Abstract
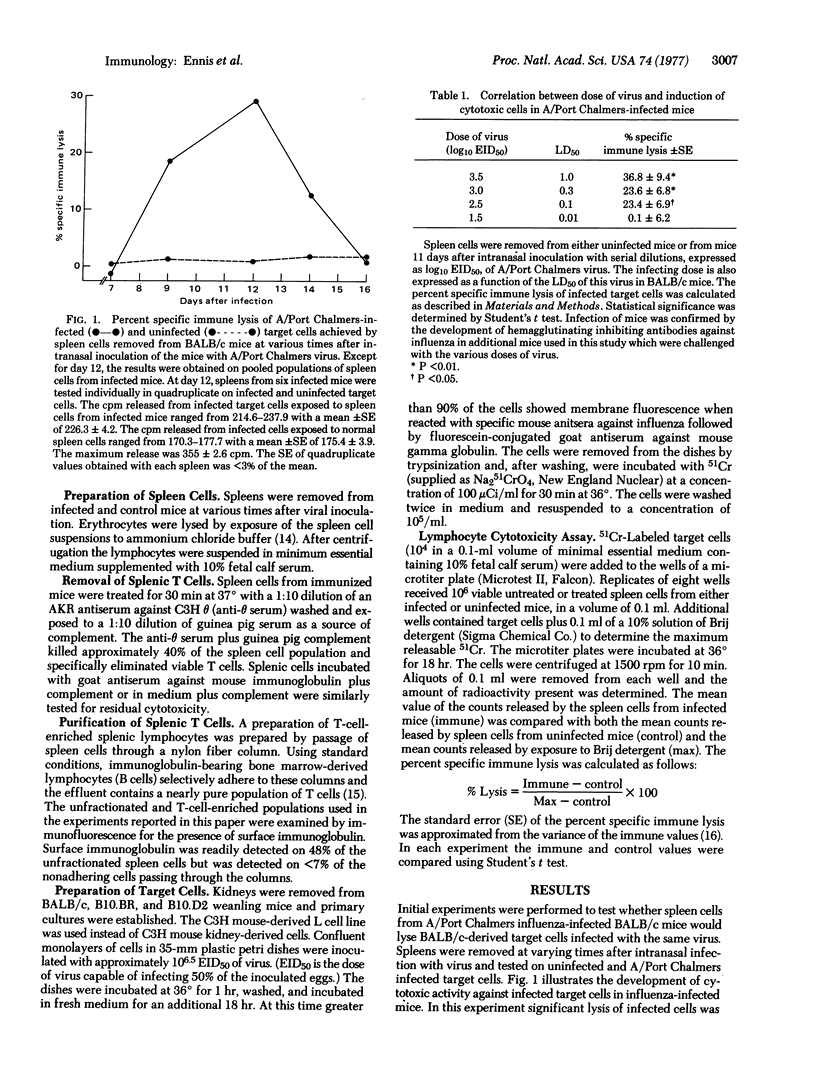
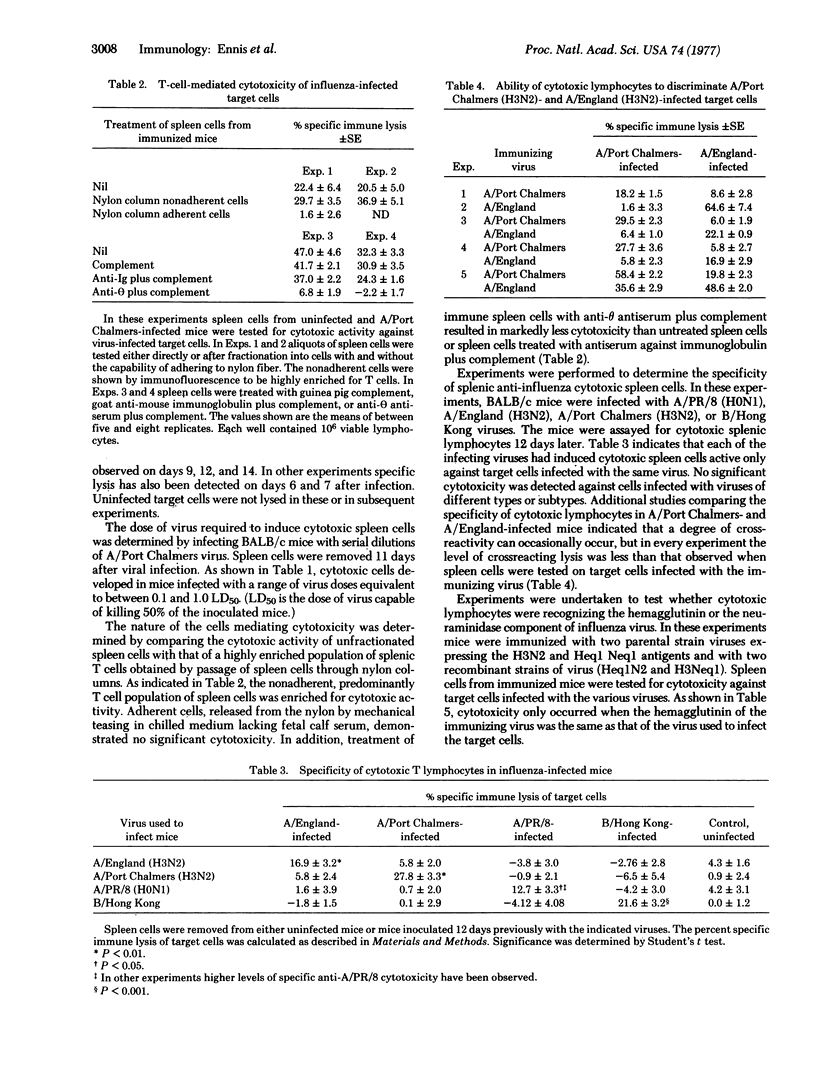
Cytotoxic thymus-derived (T) lymphocytes were readily detected in spleens of mice inoculated intranasally with mouse-adapted A/Port Chalmers (H3N2), A/England (H3N2), A/PR/8 (H0n1), and B/Hong Kong influenza viruses. T-cell-mediated lysis of H-2 compatible target cells infected with the strain of virus used to immunize the mice was considerably higher than lysis of either syngeneic cells infected with a different strain of influenza virus or allogeneic cells infected with the immunizing strain of influenza virus. The findings that cytotoxic lymphocytes can distinguish minor antigenic variants among influenza viruses and that lysis depends on H-2 histocompatibility between lymphocyte and target cell support the concept of dual recognition of visual and H-2 histocompatibility antigens in T-cell-mediated antiviral immunity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blank K. J., Freedman H. A., Lilly F. T-lymphocytes response to Friend virus-induced tumour cell lines in mice of strains congenic at H--2. Nature. 1976 Mar 18;260(5548):250–252. doi: 10.1038/260250a0. [DOI] [PubMed] [Google Scholar]
- Cambridge G., Mackenzie J. S., Keast D. Cell-mediated immune response to influenza virus infections in mice. Infect Immun. 1976 Jan;13(1):36–43. doi: 10.1128/iai.13.1.36-43.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. H-2 compatibility is required for T-cell-mediated lysis of target cells infected with lymphocytic choriomeningitis virus. J Exp Med. 1975 Feb 1;141(2):502–507. doi: 10.1084/jem.141.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. Specific immune lysis of paramyxovirus-infected cells by H-2-compatible thymus-derived lymphocytes. Immunology. 1976 Jul;31(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- Effros R. B., Doherty P. C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar 1;145(3):557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S. S., Bell H. B., Burke D. C. Abortive infection of L cells by fowl plague virus: comparison of RNA and protein synthesis in infected chick and L cells. J Gen Virol. 1971 Dec;13(3):424–432. doi: 10.1099/0022-1317-13-3-423. [DOI] [PubMed] [Google Scholar]
- Gardner I., Bowern N. A., Blanden R. V. Cell-mediated cytotoxicity against ectromelia virus-infected target cells. I. Specificity and kinetics. Eur J Immunol. 1974 Feb;4(2):63–67. doi: 10.1002/eji.1830040202. [DOI] [PubMed] [Google Scholar]
- Garrido F., Schirrmacher V., Festenstein H. H-2-like specificities of foreign haplotypes appearing on a mouse sarcoma after vaccinia virus infection. Nature. 1976 Jan 22;259(5540):228–230. doi: 10.1038/259228a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Koszinowski U., Ertl H. Lysis mediated by T cells and restricted by H-2 antigen of target cells infected with vaccinia virus. Nature. 1975 Jun 12;255(5509):552–554. doi: 10.1038/255552a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Selection of antigenic mutants of influenza viruses. Isolation and peptide mapping of their hemagglutination proteins. Virology. 1968 Feb;34(2):193–202. doi: 10.1016/0042-6822(68)90230-4. [DOI] [PubMed] [Google Scholar]
- Martin W. J., Wunderlich J. R., Fletcher F., Inman J. K. Enhanced immunogenicity of chemically-coated syngeneic tumor cells. Proc Natl Acad Sci U S A. 1971 Feb;68(2):469–472. doi: 10.1073/pnas.68.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D. B., Quigley J. P. Virus-induced modification of cellular membranes related to viral structure. Annu Rev Microbiol. 1974;28(0):325–351. doi: 10.1146/annurev.mi.28.100174.001545. [DOI] [PubMed] [Google Scholar]
- Roos D., Loos J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. I. Stimulation by phytohaemagglutinin. Biochim Biophys Acta. 1970 Dec 29;222(3):565–582. doi: 10.1016/0304-4165(70)90182-0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Cunningham B. A., Edelman G. M. Functional interactions of viral and histocompatibility antigens at tumor cell surfaces. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5066–5070. doi: 10.1073/pnas.72.12.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M., Rehn T. G., Garbarino C. A. Cell-mediated lympholysis of trinitrophenyl-modified autologous lymphocytes. Effector cell specificity to modified cell surface components controlled by H-2K and H-2D serological regions of the murine major histocompatibility complex. J Exp Med. 1975 Jun 1;141(6):1348–1364. doi: 10.1084/jem.141.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M. H-2 restriction of virus-specific cytotoxicity across the H-2 barrier. Separate effector T-cell specificities are associated with self-H-2 and with the tolerated allogeneic H-2 in chimeras. J Exp Med. 1976 Oct 1;144(4):933–945. doi: 10.1084/jem.144.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]



