). Umbilical cord blood T cells are characterized by less NFAT1 and blunted NFAT1-regulated gene expression, including a variety of cytokines and different homing receptors. These data suggested that alloreactive donor T cells functionally require high levels of NFAT proteins for pathogenic expansion and target tissue infiltration after allo-HCT.
On the contrary, the GvL and Ag-specific memory response did not involve much NFAT activity. Only DKO T cells showed some impairment in controlling tumor relapse, suggesting that the required magnitude of T-cell activation is distinct for GvL and GvHD pathology. It has been recognized early that the cytokine storm caused by the conditioning regimen and subsequent CD4+ T-cell activation including proinflammatory cytokine secretion is key to cause GvHD, while GvL is predominated by CD8+ T-cell responses (28, 29). Therefore, the preserved GvL effect likely relies on the upheld cytotoxic T-cell function and the fact that killing mechanisms during GvL are largely redundant (30). The phenotype of NFAT-deficient T cells is in line with only about 50%-reduced killing activity of Stim1−/−Stim2−/− CD8+ T cells (in these cells all NFAT activity is abolished) and almost normal tumor reactivity of Stim1−/− or Stim2−/− CD8+ T cells (with slightly reduced NFAT activity), whereas CD4+ T cells of these mice have a clear cytokine defect (31, 32).
Preclinical and clinical research focuses on exploiting nTregs as potential (immuno-) therapy. Although results are promising, nTreg therapy has its challenges, especially in terms of technical feasibility (13–16, 18). We and others had demonstrated that ablation of NFAT1 and NFAT2 does not perturb suppressive function of nTregs (and peripherally induced Tregs, iTregs) (11, 12). In particular, NFAT1 and NFAT2 are not required for general immune suppression, although NFAT2 is necessary for homing of Tregs into B-cell follicles (33). However, the latter is likely negligible in the context of GvHD. In line with Tregs operating largely independent of NFAT, CsA treatment following allo-HCT perturbed Treg function only in an indirect manner, i.e., due to impaired IL-2 production of effector T cells (34). Therefore, our data imply that the selective inhibition of NFAT does protect from GvHD immunopathogenesis in two ways. First, selective deletion of either NFAT1 or NFAT2 is sufficient to inhibit the pathogenic function(s) of alloreactive effector T cells, whereas nTregs remain operational. Second, the differential expansion of nTregs over effector cells mediates long-term tolerance toward alloantigens and, thereby, antagonizes GvHD. Several potential NFAT inhibitors with higher specificity than CsA have been developed and could be tested to modulate GvHD (35). This might limit severe side effects caused by global calcineurin inhibition. For the time being, standard CsA treatment supplemented with low-dose IL-2 may provide an effective therapy for GvHD while maintaining curative GvL effects. Indeed, in several clinical trials—including acute and chronic GvHD—IL-2 therapy is successfully administrated (36–38). Our data suggest that its effectiveness could be further boosted in combination with specific NFAT or even NFAT member inhibition.
Materials and Methods
Mice.
Nfat1−/−, Nfat2fl/fl, Tcrb−/−Tcrd−/−, DEREG, B6-Tg (Cd4cre) 1Cwi/Cwilbcm, and B6.L2G85.CD90.1 transgenic mice, were all on C57BL/6 background (H-2b) (SI Material and Methods with references). Female CD90.2+ C57BL/6 (H-2b), BALB/c (H-2d) and CB6F1 (H-2b+d) WT recipient mice were purchased from Charles River (SI Materials and Methods). All animal experiments were approved by the respective authorities (Regierung von Unterfranken) and complied with German animal protection law.
Tumor Cells.
Myc-driven B-cell lymphoma IM380 and A20 yfp/luc leukemia B cells (both H-2d background) express firefly luciferase (luc+) and B16F10OVA murine melanoma cells (H-2b) express ovalbumin (SI Materials and Methods).
BM and T-Cell Isolation.
BM cells were isolated by flushing femur and tibia bones. In GvL experiments, T cells were depleted from BM suspensions using the CD4+ and CD8+ MicroBead kit (Miltenyi Biotec). T cells were enriched using the Dynal Mouse T-cell Negative Isolation kit and CD4+CD25hi nTregs by staining negatively isolated T cells with anti-CD25-PE (PC61, BD Pharmingen) and separation of CD4+CD25hi nTregs and CD4+CD25– Tcon cells was performed using anti-PE MicroBeads (Miltenyi Biotech) following the manufacturer’s instructions (SI Materials and Methods).
Allo-HCT and Treg Depletion.
The models are individually depicted: allo-HCT (Fig. S1A), nTreg-mediated suppression of GvHD (Fig. S7A), depletion of Tregs after allo-HCT using DEREG mice (Fig. 3H), CD8+ T-cell memory response after semiallogenic HCT (Fig. S10C). In vivo and ex vivo bioluminescence imaging after allo-HCT (21) is described in SI Materials and Methods.
Tumor Models.
Myc-driven B-cell lymphoma IM380 and A20 yfp/luc leukemia B cells models are given in Figs. S7A and S8A and SI Materials and Methods.
Flow Cytometry.
Flow cytometry is described in SI Materials and Methods (12, 33).
CFSE in Vivo Proliferation Assay.
BALB/c host mice were conditioned with a total body irradiation (TBI) dose of 8.0 Gy and injected retroorbitally with sex-matched 5 × 106 C57BL/6 WT BM cells (H-2b CD90.2+) together with 1.2 × 106 CFSE-labeled (Molecular Probes) C57BL/6 T cells (H-2b CD90.1+). Three days later, allo-HCT spleen and LNs were isolated and CFSE dilution of allogenic CD90.1+CD4+ and CD90.1+CD8+ donor T cells was measured using a FACS Canto II (BD Biosciences) flow cytometer.
Cytometric Bead Array.
Serum cytokine concentrations were determined using the Mouse TH1/TH2 Cytometric Bead Array kit (BD Pharmingen) (SI Materials and Methods).
Quantitative qRT-PCR.
Primers and conditions are described in SI Materials and Methods (33).
Histopathology and Immunohistochemistry.
Colon, small bowel, liver, lungs, and skin from mice 12 d after allo-HCT were reviewed by an unbiased and experienced pathologist (A.M.) (12). The following scoring (0–4 depending on severity) was used: small bowel/large bowel: crypt apoptosis, crypt destruction, and inflammation; liver: bile duct injury, vascular injury, hepatocellular damage, and portal inflammation; lungs: periluminal infiltrate and pneumonitis; skin: apoptosis, ballooning of the basal layer, and inflammation (score 0–3) (SI Materials and Methods).
Statistical Analyses.
Statistical analyses were performed as described in SI Materials and Methods (12, 33).
Supplementary Material
Acknowledgments
We thank Anjana Rao for providing the Nfat2-floxed mice; Laurie H. Glimcher for the Nfat1−/− (Nfatc2−/−) mice; Christine Krempl and Sabine Kranz for the Tcrb−/−Tcrd−/− mice; and Nadine Winter, Miriam Eckstein, Lena Dietz, Johannes Dirks, Ana-Laura Jordán Garotte, and Sophia Hörmann for experimental support. This work was funded by the Wilhelm Sander Stiftung/2012.047.1 (to M.V. and F.B.-S.). Additional funding came from the Federal Ministry for Education and Research (Würzburg, Germany) A-167 (to F.B.-S.) and B-149 and B-233 (to A.B.); the German Research Foundation SPP1365 (to F.B.-S.), CRC52/A3 (to F.B.-S.), Z2 (to A.B.), and CRC 124/A3 (to A.B.); the Fritz Thyssen Stiftung 10.13.2.215 (to M.V. and F.B.-S.); and the Else Kröner Fresenius Stiftung 2010 Kolleg.52 (to A.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409290112/-/DCSupplemental.
References
- 1.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphanet J Rare Dis. 2007;2:35. doi: 10.1186/1750-1172-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horowitz MM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. [PubMed] [Google Scholar]
- 4.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 5.Toubert A, Glauzy S, Douay C, Clave E. Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: Never say never again. Tissue Antigens. 2012;79(2):83–89. doi: 10.1111/j.1399-0039.2011.01820.x. [DOI] [PubMed] [Google Scholar]
- 6.Warren EH, Deeg HJ. Dissecting graft-versus-leukemia from graft-versus-host-disease using novel strategies. Tissue Antigens. 2013;81(4):183–193. doi: 10.1111/tan.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogan WJ, Storb R. Use of cyclosporine in hematopoietic cell transplantation. Transplant Proc. 2004;36(2, Suppl):367S–371S. doi: 10.1016/j.transproceed.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17(18):2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 9.Müller MR, Rao A. NFAT, immunity and cancer: A transcription factor comes of age. Nat Rev Immunol. 2010;10(9):645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- 10.Serfling E, et al. The role of NF-AT transcription factors in T cell activation and differentiation. Biochim Biophys Acta. 2000;1498(1):1–18. doi: 10.1016/s0167-4889(00)00082-3. [DOI] [PubMed] [Google Scholar]
- 11.Bopp T, et al. NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4+ T lymphocytes by CD4+ CD25+ regulatory T cells. J Exp Med. 2005;201(2):181–187. doi: 10.1084/jem.20041538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaeth M, et al. Dependence on nuclear factor of activated T-cells (NFAT) levels discriminates conventional T cells from Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2012;109(40):16258–16263. doi: 10.1073/pnas.1203870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunstein CG, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Ianni M, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 15.Edinger M, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 16.Trenado A, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112(11):1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allan SE, et al. CD4+ T-regulatory cells: Toward therapy for human diseases. Immunol Rev. 2008;223:391–421. doi: 10.1111/j.1600-065X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 18.Edinger M, Hoffmann P. Regulatory T cells in stem cell transplantation: Strategies and first clinical experiences. Curr Opin Immunol. 2011;23(5):679–684. doi: 10.1016/j.coi.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: Take a billion or so and call me in the morning. Immunity. 2009;30(5):656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karwot R, Übel C, Bopp T, Schmitt E, Finotto S. Increased immunosuppressive function of CD4(+)CD25(+)Foxp3(+)GITR+ T regulatory cells from NFATc2((-/-)) mice controls allergen-induced experimental asthma. Immunobiology. 2012;217(9):905–911. doi: 10.1016/j.imbio.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Beilhack A, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106(3):1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hippen KL, Riley JL, June CH, Blazar BR. Clinical perspectives for regulatory T cells in transplantation tolerance. Semin Immunol. 2011;23(6):462–468. doi: 10.1016/j.smim.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen VH, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109(6):2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 24.Chopra M, et al. Non-invasive bioluminescence imaging to monitor the immunological control of a plasmablastic lymphoma-like B cell neoplasia after hematopoietic cell transplantation. PLoS ONE. 2013;8(12):e81320. doi: 10.1371/journal.pone.0081320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Na IK, et al. Concurrent visualization of trafficking, expansion, and activation of T lymphocytes and T-cell precursors in vivo. Blood. 2010;116(11):e18–e25. doi: 10.1182/blood-2009-12-259432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serfling E, et al. NFATc1/αA: The other face of NFAT factors in lymphocytes. Cell Commun Signal. 2012;10(1):16. doi: 10.1186/1478-811X-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaminski BA, et al. Reduced expression of NFAT-associated genes in UCB versus adult CD4+ T lymphocytes during primary stimulation. Blood. 2003;102(13):4608–4617. doi: 10.1182/blood-2003-05-1732. [DOI] [PubMed] [Google Scholar]
- 28.Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80(12):2964–2968. [PubMed] [Google Scholar]
- 29.Truitt RL, Atasoylu AA. Contribution of CD4+ and CD8+ T cells to graft-versus-host disease and graft-versus-leukemia reactivity after transplantation of MHC-compatible bone marrow. Bone Marrow Transplant. 1991;8(1):51–58. [PubMed] [Google Scholar]
- 30.MacDonald KP, Shlomchik WD, Reddy P. Biology of graft-versus-host responses: Recent insights. Biol Blood Marrow Transplant. 2013;19(1, Suppl):S10–S14. doi: 10.1016/j.bbmt.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw PJ, et al. CD4⁺ and CD8⁺ T cell-dependent antiviral immunity requires STIM1 and STIM2. J Clin Invest. 2014;124(10):4549–4563. doi: 10.1172/JCI76602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weidinger C, Shaw PJ, Feske S. STIM1 and STIM2-mediated Ca(2+) influx regulates antitumour immunity by CD8(+) T cells. EMBO Mol Med. 2013;5(9):1311–1321. doi: 10.1002/emmm.201302989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaeth M, et al. Follicular regulatory T cells control humoral autoimmunity via NFAT2-regulated CXCR5 expression. J Exp Med. 2014;211(3):545–561. doi: 10.1084/jem.20130604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeiser R, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieber M, Baumgrass R. Novel inhibitors of the calcineurin/NFATc hub: Alternatives to CsA and FK506? Cell Commun Signal. 2009;7:25. doi: 10.1186/1478-811X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bluestone JA. The yin and yang of interleukin-2-mediated immunotherapy. N Engl J Med. 2011;365(22):2129–2131. doi: 10.1056/NEJMe1110900. [DOI] [PubMed] [Google Scholar]
- 37.Koreth J, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuoka K, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5(179):179ra143. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.