Significance
In neurons, STEP61 (striatal-enriched protein tyrosine phosphatase) protein levels are tightly regulated, and the protein’s up-regulation is implicated in several neuropsychiatric disorders. Here, we demonstrate that parkin is a major E3 ligase regulating STEP61 levels through the ubiquitin proteasome system. In Parkinson’s disease, in which parkin function is compromised, STEP61 levels increase, which is associated with down-regulation of synaptic proteins required for neuronal plasticity.
Keywords: Parkinson's disease, parkin, STEP, ubiquitination, synaptic plasticity
Abstract
Parkinson’s disease (PD) is characterized by the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc). The loss of SNc dopaminergic neurons affects the plasticity of striatal neurons and leads to significant motor and cognitive disabilities during the progression of the disease. PARK2 encodes for the E3 ubiquitin ligase parkin and is implicated in genetic and sporadic PD. Mutations in PARK2 are a major contributing factor in the early onset of autosomal-recessive juvenile parkinsonism (AR-JP), although the mechanisms by which a disruption in parkin function contributes to the pathophysiology of PD remain unclear. Here we demonstrate that parkin is an E3 ligase for STEP61 (striatal-enriched protein tyrosine phosphatase), a protein tyrosine phosphatase implicated in several neuropsychiatric disorders. In cellular models, parkin ubiquitinates STEP61 and thereby regulates its level through the proteasome system, whereas clinically relevant parkin mutants fail to do so. STEP61 protein levels are elevated on acute down-regulation of parkin or in PARK2 KO rat striatum. Relevant to PD, STEP61 accumulates in the striatum of human sporadic PD and in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mice. The increase in STEP61 is associated with a decrease in the phosphorylation of its substrate ERK1/2 and the downstream target of ERK1/2, pCREB [phospho-CREB (cAMP response element-binding protein)]. These results indicate that STEP61 is a novel substrate of parkin, although further studies are necessary to determine whether elevated STEP61 levels directly contribute to the pathophysiology of PD.
Parkinson’s disease (PD) is a common motor disorder with clinical symptoms that include bradykinesia, resting tremor, rigidity, postural instability, and cognitive deficits (1–3). The pathophysiology of PD includes selective loss of dopaminergic neurons in the substantia nigra, with a progressive depletion of striatal dopamine and the presence of intraneuronal cytoplasmic inclusions known as Lewy bodies. Mutations of several genes are implicated in PD and are responsible for ∼10% of cases; the remaining cases are classified as sporadic PD. Although specific mutations in genes that include PARK2, PINK-1, LRRK2, and DJ-1 are known, the effects these mutations have on intracellular signaling and disease progression are not well understood and form an area of intense investigation (2, 4–6).
STEP61 (striatal-enriched protein tyrosine phosphatase) is a brain-specific phosphatase enriched in the striatum and in other regions, including cortex, hippocampus, and substantia nigra (7–9). STEP61 levels are elevated in several disorders, including Alzheimer’s disease, schizophrenia, and fragile X syndrome (10–12). STEP61 levels are normally regulated by the ubiquitin proteasome system, and disruption of this pathway leads to an accumulation of STEP61 in both Alzheimer’s disease and schizophrenia (10, 11).
Substrates of STEP61 include ERK1/2, Pyk2, Fyn, the GluN2B subunit of the NMDA receptor, and the GluA2 subunit of the AMPA receptor. The current model of STEP61 function is that it opposes the development of synaptic strengthening by dephosphorylating regulatory tyrosines on these substrates. In the case of the kinases, STEP61-mediated dephosphorylation of the regulatory Tyr within the activation loop inactivates these enzymes (13–16). STEP-mediated dephosphorylation of Tyr residues in the glutamate receptor subunits results in internalization of GluN1/GluN2B and GluA1/GluA2 receptor complexes (17–20). As a result, STEP KO mice have an increase in the basal Tyr phosphorylation of its substrates, including ERK1/2 and its downstream target pCREB (21, 22).
Overexpression of STEP disrupts synaptic function, and thereby contributes to cognitive and behavioral deficits (23). Consistent with this hypothesis, genetic or pharmacologic reduction of STEP activity in several disorders in which STEP levels are elevated reverses the biochemical and cognitive deficits that are present (19, 24), and STEP KO mice demonstrate enhanced hippocampal long-term potentiation and enhanced hippocampal- and amygdalar-dependent memory tasks (22, 25).
Direct mutations of the E3 ligase parkin (PARK2) result in autosomal recessive juvenile parkinsonism (AR-JP), with early onset of PD symptoms (26, 27); disruption of parkin activity is also implicated in sporadic PD (28–30). Moreover, PD toxins such as MPTP, rotenone, paraquat, and 6-hydroxydopamine alter parkin levels or its ligase activity and result in the accumulation of parkin substrates (31–35). Identification of new parkin substrates and characterization of their role or roles in synaptic function should result in a better understanding the molecular basis of PD.
Here we identify parkin as an E3 ligase that ubiquitinates STEP61. STEP61 levels are increased in human PD brains and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD models and are associated with a decrease in the phosphorylation of ERK1/2 and CREB. As an increase in STEP61 expression disrupts synaptic function and contributes to the cognitive deficits in several disorders, these findings suggest that the increase in STEP61 levels in PD may contribute to the pathophysiology of this disorder.
Results
STEP61 Interacts with Parkin.
STEP is normally degraded by the ubiquitin proteasome system, and disruption of this pathway leads to increased STEP expression in patients diagnosed with Alzheimer’s disease and schizophrenia (10, 11). A previous proteomic study identified STEP (protein tyrosine phosphatase non-receptor type 5, PTPN5) as one of several proteins up-regulated in the striatum of 6-hydroxydopamine-treated rats (36), suggestive of altered protein turnover in a PD animal model. However, little is known concerning the regulation of STEP by the ubiquitin proteasome system. We therefore set out to identify the E3 ligase that ubiquitinates STEP.
To determine whether STEP and parkin associate with each other, we performed GST pull-down experiments using lysates from STEP KO mouse brain. GST-STEP61 efficiently pulled down parkin, as well as the known STEP substrate ERK1/2. However, GST-STEP61 did not pull down GluA1, a negative control (Fig. 1A). We next overexpressed V5-tagged STEP61 and Myc-tagged parkin in HEK-293 cells and conducted immunoprecipitation studies with either V5 or Myc antibody. There was a reciprocal immunoprecipitation of STEP61 and parkin (Fig. 1B). STEP61 did not interact with a structural homolog of parkin, HHARI (Fig. 1C), or with two other E3 ligases, Mdm2 (a RING-domain containing ligase) or Nedd4 (a HECT-domain containing ligase), in HEK-293 cells (Fig. S1A). We also confirmed the interaction of STEP61 and parkin in primary corticostriatal cultures using coimmunoprecipitation experiments with parkin antibody (Fig. 1D).
Fig. 1.
Parkin interacts with STEP61. (A) GST-STEP61 efficiently pulled down parkin from STEP KO brain lysate. ERK2 served as a positive control, whereas GluA1 or use of GST alone were negative controls (n = 3). Samples in this and subsequent figures were analyzed by Western blotting, using the indicated antibodies. (B) Coimmunoprecipitation of STEP and parkin using either V5-tag or Myc-tag antibodies from cotransfected HEK lysate (n = 4). (C) STEP61 did not interact with the structural homolog of parkin, HHARI. Immunoprecipitation of STEP61 from Myc-parkin or Flag-HHARI transfected lysate demonstrated that STEP61 immunoprecipitated Myc-parkin, but not Flag-HHARI (n = 3). (D) STEP61 was coimmunoprecipitated from primary corticostriatal cultures, using parkin antibody, but not with the control mouse IgG (n = 3).
Parkin Regulates STEP61 Protein Levels.
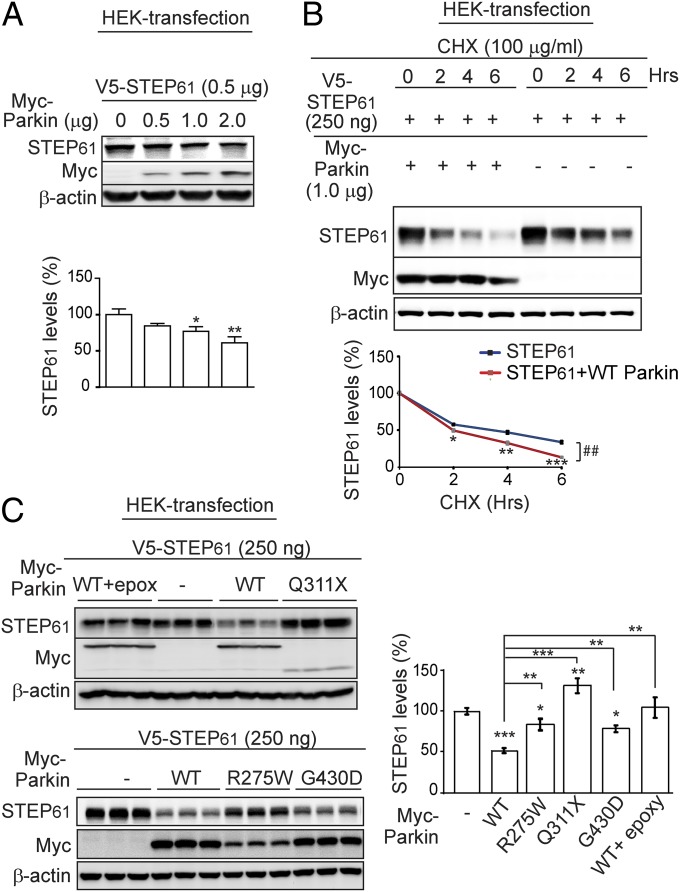
To determine the functional significance of the interaction of STEP and parkin, we analyzed STEP61 levels in the presence of increasing amounts of parkin in HEK-293 cells. There was a dose-dependent decrease in steady-state levels of STEP61 as parkin levels increased (Fig. 2A). Further, cycloheximide chase experiments indicated an accelerated decrease in steady-state levels of STEP61 when parkin was coexpressed with STEP61 (Fig. 2B).
Fig. 2.
Parkin and the ubiquitin proteasome system regulate STEP61 levels. (A) Steady-state STEP61 levels were significantly decreased by coexpressing increasing amounts of parkin in HEK cells. STEP61 levels were normalized to β-actin (n = 3; mean ± SEM; *P < 0.05, **P < 0.01, one-way ANOVA with Tukey test). (B) In the presence of cycloheximide, coexpression of parkin with STEP61 led to an accelerated decrease of STEP61 steady state levels compared with STEP61-alone transfected control. STEP61 levels were normalized to β-actin (n = 3; mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t test; ##, results of a two-way ANOVA, with treatment and time as covariants). (C) Expression of WT parkin in HEK cells decreased the steady-state level of STEP61, an effect that was blocked by parkin mutants (R275W, Q311X, and G430D), as well as by the proteasome inhibitor epoxymycin (1 μM). STEP61 levels were normalized to β-actin (n = 6; mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA with Tukey test).
We next examined the effects of clinically relevant parkin mutations on STEP61 steady state levels. STEP61 levels were significantly reduced by coexpression of WT parkin, which was blocked by the proteasome inhibitor epoxymycin, suggesting degradation of STEP61 involves the proteasome system (Fig. 2C). The expression of STEP61, along with familial-PD linked parkin mutants Q311X, R275W, or G430D, significantly increased the steady-state STEP61 levels. These results suggest parkin regulates STEP61 expression through proteasomal degradation.
Parkin Ubiquitinates STEP61 in Vivo and in Vitro.
To determine whether parkin directly ubiquitinates STEP61, we coexpressed STEP61 with hemagglutinin (HA)-ubiquitin and WT parkin or mutant parkin constructs (R275W, G430D) and conducted ubiquitin affinity pull-down experiments. Overexpression of WT parkin resulted in an increase in ubiquitinated high-molecular-weight STEP bands. In contrast, coexpression of either parkin mutant resulted in a decrease in the levels of high-molecular-weight STEP species relative to the V5-STEP61 alone control (Fig. 3A). HEK-293 cells express endogenous parkin (37), and the R275W and G430D parkin mutants had a dominant-negative effect on STEP61 ubiquitination. We then conducted in vitro ubiquitination reactions, demonstrating that STEP61 is directly ubiquitinated by parkin in the presence of the E1 ligase ubiquitin-activating enzyme E1 (UBE-1) and the E2 ligase ubiquitin-conjugating enzyme H7 (UBCH7) (Fig. 3B).
Fig. 3.
Parkin ubiquitinates STEP61 in vivo and in vitro. (A) HEK cells were transfected with STEP61, along with HA-ubiquitin or WT parkin, or with parkin mutants. Ubiquitinated proteins were enriched using tandem ubiquitin binding entity 2 (TUBE2) affinity pull-down and probed with anti-STEP antibody or anti-ubiquitin antibody. STEP61 ubiquitination was increased by WT parkin but decreased by the parkin mutants R275W or G430D (n = 3). (B) GST-STEP61 was ubiquitinated by recombinant parkin in the presence of the E1 ligase UBE1, the E2 ligase UbcH7, and HA-ubiquitin. STEP was immunoprecipitated and probed with anti-HA antibody, which shows HA immunoreactivity for higher-molecular-weight STEP–ubiquitin conjugates (n = 4).
Down-Regulation of Parkin Increases STEP61 Levels.
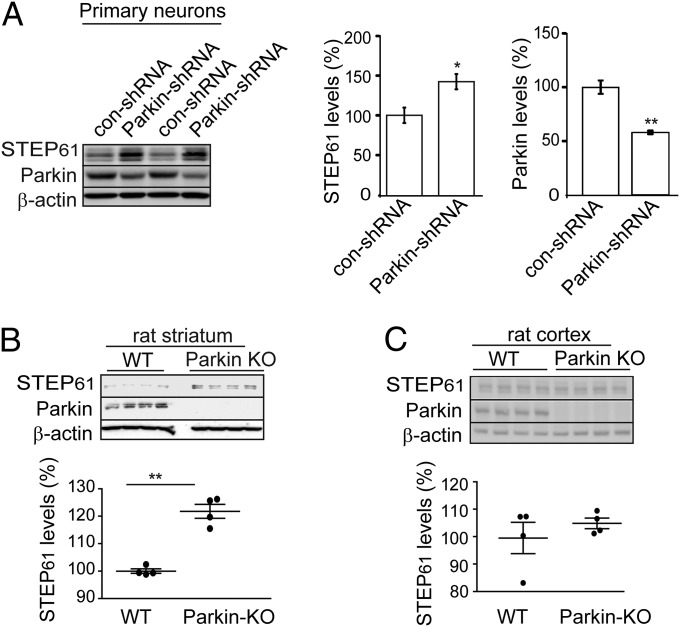
We used several complimentary approaches to examine STEP61 levels in rodent models with lower levels of parkin. We first measured STEP61 levels in rat corticostriatal cultures after shRNA down-regulation of parkin. Decreasing parkin levels (43.0 ± 1.4%) resulted in a significant increase in STEP61 expression (142.0 ± 9.0%) compared with control shRNA-treated neurons (Fig. 4A). There was no effect of reduced parkin expression on the level of STEP61 mRNA in striatal neurons (Fig. S1B) [although note that a previous study in PC12 cells exposed to ceramide suggested that parkin overexpression could influence STEP mRNA expression (38)]. We also examined STEP61 levels in a rat Park2 KO model. There was a significant increase in STEP61 in striatum of 12-mo-old Park2 KO rats (122.0 ± 2.0%) compared with WT controls (Fig. 4B). In contrast, there was no significant change in STEP61 expression in cortex (Fig. 4C).
Fig. 4.
STEP61 levels are elevated on down-regulation of parkin in primary neuronal cultures and in parkin KO rats. (A) Primary corticostriatal cultures were infected with parkin-specific shRNA, resulting in a significant increase in STEP61 levels compared with control shRNA treatment. Quantification for STEP61 and parkin is shown after normalization with β-actin (n = 6; mean ± SEM; *P < 0.05, **P < 0.01, Student’s t test). (B) STEP61 levels are increased in parkin KO rat striatum at 12 mo of age compared with WT control (n = 4; **P < 0.01, Student’s t test). β-actin served as a loading control. (C) STEP61 levels were not significantly altered in Park2 KO rat cortex (n = 4). STEP61 level was normalized to β-actin levels for quantification.
STEP61 Is Up-Regulated in MPTP-Lesioned Mice Striatum and Human PD Striatum.
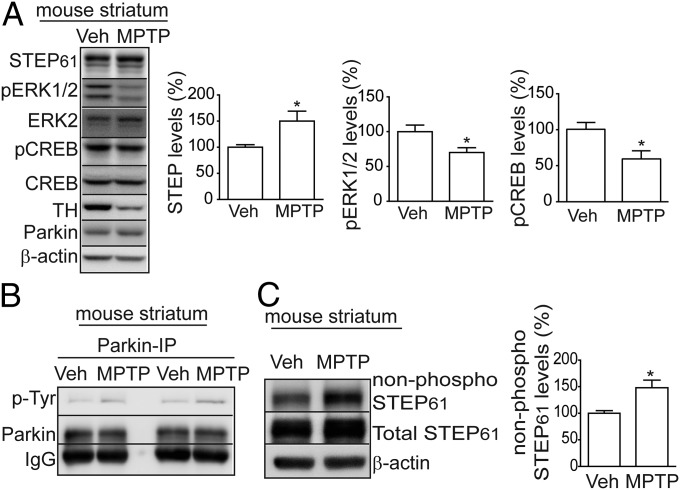
Administration of MPTP in mice is a well-accepted PD model that recapitulates many of the pathologic and behavioral symptoms of human PD (39, 40). To examine the effects of MPTP on STEP61 expression, we measured STEP61 levels, as well as the phosphorylation of STEP61 substrates in striatum. There was a significant increase in STEP61 and a concomitant decrease in pERK1/2 and pCREB phosphorylation compared with vehicle-treated controls (Fig. 5A). MPTP treatment also led to an increase in Tyr-phosphorylation of parkin (Fig. 5B), as previously shown (32). As the ability of STEP61 to bind to and dephosphorylate its substrates requires the dephosphorylation of STEP61 at a regulatory serine (Ser221) within the substrate-binding domain [kinase interaction motif (KIM) domain], we measured the phosphorylation of STEP within the KIM domain, using an antibody that detects only the nonphosphorylated form of STEP Ser221. There was a significant increase in nonphosphorylated STEP61 (more active form) in the MPTP-treated samples compared with control mice (Fig. 5C), suggesting increased STEP levels are associated with increased activity. Further, we also showed that 1-methyl-4-phenylpyridinium (MPP+) treatment of a dopaminergic cell line (SH-SY5Y) led to a significant increase in STEP61 and a decrease in the phosphorylation of pERK1/2 and pCREB (Fig. S1C).
Fig. 5.
Striatal STEP61 levels are elevated after MPTP lesion. (A) Immunoblot and quantification indicate that STEP61 expression was increased and pERK1/2 phosphorylation and pCREB levels decreased in MPTP-treated mouse striatum compared with vehicle-treated controls. STEP61 levels were normalized to β-actin for quantification (n = 8; mean ± SEM; *P < 0.05; Student’s t test). (B) Representative immunoblot indicates that parkin tyrosine phosphorylation was increased in MPTP-treated striatal lysates, after immunoprecipitation of parkin and detection with a pan phospho-tyrosine antibody (n = 2). (C) Active, non-phospho-STEP61 and total STEP61 levels were increased in MPTP-lesioned mouse striatum compared with vehicle-treated controls. STEP61 levels were normalized to β-actin (n = 8; *P < 0.05; Student’s t test).
Finally, we analyzed STEP61 levels in human sporadic PD cases. There was a significant increase of STEP61 levels in striatum and a decrease in the phosphorylation of pERK1/2 and pCREB levels compared with controls (Fig. 6). As a control, these samples showed a significant increase in aminoacyl-tRNA synthetase interacting multifunctional protein type 2 (JTV), a known parkin substrate. In addition, there was a single case of AR-JP PD among these cases that had a similar increase in STEP61 and decrease in pERK and pCREB (Fig. 6 and Fig. S1D). Of note, there were no significant changes in STEP61, pERK, or pCREB in the cortex of the human sporadic PD cases, in a single case of AR-JP PD samples tested, and in MPTP-treated mice (Fig. S2 A–C).
Fig. 6.
STEP61 is elevated in human sporadic PD and AR-JP PD striatum. Immunoblot (Left) and quantification (Right) of STEP61 expression in striatum from normal and sporadic PD. There was an increase in STEP61 levels in PD samples that was accompanied by a decrease in phosphorylation of pERK1/2, as well as the downstream target of pERK1/2, pCREB, compared with normal controls. STEP61 levels were also increased and pERK and pCREB phosphorylation were decreased in a single case of human AR-JP PD (▲) striatum compared with an age- and sex-matched control. JTV-1, another parkin substrate, was increased in the human PD striatum compared with controls. β-actin served as a loading control for quantification (n = 12; mean ± SEM; *P < 0.05 for STEP61, pERK1/2, and pCREB, as determined by ANCOVA, using PMI, age, and sex as covariates).
Discussion
Our results indicate that STEP61 is a novel substrate of parkin. Parkin associates with and ubiquitinates STEP61, thereby regulating STEP protein levels through the ubiquitin proteasome system. Several complementary techniques support this finding, including the acute shRNA-down-regulation of parkin, the data from parkin KO rats, and the findings that clinically relevant human parkin mutations all lead to an accumulation of STEP61. Parkin appears likely to be a major E3 ligase for STEP61. Partial knock-down of parkin in primary neurons had a significant effect on STEP61 protein levels independent of its mRNA levels, and other closely related or distinct E3 ligases did not interact with STEP61.
Increased expression of STEP61 was observed in human postmortem PD samples and parkin KO rats. The increase in STEP was only noted in the striatum, not in cortex. This finding parallels results from previous studies that showed that the parkin substrates JTV and parkin interacting substrate (PARIS) were elevated only in the striatum and not in the cortex of human PD cases (31, 32, 41). The increase in STEP61 levels in the human sporadic PD cases tested is of interest. There was only one case of a PARK2 mutation in the postmortem samples, suggesting that STEP61 levels are also disrupted in non-PARK2 cases, a hypothesis that is currently being tested. Taken together, the results suggest that genetic mutations or environmental toxins that disrupt parkin activity result in the accumulation of STEP61.
In striatum, STEP61 is expressed in medium spiny neurons (MSNs), which make up 90–95% of all striatal neurons (42, 43). STEP61 is also likely to be present in axonal terminals that project to striatum, including dopaminergic neurons from substantia nigra (8, 44). STEP61 expression in the human PD samples and the PD rodent KO model is presumably increased in both pre- and postsynaptic compartments in which parkin is expressed (45, 46). Little is known about its presynaptic function, but STEP may play an important role in the regulation of neurotransmitter release. STEP KO mice have elevated pERK1/2 and increased phosphorylation of synapsin I at ERK1/2-specific sites within presynaptic terminals (22), and phosphorylation of synapsin I at these sites is known to increase the probability of vesicle release (47). In this context, amperometric recordings in the striatum of PARK2 KO mice showed reduction in evoked dopamine release and impaired long-term potentiation and long-term depression in striatal MSNs (48), whereas similar analyses in KO mice for PINK-1 (an upstream regulator parkin) revealed decreases in evoked dopamine release in striatal slices (49). However, it is currently not known whether the increase in STEP expression in presynaptic terminals disrupts dopamine release, with concomitant disruption of striatal synaptic transmission.
STEP61 is highly expressed in MSNs, where it is regulated by glutamatergic and dopaminergic signaling through the protein kinase A/dopamine- and cAMP-regulated phosphoprotein MWt 32 (DARPP-32)/protein phosphatase 1 signaling pathway (42, 50). With respect to PD, the increase in STEP61 might influence dopamine signaling in MSNs. Dopamine D1 receptor stimulation in direct pathway MSNs activates protein kinase A, which phosphorylates STEP61 at ser221 within its substrate-binding domain (42). Phosphorylation at this site decreases the binding of STEP61 to substrates and prevents dephosphorylation of these targets. As a result, the Tyr phosphorylation of STEP substrates increases, including pERK1/2, and its downstream signaling pathways (14, 50). The current findings show increased STEP61 levels in human PD and animal models that are associated with a decrease in pERK and its downstream target CREB, which are involved in synaptic plasticity mechanisms by regulating gene expression (51, 52). This suggests that increased STEP61 levels in PD will negatively affect ERK- and CREB-mediated pathways in MSNs.
In PD models, the plasticity of MSNs is significantly altered. This includes loss of long-term potentiation, long-term depression, changes in glutamate receptor subunits, and dystrophic changes of dendritic terminals of MSNs (53–57). STEP dephosphorylates a number of substrates that can directly and indirectly influence synaptic plasticity. These include Fyn, Pyk2, and ERK1/2, where STEP dephosphorylation inactivates these enzymes (13–16). Other STEP substrates include the GluN2B subunit of the NMDA receptor and the GluA2 subunit of the AMPA receptor, and STEP dephosphorylation results in internalization of both GluN1/GluN2B and GluA1/GluA2 receptors (18, 58). The increase in STEP61 levels that results from loss of parkin function would therefore be expected to result in impairment in the regulation of synaptic plasticity.
An increase in STEP levels in mouse striatum after MPTP exposure is also likely to converge with other aspects of signaling in MSNs. Studies in mice suggest that MPTP treatment leads to Cdk5 activation and phosphorylation of DARPP-32 at Thr75 (59), which has an inhibitory influence on protein kinase A activity. This would be predicted to lead to a decrease in STEP61 phosphorylation and an increase in its activity (42). Consistent with this, our results show that phosphorylation of STEP61 at the regulator protein kinase A site (ser221) is decreased in MPTP-treated striatum. Protein kinase A-mediated phosphorylation of STEP was also recently found to be correlated with motor learning (60). Chagniel and colleagues reported that attenuation of striatal STEP61 activity through protein kinase A phosphorylation may be linked to a molecular pathway in the dorsal striatum that leads to the consolidation of complex motor skills during motor learning (60). PD neurotoxins such as MPTP directly inhibit parkin activity through tyrosine phosphorylation by c-Abl. Phosphorylation of parkin at a critical Tyr143 decreases its ubiquitin ligase activity, resulting in the accumulation of parkin substrates (31, 32), whereas inhibitors of c-Abl reverse this process and ameliorate motor deficits in PD mouse models (61). The increase in tyrosine phosphorylation of parkin in the MPTP-treated mouse model, coupled with the increase in STEP61 expression, suggests that STEP is not the phosphatase that normally dephosphorylates parkin.
Protein tyrosine phosphatases (PTPs) have previously been implicated in PD (62). Inhibition of PTPs protects dopaminergic neurons from PD toxins by increasing neurotrophin (BDNF) signaling and activation of ERK1/2 (63). We currently do not know whether STEP61 is the PTP that regulates this process. However, these results are intriguing, as they are consistent with our recent observation that STEP and BDNF levels are regulated by a reciprocal negative feedback mechanism. BDNF leads to a rapid ubiquitin-mediated degradation of STEP61, whereas increased STEP levels oppose pERK1/2 and pCREB-mediated expression of BDNF in psychotomimetic models.
In summary, the data identify STEP61 as a novel substrate of parkin and indicate that STEP61 is elevated in human sporadic PD samples and MPTP-treated mice. Increased STEP61 levels are known to disrupt synaptic function and contribute to the cognitive deficits in several neuropsychiatric and neurodegenerative disorders. With an already established role of STEP61 in opposing the development of synaptic strengthening, our findings suggest that the increase in STEP61 expression may contribute to the disruption of synaptic function and the motor impairments that are associated with PD (Fig. 7).
Fig. 7.
Schematic model of regulation of STEP61 by parkin in PD. The E3 ligase parkin regulates STEP61 levels through the ubiquitin proteasome pathway. STEP61 normally opposes the development of synaptic strengthening by Tyr dephosphorylation of substrates that include ERK. Genetic mutations and PD toxins result in impaired parkin function that leads to the accumulation of STEP61 and a disruption of ERK and CREB-mediated synaptic plasticity events.
Materials and Methods
Antibodies.
The antibodies used are listed in Table S1.
Animals.
The Yale Institutional Animal Care and Use Committee approved all experiments at Yale, and the Direcção Geral de Alimentação e Veterinária (Portugal) approved the MPTP experiments. Long-Evans WT and PARK2 KO rats were purchased from SAGE Labs and maintained in the animal facility for 12 mo before being killed for biochemical experiments.
Human Tissue.
Postmortem brain tissue (striatum and cortex) from patients with PD and matched control patients were obtained from the National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders (University of Maryland, Baltimore, MD) and stored at –80 °C until processed by Western blot analyses. Detailed information of human samples (age, sex, and postmortem intervals) is summarized in Table S2. For immunoblotting, frozen striatum or cortex were homogenized in Tris buffer (in mM): 10 Tris base at pH 7.6, 320 sucrose, 150 NaCl, 5 EDTA, 5 EGTA, 1 NaF, 1 Na3VO4, 1 DTT, and protease inhibitor mixture (Roche). Homogenates were centrifuged at 800 × g to remove nuclei and large debris (P1), and synaptosomal membrane (P2) fractions were prepared by centrifugation of S1 at 9200 × g for 15 min.
MPTP-Induced Lesion in Mice.
For MPTP lesion, C57BL/6 male mice (3 mo old) were used. Treatment consisted of a single intraperitoneal injection per day (30 mg/kg) for 5 consecutive days (64). Injections were performed at the same time of the day, and animals were killed 7 d after the last injection. Control mice were injected with saline (0.9% NaCl; 100 μL). Brains were quickly removed, and striatum were dissected, frozen on dry ice, and stored at −80 °C.
Western Blot Analyses.
Proteins (30–50 μg) were loaded onto 10% (wt/vol) SDS/PAGE gels and transferred to nitrocellulose membranes, held overnight at 4 °C, followed by incubation with secondary antibody. Bands were visualized by chemiluminescence, using a G:BOX system with GeneSnap image program and quantified using Image J 1.33 (NIH).
Statistical Analysis.
All experiments were repeated at least three times before statistical analyses. Data were expressed as means ± SEM. Statistical significance was determined by Student’s t test or one-way or two-way ANOVA, as appropriate, with post hoc Tukey test. P values <0.05 were considered significant. ANCOVA (IBM SPSS Statistics v19) was used for the human postmortem data analyses to determine the differences between groups (controls and PD), using PMI (postmortem interval), age, and sex as covariates.
Supplementary Material
Acknowledgments
We thank all the members of the P.J.L. laboratory for helpful discussions during the preparation of the manuscript. We thank NIH Neurobiobank (formerly National Institute of Child Health and Human Development Brain Tissue Bank (NICHD BTB) for human brain tissues. The work was funded by The Michael J. Fox Foundation for Parkinson’s Research (P.K.K.), NIH funding [MH052711 and MH091037 (to P.J.L.)], Department of Defense US Army Medical Research Acquisition Activity (DOD USAMRAA) funding [W81XWH-09-1-0434 (to A.C.N.)], Fundo Europeu de Desenvolvimento Regional Fundo Europeu de Desenvolvimento Regional via Programa Operacional Factores de Competitividade-Quadro de Referência Estratégica Nacional (to R.A.V. and G.B.), and PEst-C/SAU/UI0709/2011 (to G.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417423112/-/DCSupplemental.
References
- 1.Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: Molecules to medicine. J Clin Invest. 2006;116(7):1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas B, Beal MF. Parkinson's disease. Hum Mol Genet. 2007;16(R2):R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 3.Aarsland D, Beyer MK, Kurz MW. Dementia in Parkinson’s disease. Curr Opin Neurol. 2008;21(6):676–682. doi: 10.1097/WCO.0b013e3283168df0. [DOI] [PubMed] [Google Scholar]
- 4.Saiki S, Sato S, Hattori N. Molecular pathogenesis of Parkinson’s disease: Update. J Neurol Neurosurg Psychiatry. 2012;83(4):430–436. doi: 10.1136/jnnp-2011-301205. [DOI] [PubMed] [Google Scholar]
- 5.Singleton AB, Farrer MJ, Bonifati V. The genetics of Parkinson’s disease: Progress and therapeutic implications. Mov Disord. 2013;28(1):14–23. doi: 10.1002/mds.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasser T. Update on the genetics of Parkinson’s disease. Mov Disord. 2007;22(Suppl 17):S343–S350. doi: 10.1002/mds.21676. [DOI] [PubMed] [Google Scholar]
- 7.Lombroso PJ, Murdoch G, Lerner M. Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. Proc Natl Acad Sci USA. 1991;88(16):7242–7246. doi: 10.1073/pnas.88.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulanger LM, et al. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15(2):1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SY, et al. Striatal-enriched protein tyrosine phosphatase regulates dopaminergic neuronal development via extracellular signal-regulated kinase signaling. Exp Neurol. 2008;214(1):69–77. doi: 10.1016/j.expneurol.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Kurup P, et al. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30(17):5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carty NC, et al. The tyrosine phosphatase STEP: Implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl Psychiatr. 2012;2:e137. doi: 10.1038/tp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goebel-Goody SM, et al. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 2012;11(5):586–600. doi: 10.1111/j.1601-183X.2012.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muñoz JJ, Tárrega C, Blanco-Aparicio C, Pulido R. Differential interaction of the tyrosine phosphatases PTP-SL, STEP and HePTP with the mitogen-activated protein kinases ERK1/2 and p38alpha is determined by a kinase specificity sequence and influenced by reducing agents. Biochem J. 2003;372(Pt 1):193–201. doi: 10.1042/BJ20021941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6(1):34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, et al. Striatal-enriched protein-tyrosine phosphatase (STEP) regulates Pyk2 kinase activity. J Biol Chem. 2012;287(25):20942–20956. doi: 10.1074/jbc.M112.368654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277(27):24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- 17.Pelkey KA, et al. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34(1):127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 18.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, et al. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. Proc Natl Acad Sci USA. 2010;107(44):19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, et al. Reduced levels of the tyrosine phosphatase STEP block β amyloid-mediated GluA1/GluA2 receptor internalization. J Neurochem. 2011;119(3):664–672. doi: 10.1111/j.1471-4159.2011.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkitaramani DV, et al. Knockout of striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse. 2009;63(1):69–81. doi: 10.1002/syn.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkitaramani DV, Moura PJ, Picciotto MR, Lombroso PJ. Striatal-enriched protein tyrosine phosphatase (STEP) knockout mice have enhanced hippocampal memory. Eur J Neurosci. 2011;33(12):2288–2298. doi: 10.1111/j.1460-9568.2011.07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goebel-Goody SM, et al. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev. 2012;64(1):65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, et al. Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of alzheimer’s disease. PLoS Biol. 2014;12(8):e1001923. doi: 10.1371/journal.pbio.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olausson P, et al. The tyrosine phosphatase STEP constrains amygdala-dependent memory formation and neuroplasticity. Neuroscience. 2012;225:1–8. doi: 10.1016/j.neuroscience.2012.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka K, Suzuki T, Hattori N, Mizuno Y. Ubiquitin, proteasome and parkin. Biochim Biophys Acta. 2004;1695(1-3):235–247. doi: 10.1016/j.bbamcr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Shimura H, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25(3):302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, et al. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci USA. 2000;97(24):13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sriram SR, et al. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet. 2005;14(17):2571–2586. doi: 10.1093/hmg/ddi292. [DOI] [PubMed] [Google Scholar]
- 30.Dawson TM, Dawson VL. The role of parkin in familial and sporadic Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S32–S39. doi: 10.1002/mds.22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko HS, et al. Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin’s ubiquitination and protective function. Proc Natl Acad Sci USA. 2010;107(38):16691–16696. doi: 10.1073/pnas.1006083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imam SZ, et al. Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: Implications for Parkinson’s disease. J Neurosci. 2011;31(1):157–163. doi: 10.1523/JNEUROSCI.1833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali SF, Binienda ZK, Imam SZ. Molecular aspects of dopaminergic neurodegeneration: Gene-environment interaction in parkin dysfunction. Int J Environ Res Public Health. 2011;8(12):4702–4713. doi: 10.3390/ijerph8124702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, et al. ATF4 protects against neuronal death in cellular Parkinson’s disease models by maintaining levels of parkin. J Neurosci. 2013;33(6):2398–2407. doi: 10.1523/JNEUROSCI.2292-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson TM, Dawson VL. Parkin plays a role in sporadic Parkinson’s disease. Neurodegener Dis. 2014;13(2-3):69–71. doi: 10.1159/000354307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park B, et al. Proteomic analysis of expression and protein interactions in a 6-hydroxydopamine-induced rat brain lesion model. Neurochem Int. 2010;57(1):16–32. doi: 10.1016/j.neuint.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Pawlyk AC, et al. Novel monoclonal antibodies demonstrate biochemical variation of brain parkin with age. J Biol Chem. 2003;278(48):48120–48128. doi: 10.1074/jbc.M306889200. [DOI] [PubMed] [Google Scholar]
- 38.Unschuld PG, et al. Parkin modulates gene expression in control and ceramide-treated PC12 cells. Mol Biol Rep. 2006;33(1):13–32. doi: 10.1007/s11033-005-3961-5. [DOI] [PubMed] [Google Scholar]
- 39.Gerlach M, Riederer P. Animal models of Parkinson’s disease: An empirical comparison with the phenomenology of the disease in man. J Neural Transm. 1996;103(8-9):987–1041. doi: 10.1007/BF01291788. [DOI] [PubMed] [Google Scholar]
- 40.Przedborski S, Vila M. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: A tool to explore the pathogenesis of Parkinson’s disease. Ann N Y Acad Sci. 2003;991:189–198. [PubMed] [Google Scholar]
- 41.Shin JH, et al. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144(5):689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul S, et al. The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci. 2000;20(15):5630–5638. doi: 10.1523/JNEUROSCI.20-15-05630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matamales M, et al. Striatal medium-sized spiny neurons: Identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS ONE. 2009;4(3):e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13(7):3064–3074. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horowitz JM, Myers J, Stachowiak MK, Torres G. Identification and distribution of Parkin in rat brain. Neuroreport. 1999;10(16):3393–3397. doi: 10.1097/00001756-199911080-00025. [DOI] [PubMed] [Google Scholar]
- 46.Stichel CC, et al. Parkin expression in the adult mouse brain. Eur J Neurosci. 2000;12(12):4181–4194. [PubMed] [Google Scholar]
- 47.Jovanovic JN, et al. Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J Neurosci. 2001;21(20):7944–7953. doi: 10.1523/JNEUROSCI.21-20-07944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of parkin-/- mice. J Neurochem. 2009;110(2):613–621. doi: 10.1111/j.1471-4159.2009.06152.x. [DOI] [PubMed] [Google Scholar]
- 49.Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci USA. 2007;104(27):11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valjent E, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102(2):491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5(3):173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 52.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 53.Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13(6):1071–1077. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- 54.Dunah AW, et al. Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson’s disease. Mol Pharmacol. 2000;57(2):342–352. [PubMed] [Google Scholar]
- 55.Day M, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9(2):251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 56.Deutch AY, Colbran RJ, Winder DJ. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism Relat Disord. 2007;13(Suppl 3):S251–S258. doi: 10.1016/S1353-8020(08)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Picconi B, Piccoli G, Calabresi P. Synaptic dysfunction in Parkinson’s disease. Adv Exp Med Biol. 2012;970:553–572. doi: 10.1007/978-3-7091-0932-8_24. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, et al. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28(42):10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamura Y, et al. Dopamine signaling negatively regulates striatal phosphorylation of Cdk5 at tyrosine 15 in mice. Front Cell Neurosci. 2013;7:12. doi: 10.3389/fncel.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chagniel L, Bergeron Y, Bureau G, Massicotte G, Cyr M. Regulation of tyrosine phosphatase STEP61 by protein kinase A during motor skill learning in mice. PLoS ONE. 2014;9(1):e86988. doi: 10.1371/journal.pone.0086988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanabe A, et al. A novel tyrosine kinase inhibitor AMN107 (nilotinib) normalizes striatal motor behaviors in a mouse model of Parkinson’s disease. Front Cell Neurosci. 2014;8:50. doi: 10.3389/fncel.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang P, Dankowski A, Hagg T. Protein tyrosine phosphatase inhibition reduces degeneration of dopaminergic substantia nigra neurons and projections in 6-OHDA treated adult rats. Eur J Neurosci. 2007;25(5):1332–1340. doi: 10.1111/j.1460-9568.2007.05384.x. [DOI] [PubMed] [Google Scholar]
- 63.Lu X, Maysinger D, Hagg T. Tyrosine phosphatase inhibition enhances neurotrophin potency and rescues nigrostriatal neurons in adult rats. Exp Neurol. 2002;178(2):259–267. doi: 10.1006/exnr.2002.8042. [DOI] [PubMed] [Google Scholar]
- 64.Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77(4):1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.