Abstract
ATM, ATR and DNA-PK are critical for DNA damage response (DDR) and sequential repair, leading to genomic stability. In this study, we found the expression of these proteins is markedly induced by PMA during THP1 differentiation without the change in the level of transcripts. Also, inhibitors of these protein activity suppressed PMA-induced morphological change of THP1 cells. Our results suggest the potential roles of these DDR proteins in cellular differentiation.
Keywords: DNA damage response, Severe combined immunodeficiency, Antigen presenting cells
INTRODUCTION
Mechanisms of detecting and repairing damaged DNA are conserved and controlled through the DNA damage response (DDR). DDR is essential to maintain genome stability and suppress malignant progresses [1]. ATM (ataxia telangiectasiamutated), ATR (ataxia telangiectasia and Rad3-related) are key DDR signaling components, which are activated by DNA damages to lead to downstream effectors, such as Chk1/2, SMC1, BRCA1, and p53 [2]. The DNA-dependent protein kinase (DNA-PK) plays key roles in the repair through the non-homologous end-joining (NHEJ) pathway [3]. These DDR proteins are included in the phsophatidylinositol 3′-kinase (PI3K) -related kinase (PIKK) family, which have multiple roles in cell growth, metabolism and differentiation [4]. For examples, ATM plays a role in the proliferation of neuronal stem cell (NSC) [5], and mice deleted ATM gene displayed impaired T cell development leading to decreased total T cell number [6,7]. It has be well known that mouse severe combined immunodeficiency (SCID) is induced by DNA-PK deficiency [8]. However, it remains unclear whether the expression of these proteins is required for development of antigen presenting cells (APCs), such as DCs and MPs, or how these proteins are regulated during development of these cells. To address these questions, we have investigated expression patterns of these proteins during mitogen induced differentiation of THP1. To test if inhibition of these proteins can effect on cell differentiation, we checked the morphological change after treatment with specific inhibitors of ATM, ATR and DNA-PK. We found that protein expression of these PIKKs significantly increased (over 100 fold) after mitogen treatment through mainly posttranscriptional regulation, and that three different specific inhibitors block the differentiation. These finding implicated that protein expression of PIKKs is required and mechanisms by which PIKKs is regulated during cellular differentiation, are critical for differentiation/development.
MATERIALS AND METHODS
Cells and reagents
Human monocytic cell line THP1 was purchased from ATCC (Manassas, VA), and maintained in RPMI medium containing 10% FBS (Invitrogen, Carlsbad, CA) and penicillin/Streptomycin. Phorbal 12-myristate 13-acetate (PMA), Neocarzinostatin (NCS) and MG 132 were purchased from Sigma (St. Louis, MO), and dissolved in DMSO. KU55933 and NU7441 were obtained from TOCRIS (Bristol, UK). Schisandrin B (Sch-B) was gratefully provided by Dr. Konishi (Niigata University, Japan)
Western blotting
Cells were treated with indicated chemicals for indicated times. Total cell lysate (20 or 30 mg/lane) was loaded and separated by SDS-PAGE. Transfer to a PVDF membrane (Millipore, Billerica, MA) was performed using semi-dry transfer method. Primary antibodies used in this study were anti-DNA-PK, anti-ATM (GeneScript, Piscataway, NJ), Anti-SMC1, anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-BRAT1 (abcam, Cambridge, MA). To detect phosphorylation of some proteins, we used anti-phospho-DNA-PK (Thr 2609, Rockland, Gilbertsville, PA), anti-phospho-ATM (Ser1981, Cell signaling, Danvers, MA), and anti-phospho-SMC1 (Ser966, Bethyl Laboratories, Montgomery, TX).
RNA isolation and RT-PCR
Total RNA was isolated using TRIzol Reagent (Invitorgen) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 4 mg total RNA using MMLV reverse transcriptase Kit (Invitrogen). Primers were obtained from Integrated DNA Technologies (Coralville, IA) as follows: DNA-PK: 5′ACCAGCATGAGCCCAGATTATCCA3′ and 5′CGGCCGCACCTTTCACTTTGTTAT3′, ATM: 5′-AACTCTTGTCCGGTGTTCACGTCT3′ and 5′ACTTTGGCTCTCTCCAGGTTCGTT3′, BRAT1: 5′AAACGGTCACTGAAGGAGAGTCCA3′ and 5′ACGTGATCCATGATCTTCTGGGCA3′, GAPDH: 5′-AAGGTCGGAGTCAACGGATTTGGT-3′ and 5′-AGTGATGGCATGGACTGTGGTCAT-3′. GAPDH expression was assessed as an internal reference for normalization.
Microscopy
THP1 cells were treated with indicated chemical for 24 h, and morphological change was observed by inverted microscope (×40), and analyzed by Spot software (Sterling Heights, MI).
RESULTS AND DISCUSSIONS
DDR proteins are required for THP1 differentiation
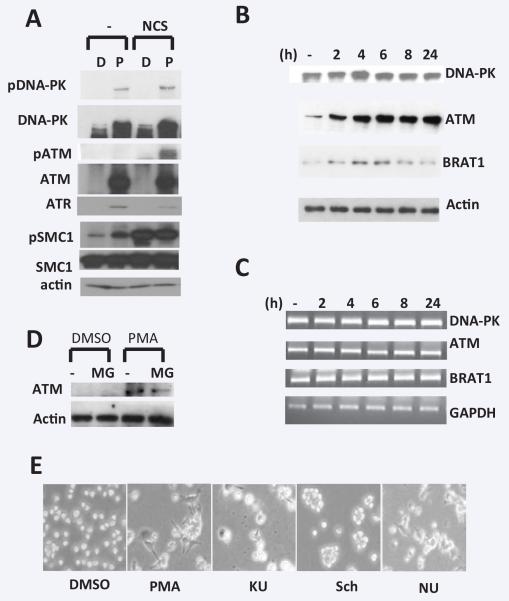
Earlier studies described that the level of ATM protein markedly increases in phytohemagglutinin (PHA) treated peripheral blood mononuclear cells (MNCs) without change in ATM mRNA and protein stability [9]. Although variation in expression of ATM transcripts between different tissues has been observed [10], ATM expression in specific cell types during differentiation has not been analyzed. The human monocytic cell line THP-1 can be differentiated to cell with macrophage-like characteristics by PMA. To investigate the role of PIKKs in PMA-induced THP1 differentiation, we examined the level of proteins and activity of DNA-PK, ATM and ATR. (Figure 1A). THP1 cells were treated with PMA for 48 h to differentiate into macrophage. Immunoblotting data indicate that the protein expression of DNA-PK, ATM and ATR is almost undetectable in undifferentiated THP1 cells, but significantly increased after differentiation. In contrast, the level of SMC1 protein was not changed during differentiation. DNA-damaging agent (NCS) treatment didn’t change PIKK protein level in THP1, while we found the treatment with PMA induces phosphorylation of DNA-PK and SMC1, suggesting that DDR and DNA repair may be happened during PMA-induced differentiation, but it remains to be clear. Next, we examined mRNA and protein expression of DNA-PK, ATM and BRAT1 in different times during PMA-induced differentiation. BRAT1 was recently identified as regulator of ATM/DNA-PK-mediated DDR [11]. THP-1 cells were treated with relatively low concentration (10 ng/ml) at the time of 2, 4, 6, 8, 24 hours. Total RNA and total extract were prepared from cells for RT-PCR and western blot analysis, respectively (Figure 1B and 1C). While all observed proteins were very low level in untreated THP-1, it dramatically increased by 2 h and reached to maximal at 6 h. Because the amount of proteins might be reflected in transcripts level, we also examine mRNA level of these genes during differentiation. As shown in Figure 1C, mRNA levels in untreated and PMA-stimulated THP-1 cells were not significantly different over 0 to 24 h and could not account for the increased ATM protein in PMA-stimulated cells. Another explanation for the higher level of PIKK’s proteins in PMA-treated cells could be decreased function of protein degradation by proteasome. However, as shown in Figure 1D, the treatment of MG132, specific proteasome inhibitor, couldn’t change ATM protein levels in differentiated THP-1 cells, compared to untreated cells. Our results indicate that the increase in PIKK protein in PMA-induced differentiation of monocytic cell lines is due to posttranscriptional regulation including translational regulation and protein stabilization. Next, we used a series of PIKK inhibitors to suppress kinase activity of ATM, ATR, and DNA-PK in THP1 cells. The ATM inhibitor KU-55933 (KU) suppresses cell proliferation by blocking overactivated Akt [12]. Schisandrin B (SchB) was recently isolated from an active ingredient of Fructus schisandrae, inhibiting ATR kinase activity following DNA damage [13]. NU7441 is a highly selective DNA-PK inhibitor, which increases the persistence of gH2AX foci after IR-induced DNA damage [4]. THP1 were treated with these inhibitors after PMA treatment (6h post-treatment), and morphological change was detected under bright microscope (Figure 1D). As we know, PMA-treated THP1 showed typical macrophage shape (elongated, flattened). The inhibition of kinase activity suppressed PMA-induced morphological changed, suggesting PIKK are required for mitogen-induced THP1 differentiation.
Figure 1. The role of DDR proteins in PMA-induced THP1 differentiation.
A. THP1 cells were treated without (D, DMSO) or with PMA (P, 50 ng/ml). After 48 h, NCS (0.5 μg/ml) was added into media and cells were cultured for 1 h more. Total extracts were isolated for western blot analysis. B and C. Cells were treated with PMA (10 ng/ml) for indicated times and total lysates and total RNA were isolated for western blot (B) and RT-PCR analysis (C), respectively. Actin protein and GAPDH transcript were used as internal control, respectively. D. Cells were pre-treated with or without MG132 (MG, 5 mM) for 3 h prior to PMA treatment for 24 h. Total lysates were isolated to detect human ATM as above. E. Cells were post-treated indicated inhibitors (KU: KU55933 5 mM, Sch: Schisandrin B 30 mM, NU: NU7441 5 mM), after PMA 6 h treatment. Cells in culture plates were visualized by bright microscope (×40) and taken picture using Spot imaging systems. This picture was representative of two independent experiments.
In conclusion, our data suggests the novel role in cell differentiation of PIKK, which are well-known DDR proteins. Which of the mechanisms are involved in this regulation will be studied in future experimental projects.
ACKNOWLEDGE
We thank all the members of the Ouchi laboratory for discussion of the results. This work is supported by NIH R01CA90631, Susan G Komen Breast Cancer Grant and Matsutani America Cancer Research Fund.
REFERENCES
- 1.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 3.Dobbs TA, Tainer JA, Lees-Miller SP. A structural model for regulation of NHEJ by DNA-PKcs autophosphorylation. DNA Repair (Amst) 2010;9:1307–1314. doi: 10.1016/j.dnarep.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman MH, Bassing CH, Teitell MA. Regulation of cell differentiation by the DNA damage response. Trends Cell Biol. 2011;21:312–319. doi: 10.1016/j.tcb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J, Wong PK. Loss of ATM impairs proliferation of neural stem cells through oxidative stress-mediated p38 MAPK signaling. Stem Cells. 2009;27:1987–1998. doi: 10.1002/stem.125. [DOI] [PubMed] [Google Scholar]
- 6.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 7.Bagley J, Cortes ML, Breakefield XO, Iacomini J. Bone marrow transplantation restores immune system function and prevents lymphoma in Atm-deficient mice. Blood. 2004;104:572–578. doi: 10.1182/blood-2003-12-4226. [DOI] [PubMed] [Google Scholar]
- 8.Jhappan C, Morse HC, 3rd, Fleischmann RD, Gottesman MM. Nat Genet. 1997;17:483–486. doi: 10.1038/ng1297-483. [DOI] [PubMed] [Google Scholar]
- 9.Fukao T, Kaneko H, Birrell G, Gatei M, Tashita H, Yoshida T, et al. ATM is upregulated during the mitogenic response in peripheral blood mononuclear cells. Blood. 1999;94:1998–2006. [PubMed] [Google Scholar]
- 10.Starczynski J, Simmons W, Flavell JR, Byrd PJ, Stewart GS, Kullar HS, et al. Variations in ATM protein expression during normal lymphoid differentiation and among B-cell-derived neoplasias. Am J Pathol. 2003;163:423–432. doi: 10.1016/S0002-9440(10)63672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aglipay JA, Martin SA, Tawara H, Lee SW, Ouchi T. ATM activation by ionizing radiation requires BRCA1-associated BAAT1. J Biol Chem. 2006;281:9710–9718. doi: 10.1074/jbc.M510332200. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Yang DQ. The ATM inhibitor KU-55933 suppresses cell proliferation and induces apoptosis by blocking Akt in cancer cells with overactivated Akt. Mol Cancer Ther. 2010;9:113–125. doi: 10.1158/1535-7163.MCT-08-1189. [DOI] [PubMed] [Google Scholar]
- 13.Nishida H, Tatewaki N, Nakajima Y, Magara T, Ko KM, Hamamori Y, et al. Inhibition of ATR protein kinase activity by schisandrin B in DNA damage response. Nucleic Acids Res. 2009;37:5678–5689. doi: 10.1093/nar/gkp593. [DOI] [PMC free article] [PubMed] [Google Scholar]