Significance
Classical studies have been focused on understanding the role of gustatory areas in evaluating the sensory component of taste. However, recent studies have suggested an involvement of gustatory areas, specifically the insular cortex, in guiding behavior on the basis of anticipation of food. At present, however, no causal link between gustatory cortex anticipatory activity and feeding behaviors has been demonstrated. The experiments in this study combine electrophysiological, pharmacological, and optogenetic approaches to establish, for the first time to our knowledge, that cue-evoked activity in the gustatory cortex is necessary to drive food-oriented behaviors. These results demonstrate that the integration between perception and action can occur also in primary sensory areas.
Keywords: insular cortex, reward, anticipation, cue, learning
Abstract
Reward-related circuits are fundamental for initiating feeding on the basis of food-predicting cues, whereas gustatory circuits are believed to be involved in the evaluation of food during consumption. However, accumulating evidence challenges such a rigid separation. The insular cortex (IC), an area largely studied in rodents for its role in taste processing, is involved in representing anticipatory cues. Although IC responses to anticipatory cues are well established, the role of IC cue-related activity in mediating feeding behaviors is poorly understood. Here, we examined the involvement of the IC in the expression of cue-triggered food approach in mice trained with a Pavlovian conditioning paradigm. We observed a significant change in neuronal firing during presentation of the cue. Pharmacological silencing of the IC inhibited food port approach. Such a behavior could be recapitulated by temporally selective inactivation during the cue. These findings represent the first evidence, to our knowledge, that cue-evoked neuronal activity in the mouse IC modulates behavioral output, and demonstrate a causal link between cue responses and feeding behaviors.
In natural environments, animals use sensory information from various sources to predict the availability of food (1–3). Repeated pairings of a neutral stimulus with the availability of food leads to the formation of associations. Upon association, food-predicting cues become capable of triggering the expectation of food. These expectations drive motivation for food seeking and food consumption (4–6). It is generally believed that cues drive behavior by activating reward-related circuits responsible for coordinating food seeking and food consumption. A large body of evidence shows that regions like the amygdala, ventral striatum, orbitofrontal and prefrontal cortices, and ventral tegmental area can be activated by anticipatory cues (7–12). Although the reward circuitry involved in cue-triggered, food-related behaviors has been extensively studied, relatively little attention has been devoted to the role of sensory cortical areas in this process. The insular cortex (IC), for instance, has traditionally been studied for its role in the consummatory and postconsummatory phases of feeding (13–17). Neuronal ensembles in the IC are involved in taste processing and learning (18–21), and IC function is believed to be limited to the evaluative and sensory aspects of food consumption (22, 23). More recent evidence, however, has suggested that the IC can also be involved in processing cues associated with food availability or delivery of addictive drugs (24–28). The presence of neurons that encode for both anticipatory cues and taste suggests a functional integration of reward and expectation processing. The prediction emerging from these studies is that manipulations of IC anticipatory activity might have an impact on food-directed and, in general, reward-directed behaviors. Although pharmacological manipulations of the IC have been shown to have an impact on reward-guided behaviors, no study to date has focused directly on the role of IC cue-related activity in driving those behaviors.
Here, we examined the hypothesis that IC neuronal activity triggered by food-predictive cues modulates behavioral responses to obtain food. Mice were trained in a Pavlovian learning paradigm in which a sensory cue anticipates the delivery of a food pellet. Electrophysiological recordings of single units were used to investigate whether IC activity tracked anticipation in this task. Pharmacological and optogenetic inactivation of the IC allowed us to determine the behavioral role of IC’s anticipatory activity in mediating food port approach response on presentation of the anticipatory cue.
Results
Conditioned Reward Approach Behavior as an Indicator of Motivation to Food.
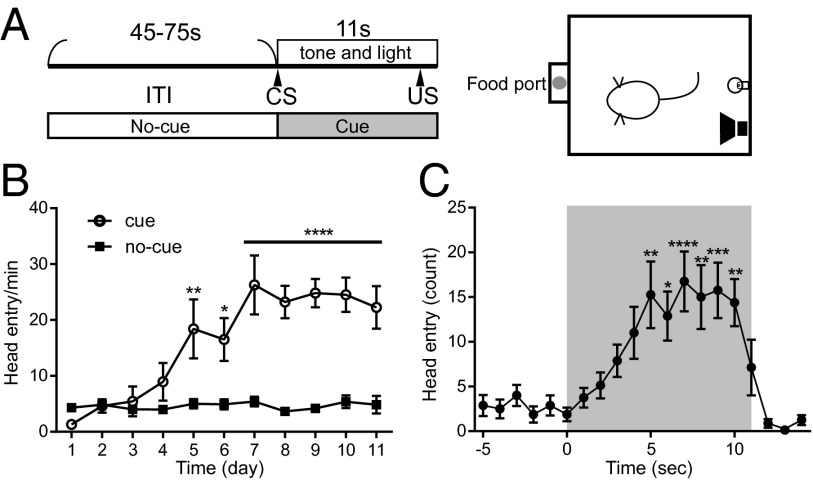
A classical Pavlovian paradigm was used to induce the anticipation of food in food-restricted mice (Fig. 1A). A compound visual and auditory cue was associated with the delivery of a food pellet at a receptacle. The cue lasted 11 s, and the pellet was delivered at 10 s. Each session comprised 25 trials, and the intertrial interval (ITI) was variable (45–75 s) such that the animal could not predict the onset of the cue. Mice did not show any response to the cue at the beginning of training; in the first 4 d, the number of food magazine entries during the cue was not significantly different from head entries observed during the ITI. A significant increase in number of entries during cue presentation could be seen after 5 d of training [Fig. 1B; F(1,14) = 34.11669, P < 1E-4 by repeated measures two-way ANOVA (cue vs. no-cue), P < 0.01 by Bonferroni’s multiple comparisons test at the time point of session day 5]. The emergence of a conditioned response indicates that mice acquired the associative and anticipatory value of the sensory cue. Upon learning of the association (i.e., from day 5), mice showed robust head entry responses during cue presentation in each training day (Fig. 1B). Averaged head entry activity during trials is shown in Fig. 1C. There was a significant effect of averaged head entry activity (Fig. 1C; F(19,133) = 9.994012, P < 1E-4 by repeated measures one-way ANOVA). Overall head entry activity was significantly higher than baseline level after 5 s of cue onset (head entry activity in −5 s vs. 5–10 s after onset of the reward-predicting cue, P < 0.05 by Bonferroni’s multiple comparisons test).
Fig. 1.
Behavioral paradigm used for assessing reward-approach behavior. (A, Left) Design of one trial. Each session contained 25 trials. (A, Right) Operant chamber. Food delivery was associated with tone and light presentation. (B) Learning of cue-food association and development of food magazine approach behavior. Mean (± SEM) head entry activity across sessions for eight subjects is shown. Head entry activity during the cue presentation period (○) and head entry during the ITI (■) are shown. The x axis represents training days, and the y axis represents averaged head entry activity. (C) Mean (± SEM) head entry activity during the prepresentation, cue presentation, and postpresentation periods (n = 8). Each dot represents the cumulated number of head entries observed in one session. The x axis represents seconds from the onset of the cue, and the y axis represents averaged head entry activity. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Neuronal Activity of Mouse IC During Reward-Predicting Cue.
Recent studies using rats have shown that neurons in the IC can change their firing in response to auditory cues anticipating the availability of taste (26, 29, 30). To determine whether IC activity could also be modulated by anticipatory cues in the present experimental conditions, we implanted 14 mice with movable bundles of 8 or 16 electrodes. Recording of single-neuron activity revealed that different phases of a trial could result in significant changes in firing rates relative to precue baseline. Firing rates could either increase or decrease relative to baseline. A total of 35.5% (39 of 110) of the neurons were modulated by the cue (Fig. 2A, Left), 25.5% (28 of 110) by food delivery (i.e., dropping of the pellet in the last second of the cue; Fig. 2A, Middle), and 45.5% (50 of 110) by food consumption (Fig. 2A, Right). Some neurons could be modulated by more than one event [12.7% (14 of 110) for cue and food delivery, 18.2% (20 of 110) for cue and food consumption, 13.6% (15 of 110) for food delivery and consumption, and 7.2% (8 of 110) for all three]. We focused our attention on the group of cue-modulated neurons. Fig. 2A (Left) shows the population response (normalized as the area under the receiver operating characteristic curve; Methods) for neurons that were excited [14.5% (16 of 110)] and for neurons that were inhibited by the cue [21.0% (23 of 110)]. The average change in firing rates was 1.22 ± 0.17 Hz relative to baseline firing of 6.33 ± 1.38 Hz [corresponding to a modulation index (MI) of 0.20 ± 0.01; Fig. 2C] for excitatory responses. Neurons inhibited by the cue showed a reduction of 1.94 ± 0.36 Hz from a baseline firing of 9.67 ± 2.02 Hz (corresponding to an MI of −0.20 ± 0.02; Fig. 2C). In addition, the peak/trough of cue-evoked activity was computed in the two groups of neurons; peak response was 2.41 ± 1.52 Hz above baseline (corresponding to an MI of 0.16 ± 0.009) for neurons excited by cue. The trough of the response was 3.07 ± 1.73 Hz below baseline (corresponding to an MI of −0.17 ± 0.01) for neurons suppressed by cue. Population activity of cue-modulated neurons did not show strong responses to food delivery or food consumption. This observation suggests that cue modulations do not relate to conditioned mouth movements similar to those mouth movements evoked by food consumption. Fig. 2B shows raster plots and peristimulus time histograms for two representative units. Additional analyses were performed to determine whether cue-related modulations depended on the act of entering the food port. To track head entry behavior, the rate of head entries was computed for each session. On average, the onset of cue responses was significantly faster than the onset of head entry rate increase (0.73 ± 0.07 vs. 1.88 ± 0.16 s; P < 1E-7 by paired t test), suggesting that cue responses preceded the increase of head entry rate. To investigate the potential contribution of head entries to cue-related modulation further, we compared spiking activity before and after head entries, during both cue and ITI. We applied an unbalanced two-way ANOVA to investigate modulations related to cue, head entries, and the interaction of the two. This analysis revealed that of 110 neurons, 51 showed modulation due to cue, 21 showed modulation by head entries (11 overlapped with the previous category), and only 2 showed a significant interaction term (P < 0.05). Then, we focused on the group that showed head entry-related modulation (n = 21), and we computed an MI. The absolute MI, a measure of modulation strength, was significantly larger for head entries performed during the cue compared with head entries performed during the ITI (0.11 ± 0.01 vs. 0.05 ± 0.01; P < 1E-6 by paired t test). This result suggests that the cue enhances the head entry-related modulation (if there was any). To support further the evidence that cues are influencing neural activity regardless of head entry, we computed the Fano factor (a measure of neural variability) around head entries during the cue and during the ITI. The Fano factor was significantly smaller when the animal entered the port during the cue, relative to ITI entries (1.44 ± 0.8 vs. 1.63 ± 0.06; P < 1E-3 by paired t test), implying that cues reduced the variability of neural activity preceding head entry.
Fig. 2.
Neuronal activity in the mouse IC. (A) Population peristimulus time histogram (PSTH) of IC neurons significantly changing their firing in response to the cue (Left), to food delivery (Middle), and to food consumption (Right). Activity is aligned to cue onset (time 0). Solid lines represent excitatory modulations, and dashed lines represent inhibitory modulations. Dark gray-shaded areas indicate SEM. Light gray-shaded areas indicate the cue period (Left), the pellet-dropping period (Middle), and the 5-s period after cue offset (Right), when food pellet is consumed by the animal. The x axis represents time, and the y axis represents normalized firing activity. (B) Representative example of excitatory (Left) and inhibitory (Right) modulations during the cue period. A raster plot and PSTH are shown. Time 0 is the cue onset. Light gray-shaded areas indicate the cue period. The x axis represents time, and the y axis represents firing rates (FR). (C) Comparison of MI during the cue period for neurons with excitatory responses (CueE; solid lines, n = 16), inhibitory responses (CueI; dashed lines, n = 23), and not significantly modulated (NonCue; empty, n = 71). Bars indicate SEM.
Altogether, these data show that IC neurons are active throughout different phases of the task, including the anticipatory phase during playback of the cue.
Pharmacological Inactivation of the IC Decreases Food-Oriented Behavior.
To examine whether neuronal activity in the IC had an effect on feeding behavior, we tested the effects of pharmacological inactivation of the IC on conditioned responses. We performed local infusions of the GABA_A receptor agonist muscimol and the GABA_B receptor agonist baclofen. A mixture of muscimol and baclofen was infused locally and bilaterally in the IC before starting the session. In inactivation sessions, we observed a significant decrease in the number of head entries during the cue period compared with a control saline injection session (Fig. 3A; repeated measures one-way ANOVA: F(3,21) = 54.64, P < 1E-4; P < 1E-4 by Bonferroni’s multiple comparisons test between drug cue and saline cue). Fig. 3B shows the average time course of head entries before and during the cue. On the day of drug injection, mice showed very little head entry response throughout the cue presentation period compared with the saline session (Fig. 3B; repeated measures two-way ANOVA: F(1,14) = 56.32, P < 1E-4; P < 1E-4 for 2–10 s by Bonferroni’s multiple comparisons test). One of the potential disadvantages of local drug injection is the possibility of drug diffusion beyond the site of injection. To confirm that the effect we observed in Fig. 3 A and B is due to specific inactivation of the IC, we performed a new series of experiments where the same volume of drug mixture was injected in the adjacent somatosensory cortex. Statistical comparison of head entry activity during the cue period between the control (saline session) and the drug (muscimol/baclofen session) did not reveal significant differences (Fig. 3C; repeated measures one-way ANOVA: F(3,21) = 33.34 by Bonferroni’s multiple comparisons test; cue-control vs. cue-drug: t = 2.192, P > 0.05). There was also no significant difference in the time course of the port entry behavior (Fig. 3D; repeated measures two-way ANOVA: F(1,14) = 0.9189, P > 0.05). Thus, these results indicate that the inhibition of port entry activity during the cue period was caused by silencing neuronal activity in the IC.
Fig. 3.
Behavioral effects of pharmacological inactivation of the IC. (A) Head entry responses during the cue period and ITI after injection of muscimol and baclofen (Mus/Bac) or saline into the IC (n = 8). Mean ± SEM. The y axis represents the response rate. (B) Time course of head entry response during Mus/Bac injection day or saline injection day (n = 8). Each dot represents the total number of head entries for each 1-s-long bin. The cue period is shown with a gray shadow. Mus/Bac (●) and saline (▲) are shown. The x axis represents the time from cue, and the y axis represents count head entries (C) Head entry responses during cue and no-cue periods after injection of Mus/Bac or saline into the somatosensory cortex (n = 8). Responding is shown as a rate in a minute. (D) Time course of head entry activity after injection of Mus/Bac or saline into the somatosensory cortex (n = 8). Each dot represents the total count of head entries during one session. Conventions are as in B. Mean ± SEM. ****P < 0.0001; n.s., not signficant.
Temporal Inactivation of IC Decreases Port Entry Activity During Cue Presentation Period.
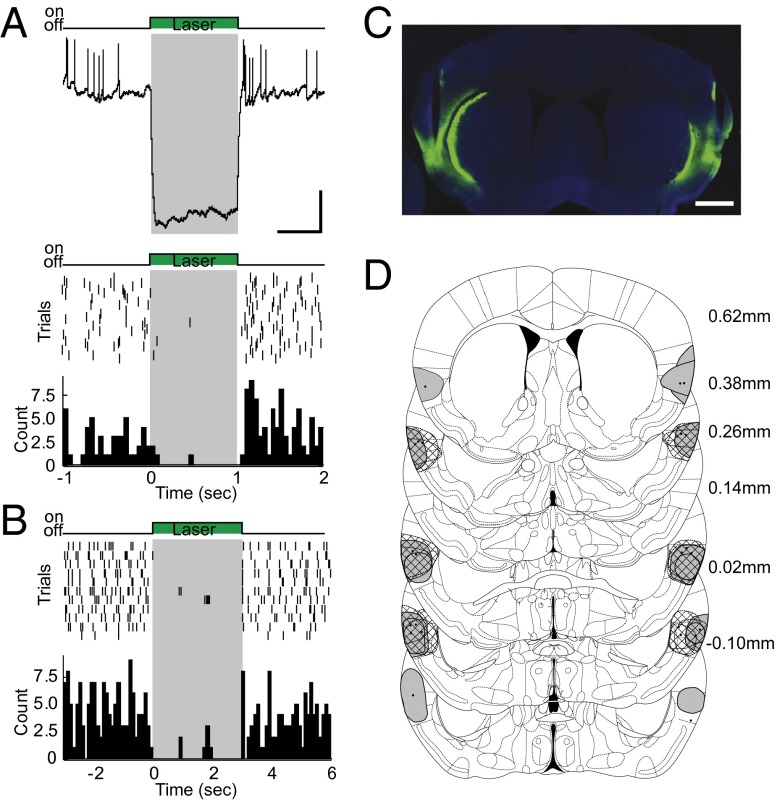
To determine whether the role of the IC in mediating conditioned responses was related to the cue-triggered activity, a series of experiments was performed using optogenetic inactivation of the IC, exclusively during the cue presentation. Insular neurons were infected by adeno-associated virus (AAV) carrying either halorhodopsin (eNpHR3.0) with enhanced yellow fluorescent protein (EYFP) or EYFP alone. First, we determined the effectiveness of green laser light (wave length = 532 nm) in silencing transfected IC neurons. Whole-cell patch-clamp recordings in slices showed that neurons in the IC were reliably hyperpolarized by a 1-s-long constant pulse of green light at 10 mW (Fig. 4A, Top). A similar result was obtained with cell-attached recordings in slices (Fig. 4A, Bottom). Consistent with this result, extracellular recordings in anesthetized rodents demonstrated a reliable suppression of ongoing firing by 3 s of constant photostimulation at 10 mW (Fig. 4B). Consistent with other reports (31, 32), a small increase in firing was occasionally observed at the offset of photostimulation; however, it was short-lasting. Optic fibers were chronically implanted in the IC of transfected mice to manipulate neuronal activity selectively during the cue presentation period (Fig. 4 C and D). Fig. 4 C and D shows the viral injection sites and the histological verification of positions of optic fibers. Inactivation of the IC in behaving mice relied on a constant single pulse of 11 s at 10 mW. Quantification of the port entry activity during the cue revealed a significant decrease in the halorhodopsin group compared with control animals (Fig. 5A; halorhodopsin: 71.13 ± 5.031, control: 97.32 ± 4.066, n = 8 for each group; unpaired t test, P < 0.01). No significant difference was observed in the no-cue period (Fig. 5B; halorhodopsin: 88.91 ± 22.81, control: 142.2 ± 20.69, n = 8 for each group; unpaired t test, P > 0.05). In addition, no significant difference in port entry activity was observed for 1 s (paired t test, P > 0.05) or 10 s (paired t test, P > 0.05) after the offset of the light (Fig. 5C; 12–25 s). This result suggests that rebound activity has no influence on conditioned responses. Comparison of the time course of port entry activity between the prestimulation session and laser stimulation session in halorhodopsin-expressing animals revealed a significant effect of laser stimulation (Fig. 5C; repeated measures of two-way ANOVA: F(1,14) = 13.21, P < 0.01). No effect was observed for laser stimulation in control animals (Fig. 5D; repeated measures of two-way ANOVA: F(1,14) = 9.556e-005, P > 0.05). Altogether, these data suggest that neuronal activity in the IC triggered by a reward-predicting cue plays an important role in modulating approach behavior to a food reward.
Fig. 4.
Optogenetic silencing in the IC. (A) Effectiveness of photostimulation in slices of the IC. (Top) In vitro whole-cell, current-clamp recording showing the average hyperpolarizing response to photostimulation in a halorhodopsin-expressing neuron. (Scale bar: vertical, 10 mV; horizontal, 0.5 s.) (Bottom) Raster plot and PSTH from an in vitro cell-attached recording showing silencing of spontaneous spiking as recorded in a cell-attached mode. The x axis represents time, and the y axis represents trials for the raster plot and counts for the PSTH. Gray shading and the green box illustrate the timing of laser stimulation. Photostimulation was performed with a 1-s-long single pulse of green light at 10 mW. (B) Raster plot and PSTH showing spiking responses recorded from an IC neuron in vivo from an anesthetized mouse. Gray shading and the green box illustrate the timing of laser stimulation. Photostimulation was performed with a 3-s-long single pulse of green light at 10 mW. (C) Representative section showing viral injection sites and fiber tracks in ICs. (Scale bar: 1 mm.) (D) Reconstruction of placements of viral injection sites and fiber tips (dots). Expression of GFP fluorescence was drawn either as a gray round shape (halorhodopsin) or a round shape filled with diamond (♢, control).
Fig. 5.
Behavioral effects of optogenetic inactivation of the IC during the cue period. (A) Comparison of head entry activity during the cue between halorhodopsin (Halo)-expressing animals and control animals (EYFP). Each data point represents the ratio of head entry activity between sessions with laser stimulation and sessions with no laser stimulation during the cue. Control animals (gray square; n = 8) and Halo animals (filled square; n = 8) are shown. The y axis represents the ratio of head entries. (B) Head entry activity during ITI. Conventions are as in A. (C) Time course of head entry activity during the prepresentation, cue presentation, and postpresentation periods in animals that have Halo expression in the IC. Animals were well trained to show stable port entry activity (learned), and results were compared with data from sessions with laser stimulation during the cue (n = 8). Performance without manipulation (●) and performance with laser stimulation (■) are shown. Gray shading illustrates the period of cue presentation. The x axis represents time around cue presentation, and the y axis represents head entries. (D) Head entry activity of control animals. Those animals received control virus injection and have only EYFP expression in the IC. Conventions are as in C.
Discussion
The experiments presented here demonstrate that IC activity in mice is significantly modulated by cues for food anticipation. Furthermore, this study shows that silencing the IC during the cue interferes with food-directed behavior. To investigate the role of the IC in mediating cue-triggered behaviors, we used a Pavlovian conditioning paradigm in which a sensory cue (light and tone) was associated with the delivery of a food pellet. Mice reliably learned the association between the cue and food, as evidenced by the consistent observation of food port entries. Electrophysiological recordings of single units in the IC of mice involved in the paradigm revealed a significant contingent of neurons that were modulated by different phases of the task. Neurons changed their firing rates in response to cues, food delivery, and food consumption. Responses to food delivery and food consumption were expected, because they are consistent with the role in taste processing that is traditionally assigned to the IC in rodents. Indeed, the rodent IC is generally considered to be the site of the primary gustatory cortex across species (19, 33–35). Although evidence for responses to anticipatory cues is less in line with classical reports on the function of the IC in rodents, the evidence is entirely consistent with more recent evidence suggesting the involvement of the IC in processing the anticipation of food and reward in general (18, 24, 25). Analysis of single-unit activity in rats has confirmed the importance of anticipatory cues in shaping neuronal firing. Neurons in the IC can be either excited or inhibited by an auditory cue predicting the availability of tasting solutions (26, 28). In addition, cues quench the variability of neural responses (26). Our results confirm and extend this evidence to mice engaged in a Pavlovian task. A difference between this and prior studies in rodents is the reliance of our experiments on longer lasting cues. The use of a 10-s-long cue allowed for an accurate and robust assessment of conditioned responses. However, the use of longer cues is likely to have an impact on the dynamics of cue responses. Indeed, in these experiments, cue responses appeared to be smaller than the strong, phasic responses seen in previous reports relying on shorter cues (26). The presence of cue-related activity in the IC begs the question of its functional role. Can this type of activity, known to be integrated with taste coding (26, 28), reflect a role of the IC in guiding behavior on the basis of expected outcomes? The IC is a highly interconnected area, and its connections to the ventral striatum and to prefrontal cortices (36–38) make it an ideal candidate to modulate behavioral output directly.
Classically, the function of the IC has been investigated by relying on permanent lesions or reversible pharmacological manipulations (14, 18, 24, 39). Inactivation of the IC interferes with an animal’s ability to retrieve expected outcomes and perform food-directed behaviors when outcomes are devaluated (40). Such a functional role of the IC in flexibly guiding behavior was further supported, and extended to nondietary rewards, by experiments showing that inhibition of orexinergic transmission in the IC reduces nicotine self-administration (25). Lesions and inactivation studies have played a fundamental role in suggesting the importance of the IC in reward-oriented behaviors. However, due to intrinsic limitations of these techniques, it has been difficult to relate the outcome of these experiments to the role of the IC in encoding anticipatory cues. To bypass this technical limitation and directly investigate the behavioral role of cue-related activity in the IC, we took advantage of optogenetics and silenced the IC exclusively during the presentation of the cue. Such a temporally and spatially restricted manipulation resulted in a significant reduction of the conditioned response, providing the first direct evidence, to our knowledge, that IC cue-related activity is necessary for the full expression of conditioned responses to the food-anticipating cue. The behavioral effects observed with optogenetic silencing were smaller than those behavioral effects seen with pharmacological inactivation, likely a consequence of its temporal and spatial selectivity compared with drug infusions. A difference between this and previous studies investigating the role of the IC in mediating associations between cues and outcomes lies in the behavioral task. Whereas previous studies relied on instrumental conditioning, requiring rodents to make associations between action and outcome, the experiments presented here are evaluating stimulus-outcome associations. In the context of instrumentally learned food-oriented actions, lesions and inactivation of the IC have an impact on performance only when animals are required to encode changes in the value of the outcome (i.e., outcome devaluation). When the motivational value of the cue is stable, lesions of the IC have little impact on instrumentally conditioned responses toward food (18). On the contrary, in the case of classical stimulus-response conditioning, optogenetic silencing during the cue disrupts conditioned responses even in motivationally stable contexts. This task-dependent difference in the effects of IC inactivation might reflect a differential involvement of this area in the two behavioral paradigms. Our results predict a strong role of the IC in expressing classical conditioning.
How do the results presented here relate to the large body of literature on the function of the human IC? Our data are entirely consistent with imaging studies, reporting the IC as one of the brain areas that respond to food and reward-predicting cues (27). The human IC has also been studied for its role in processing interoceptive signals related to hunger, satiety (41), disgust (42), cravings (43), and homeostasis in general (44). Although more experiments will be needed to understand the significance of our data for each of these processes, the results presented here suggest a profound reconceptualization of the IC. Indeed, the IC should no longer be considered just as a sensor of external and internal information. Instead, it should be viewed as a key driver of behaviors based on motivation and retrieval of learned responses. As such, the IC might represent a previously unidentified target for shaping and adjusting maladaptive behaviors.
Methods
Subjects.
Adult male and female C57BL/6J mice (8 wk old at the start of experiments) were used. Mice were housed in a 12:12-h light/dark, reverse-cycled room with unlimited access to food and water. Two days before the start of training, food delivery was restricted to maintain mice at ∼85% of free-feeding weight. Water was available at all times during the food-restricted period in the home cages. All experiments were reviewed and approved by the National Institute on Drug Abuse Institutional Animal Care and Use Committee and by the Institutional Animal Care and Use Committee of Stony Brook University.
Cannula Implantation.
Mice were anesthetized with ketamine and xylazine i.p. injected (80 mg/kg, and 20 mg/kg, respectively) and fixed on a stereotaxic device (David Kopf Instruments). The surface of the skull was exposed, and small holes were drilled above the IC (0.26 mm anteroposterior and 3.9 mm mediolateral, relative to bregma). Stainless-steel guide cannulae (26-gauge; Plastics One) were implanted bilaterally through the holes at a depth of 2.8 mm from the bregma. Cannulae were secured in place with dental acrylic, and a stylet was inserted beyond the end of the cannula (0.2-mm extension). The recovery period was set as 1 wk, before starting food restriction and habituation.
Virus Injection and Optic Fiber Implantation in the Insula.
To allow maximum gene expression, we performed surgery in 5-wk-old mice. Mice were anesthetized (as above), and a hole was drilled in the skull just above the IC. A 29-gauge stainless-steel cannula was lowered to the depth of 3.8 mm relative to the bregma, and 0.5 μL of either AAV1-alpha-calcium/calmodulin-dependent protein kinase II promoter (CaMKIIα)-eNpHR3.0-EYFP (n = 8) or AAV1-CaMKIIα-EYFP (n = 8) (National Institute on Drug Abuse Intramural Research Program Optogenetics and Transgenic Technology Core) was infused into the target site at 0.1 μL⋅min−1. Five minutes after viral infusion, the cannula was removed and a custom-made implantable optic fiber [4-mm length below ferrule, 200-μm core multimode (Thorlabs), 1.25 mm-outside diameter ceramic zirconia ferrule (Precision Fiber Products)] was lowered into place 0.3 mm above the site of injection and secured with dental acrylic. All mice were allowed to recover for at least 2 wk after surgery and were started on food restriction and training 4 wk after surgery.
Electrode Implantation.
Mice were anesthetized using an i.p.-injected ketamine/xylazine mixture (100 mg/kg and 10 mg/kg, respectively) with supplemental doses (30% of induction dose) to maintain surgical levels of anesthesia. After placing mice in a stereotaxic device, the scalp was sterilized with 0.1% iodine and excised to reveal the skull. Holes were drilled for anchoring screws and electrode bundles. Microdrivable electrode bundles, consisting of 16 or eight 25-μm formvar-coated nichrome microwires, were inserted dorsal to the IC in one or both hemispheres. All implants were cemented to the skull with dental acrylic. Mice were allowed 7–10 d of recovery before beginning behavioral training. Proper placement of electrodes was histologically verified using standard procedures.
Behavioral Training.
Training took place in an operant chamber (ENV-307W; 8.5 inches long × 7.0 inches wide × 5.0 inches high; Med Associates, Inc.) housed within a light-resistant and sound-attenuating cubicle. The chamber was equipped with a food magazine that received 20-mg pellets (5TUL; Test Diet) from a pellet dispenser, and it had a house light and two sound speakers on the wall opposite the magazine. Computers running the Med-PC-IV program (Med Associates, Inc.) were used to control the chambers and record behavior. After a recovery period from surgery, mice were trained to get food pellets from the food magazine. Fifteen food pellets were delivered randomly over 30 min for 2 d (habituation sessions). On subsequent days, mice underwent Pavlovian conditioning. The combination of tone and house light cues [conditioned stimulus (CS)] was paired with delivery of a 20-mg food pellet [unconditioned stimulus (US)]. The association learning task was adapted from a task described before (45). Each training session began with a 45- to 75-s ITI. After the interval period, the CS was turned on for 11 s. A pellet was delivered following 10 s of the CS onset. Mice were allowed to access the food magazine freely, and both the number and timing of head entry to the magazine were recorded. After 11 s of the CS presentation, the ITI was started and the trial was repeated 25 times in each session. Each session lasted for 30 min, and the sessions were continued until the mice showed a stable head entry response. Learning was assessed by comparing anticipatory head entries to the food magazine during the CS and the ITI. Head entry rate was obtained by dividing the number of head entries during each period by time and was converted to rate per minute. For experiments using local drug infusion in the IC, mice were injected with 0.3 μL of muscimol/baclofen solution (0.05 μg/μL and 0.02 μg/μL, respectively) or saline at a rate of 0.1 μL⋅min−1. Drugs were infused on alternating days through a 33-gauge stainless-steel cannula that was inserted in the chronic guide cannula and protruded 1 mm below the tip. Five minutes after infusion, the injection cannula was removed, and the mice were allowed to recover in their home cage for 15 min before starting the session.
Optogenetic Stimulation.
For all optogenetic experiments, either eNpHR3.0 with EYFP or EYFP-tagged viral vector under control of the CaMKIIα promoter was used. These viruses provide eNpHR3.0 (or EYFP) expression that is highly specific to pyramidal cells in a cortical structure (46). Mice started behavioral training with optical cables connected on their head cap with a sleeve (Precision Fiber Products). The fiber was connected to a 532-nm laser (OEM Laser) located outside the operant chamber. The laser was controlled with Master-9 (AMPI) and Med-PC-IV. Animals performed the same task described above, but the laser was activated for 11 s of each cue presentation period. We used a single laser pulse, which lasted for the entire duration of the cue period. Before testing, the final output of the laser was adjusted to 10 mW. According to the literature (47) and our laboratory’s prior experience (48), this level of output, combined with the characteristics of the fiber used, guarantees a conical spread that can reach a depth of 1 mm from the tip of the fiber.
In Vitro Electrophysiology.
For whole-cell recording, 250-μm coronal slices were cut from virus-injected mice (n = 2). We waited at least 4 wk after virus injection before starting the slice recording experiments. Brains were quickly removed and placed into ice-cold solution bubbled with 95% O2 and 5% (vol/vol) CO2 containing the following: 75 mM sucrose, 55 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 10 mM MgSO4, 0.5 mM CaCl2, 26 mM NaHCO3, and 25 mM glucose. After 5 min, brains were blocked and coronal slices were taken. During the recovery period, slices were placed at 35 °C with oxygenated artificial cerebrospinal fluid (aCSF) solution containing the following: 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 1.0 mM MgCl2, 2.4 mM CaCl2, 26 mM NaHCO3, and 11 mM glucose. All recordings were made under continuous perfusion of aCSF at 32 °C with a flow rate of 2 mL⋅min−1. Pipettes (2.5–5 MOhm) for voltage and current-clamp experiments contained the following: 140 mM potassium methylsulfate (KMeSO4), 5 mM KCl, 0.05 mM EGTA, 10 mM HEPES, 2 mM MgCl2, 2 mM ATP, and 0.4 mM GTP (pH 7.2–7.3; 280 mOsm). Recordings were made in the IC from visually identified pyramidal neurons. For recordings of pyramidal neurons, slices were stimulated with 532 nm green light. During recordings, 1 s of light was delivered with a power of 10 mW.
Behavioral Electrophysiology.
Recordings from all microwires were amplified, band pass-filtered at 300--8,000 Hz, and digitized (Plexon). Single-neuron action potentials of >3:1 signal-to-noise ratio were isolated using a waveform template algorithm from off-line cluster sorting software (Offline Sorter; Plexon).
Recordings from Anesthetized Animals.
A few animals (n = 3) were used to check the effects of neuronal inhibition by halorhodopsin. Animals were anesthetized with i.p.-injected ketamine and xylazine. The surface of the IC was exposed from the lateral side of the brain. The skull was opened above the IC, and a recording electrode (Neuronexus) was inserted. Stimulation light (532 nm) was delivered through the implanted optic fiber. All recordings were performed with a Blackrock Cerebus system.
Histology.
After the completion of experiments, cannula- or fiber-implanted mice were anesthetized with euthasol and transcardially perfused with 0.1 M phosphate buffer (PB), followed by 4% (wt/vol) paraformaldehyde in 0.1 M PB. The brains were sliced into 50-μm coronal sections with a vibratome (VT-1200; Leica), and Nissl staining was performed for cannula-implanted animals. To verify EYFP expression by virus injection, we used 2% Neurotrace (Life Technologies) or 4′,6-diamidino-2-phenylindole staining to visualize layer structure. After completion of chronic recordings, mice were deeply anesthetized. A 10-s, 10-μA direct current was passed through several electrodes of the bundle to make lesion markers for histological examination. Then, mice were perfused with 3.7% (wt/vol) formaldehyde. Eighty-micrometer coronal sections were made through the IC area. All recording positions were confirmed to be within the IC region, and mice with electrode tracts falling outside of the IC region were excluded from the study.
Data Analysis and Statistics.
All data from behavior experiments were analyzed with Microsoft Excel and NeuroExplorer. Data from electrophysiology experiments were analyzed with NeuroExplorer and Spike2. Graphpad Prism was used for statistical analysis. Data analyses of chronic recordings were performed with custom MATLAB scripts (MathWorks). Details on analyses of chronic recordings are provided in SI Methods.
Supplementary Material
Acknowledgments
We thank K. Deisseroth for providing the NpHR3.0 vectors and the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP) Optogenetics and Transgenic Technology Core for assistance with the AAV vectors. We thank Joo J. and Goddard S. for technical support. We also thank members of the Synaptic Plasticity Section in NIDA IRP for discussion. This work was supported by funds from the Rotary Foundation (I.K.-Y.), Japan Society for the Promotion of Science Research Fellowship for Japanese Biomedical and Behavioral Researchers at NIH (I.K.-Y.), and NIDA IRP and National Institute of Deafness and Other Communication Disorders–National Institutes of Health Grant R01 - DC012543 (to A.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416573112/-/DCSupplemental.
References
- 1.Cheng K, Newcombe NS. Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychon Bull Rev. 2005;12(1):1–23. doi: 10.3758/bf03196346. [DOI] [PubMed] [Google Scholar]
- 2.Garber PA, Hannon B. Modeling monkeys—A comparison of computer-generated and naturally-occurring foraging patterns in 2 species of neotropical primates. Int J Primatol. 1993;14(6):827–852. [Google Scholar]
- 3.Stevens M. Sensory Ecology, Behaviour, and Evolution. 1st Ed Oxford Univ Press; Oxford: 2013. [Google Scholar]
- 4.Berridge KC. From prediction error to incentive salience: Mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35(7):1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- 6.Petrovich GD. Learning and the motivation to eat: Forebrain circuitry. Physiol Behav. 2011;104(4):582–589. doi: 10.1016/j.physbeh.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bissonette GB, Gentry RN, Padmala S, Pessoa L, Roesch MR. Impact of appetitive and aversive outcomes on brain responses: Linking the animal and human literatures. Front Syst Neurosci. 2014;8:24. doi: 10.3389/fnsys.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jodo E, Suzuki Y, Kayama Y. Selective responsiveness of medial prefrontal cortex neurons to the meaningful stimulus with a low probability of occurrence in rats. Brain Res. 2000;856(1-2):68–74. doi: 10.1016/s0006-8993(99)02386-0. [DOI] [PubMed] [Google Scholar]
- 9.Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol. 2004;91(4):1840–1865. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- 10.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1(2):155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 11.Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23(2):229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Duuren E, et al. Single-cell and population coding of expected reward probability in the orbitofrontal cortex of the rat. J Neurosci. 2009;29(28):8965–8976. doi: 10.1523/JNEUROSCI.0005-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bermudez-Rattoni F, McGaugh JL. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Res. 1991;549(1):165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- 14.Braun JJ, Slick TB, Lorden JF. Involvement of gustatory neocortex in the learning of taste aversions. Physiol Behav. 1972;9(4):637–641. doi: 10.1016/0031-9384(72)90023-6. [DOI] [PubMed] [Google Scholar]
- 15.de Araujo IE, et al. Neural ensemble coding of satiety states. Neuron. 2006;51(4):483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira-Maia AJ, et al. The insular cortex controls food preferences independently of taste receptor signaling. Front Syst Neurosci. 2012;6:5. doi: 10.3389/fnsys.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Sensory inputs from the oral region to the cerebral cortex in behaving rats: An analysis of unit responses in cortical somatosensory and taste areas during ingestive behavior. J Neurophysiol. 1988;60(4):1303–1321. doi: 10.1152/jn.1988.60.4.1303. [DOI] [PubMed] [Google Scholar]
- 18.Balleine BW, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: Evidence for a role in incentive memory. J Neurosci. 2000;20(23):8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jezzini A, Mazzucato L, La Camera G, Fontanini A. Processing of hedonic and chemosensory features of taste in medial prefrontal and insular networks. J Neurosci. 2013;33(48):18966–18978. doi: 10.1523/JNEUROSCI.2974-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maier JX, Katz DB. Neural dynamics in response to binary taste mixtures. J Neurophysiol. 2013;109(8):2108–2117. doi: 10.1152/jn.00917.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto T, Yuyama N, Kawamura Y. Responses of cortical taste cells and chorda tympani fibers to anodal d.c. stimulation of the tongue in rats. Exp Brain Res. 1980;40(1):63–70. doi: 10.1007/BF00236663. [DOI] [PubMed] [Google Scholar]
- 22.Frank GK, Shott ME, Hagman JO, Mittal VA. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013;170(10):1152–1160. doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maffei A, Haley M, Fontanini A. Neural processing of gustatory information in insular circuits. Curr Opin Neurobiol. 2012;22(4):709–716. doi: 10.1016/j.conb.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318(5850):655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 25.Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci USA. 2008;105(49):19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuelsen CL, Gardner MP, Fontanini A. Effects of cue-triggered expectation on cortical processing of taste. Neuron. 2012;74(2):410–422. doi: 10.1016/j.neuron.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiol Behav. 2012;106(3):317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Gardner MP, Fontanini A. Encoding and tracking of outcome-specific expectancy in the gustatory cortex of alert rats. J Neurosci. 2014;34(39):13000–13017. doi: 10.1523/JNEUROSCI.1820-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuelsen CL, Gardner MP, Fontanini A. Thalamic contribution to cortical processing of taste and expectation. J Neurosci. 2013;33(5):1815–1827. doi: 10.1523/JNEUROSCI.4026-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saddoris MP, Holland PC, Gallagher M. Associatively learned representations of taste outcomes activate taste-encoding neural ensembles in gustatory cortex. J Neurosci. 2009;29(49):15386–15396. doi: 10.1523/JNEUROSCI.3233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goshen I, et al. Dynamics of retrieval strategies for remote memories. Cell. 2011;147(3):678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 32.Madisen L, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15(5):793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto T. Taste responses of cortical neurons. Prog Neurobiol. 1984;23(4):273–315. doi: 10.1016/0301-0082(84)90007-8. [DOI] [PubMed] [Google Scholar]
- 34.Soares ES, et al. Behavioral and neural responses to gustatory stimuli delivered non-contingently through intra-oral cannulas. Physiol Behav. 2007;92(4):629–642. doi: 10.1016/j.physbeh.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackey WB, Keller J, van der Kooy D. Visceral cortex lesions block conditioned taste aversions induced by morphine. Pharmacol Biochem Behav. 1986;24(1):71–78. doi: 10.1016/0091-3057(86)90047-x. [DOI] [PubMed] [Google Scholar]
- 36.Wright CI, Groenewegen HJ. Patterns of overlap and segregation between insular cortical, intermediodorsal thalamic and basal amygdaloid afferents in the nucleus accumbens of the rat. Neuroscience. 1996;73(2):359–373. doi: 10.1016/0306-4522(95)00592-7. [DOI] [PubMed] [Google Scholar]
- 37.Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311(1):1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- 38.Kelley AE. Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27(8):765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010;68(3):265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 40.Parkes SL, Balleine BW. Incentive memory: Evidence the basolateral amygdala encodes and the insular cortex retrieves outcome values to guide choice between goal-directed actions. J Neurosci. 2013;33(20):8753–8763. doi: 10.1523/JNEUROSCI.5071-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tataranni PA, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA. 1999;96(8):4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wicker B, et al. Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- 43.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: Food-craving activation during fMRI. Neuroimage. 2004;23(4):1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 44.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 45.Parker JG, et al. Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning. Proc Natl Acad Sci USA. 2010;107(30):13491–13496. doi: 10.1073/pnas.1007827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calu DJ, et al. Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. J Neurosci. 2013;33(1):214–226. doi: 10.1523/JNEUROSCI.2016-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tye KM, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471(7338):358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen BT, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496(7445):359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.