Abstract
BACKGROUND
No single standard treatment exists for patients with small, node-negative, human epidermal growth factor receptor type 2 (HER2)–positive breast cancers, because most of these patients have been ineligible for the pivotal trials of adjuvant trastuzumab.
METHODS
We performed an uncontrolled, single-group, multicenter, investigator-initiated study of adjuvant paclitaxel and trastuzumab in 406 patients with tumors measuring up to 3 cm in greatest dimension. Patients received weekly treatment with paclitaxel and trastuzumab for 12 weeks, followed by 9 months of trastuzumab monotherapy. The primary end point was survival free from invasive disease.
RESULTS
The median follow-up period was 4.0 years. The 3-year rate of survival free from invasive disease was 98.7% (95% confidence interval [CI], 97.6 to 99.8). Among the 12 relapses seen, 2 were due to distant metastatic breast cancer. Excluding contra-lateral HER2-negative breast cancers and nonbreast cancers, 7 disease-specific events were noted. A total of 13 patients (3.2%; 95% CI, 1.7 to 5.4) reported at least one episode of grade 3 neuropathy, and 2 had symptomatic congestive heart failure (0.5%; 95% CI, 0.1 to 1.8), both of whom had normalization of the left ventricular ejection fraction after discontinuation of trastuzumab. A total of 13 patients had significant asymptomatic declines in ejection fraction (3.2%; 95% CI, 1.7 to 5.4), as defined by the study, but 11 of these patients were able to resume trastuzumab therapy after a brief interruption.
CONCLUSIONS
Among women with predominantly stage I HER2-positive breast cancer, treatment with adjuvant paclitaxel plus trastuzumab was associated with a risk of early recurrence of about 2%; 6% of patients withdrew from the study because of protocol-specified adverse events. (Funded by Genentech; ClinicalTrials.gov number, NCT00542451.)
Overexpression of the human epidermal growth factor receptor type 2 (HER2) occurs in approximately 15 to 20% of invasive breast cancers and was historically associated with poor clinical outcomes.1–4 Trastuzumab, a humanized monoclonal antibody that binds HER2, improves the outcomes for patients with HER2-positive breast cancer. Four phase 3 randomized trials involving more than 8000 patients showed that when trastuzumab was administered in combination with or after chemotherapy, the risk of recurrence was decreased by approximately 50% and overall survival improved.5–9 These trials focused largely on patients with stage II or stage III HER2-positive breast cancers.
Although patients with stage I HER2-positive tumors are expected to derive a smaller absolute benefit from adjuvant therapy than those with larger or node-positive tumors, they remain at more than minimal risk for a recurrence of breast cancer.10–14 However, given the more limited benefit from adjuvant treatment in these patients, the decision to use trastuzumab and chemotherapy is influenced by the toxicity of the treatment regimen.
Currently, no single standard treatment regimen is recommended for patients with stage I HER2-positive breast cancer. We conducted a single-group, multicenter, investigator-initiated study to characterize the prospective outcomes in a group of patients uniformly treated with paclitaxel and trastuzumab, a regimen that is expected to be less toxic than the traditional adjuvant regimens.
METHODS
ELIGIBILITY AND ENROLLMENT
Enrollment required a pathological diagnosis of adenocarcinoma of the breast, with immunohistochemical staining for the HER2 protein of 3+ intensity or amplification of the HER2 gene on fluorescence in situ hybridization (ratio of HER2 to chromosome 17 centromere [CEP17], ≥2.0). The invasive tumor had to measure no more than 3 cm in the greatest dimension; there was no lower limit on tumor size. Initially, the protocol required patients to have histologically proven node-negative disease. The protocol was amended to allow entry of patients who had one lymph-node micrometastasis if an axillary dissection was completed and no further lymph-node involvement was detected. Other requirements included adequate hematopoietic and liver function and a left ventricular ejection fraction of 50% or greater. The institutional review board at each participating institution approved the study. Written informed consent was provided by all the participants.
The study was designed by the first author and the last two authors. The data were collected by the Dana–Farber Cancer Institute and analyzed by the lead and assistant statisticians (the second and the fifteenth authors, respectively) in collaboration with the first and last authors, both of whom vouch for the completeness and accuracy of the data and analyses and for the fidelity of the study to the protocol. No one who was not an author contributed to the writing of the manuscript. Genentech provided funding for the study but did not provide paclitaxel or trastuzumab; these agents were commercially supplied, and the costs were billed to insurance companies. The protocol is available with the full text of this article at NEJM.org.
TREATMENT REGIMEN
Treatment consisted of the intravenous administration of 80 mg of paclitaxel per square meter of body-surface area weekly for 12 weeks and a loading dose of 4 mg of intravenous trastuzumab per kilogram of body weight on day 1, followed by 2 mg per kilogram weekly, for a total of 12 doses. After the completion of 12 weeks of treatment with trastuzumab, the dosing of trastuzumab could be continued on a weekly basis, or the regimen could be changed to 6 mg per kilogram every 3 weeks for 40 weeks to complete a full year of intravenous treatment with trastuzumab.
Patients who underwent lumpectomy were required to receive either partial-breast radiation, which was performed before the initiation of the protocol therapy, or radiation of the whole breast, which was initiated after the completion of treatment with paclitaxel. Treatment with trastuzumab was continued during the time the patient was receiving radiation therapy. Adjuvant hormonal therapy was recommended for women with hormone-receptor–positive tumors after the completion of paclitaxel therapy.
ASSESSMENT OF CARDIAC FUNCTION
The protocol required assessment of the left ventricular ejection fraction with echocardiography or multigated acquisition scanning at baseline and at 12 weeks, 6 months, and 1 year after the start of protocol therapy. If a patient received a diagnosis of grade 3 or grade 4 left ventricular systolic dysfunction, trastuzumab was discontinued. Interruption of dosing with trastuzumab was required if either of the following conditions was met: a decrease in the ejection fraction of 10 to 15 percentage points from baseline, with the ejection fraction at least 1 percentage point below the lower limit of the normal range at the radiology facility in which the assessment was performed; or a decrease of 16 or more percentage points from baseline. If either of these conditions was met, another assessment of ejection fraction was required after 4 weeks; if the ejection fraction did not increase substantially and two consecutive interruptions in the protocol therapy were required, the patient was withdrawn from the study treatment.
STATISTICAL ANALYSIS
The primary measure of efficacy was the number of events of invasive disease (recurrence and new invasive disease) and death from any cause, as defined by the standardized efficacy end-points (STEEP) criteria.15 We used a group-sequential Poisson test that was based on the total patient-years of follow-up. In designing the trial, a 3-year event rate of 9.2% was deemed to be unacceptable in this patient population. In contrast, a 3-year rate of invasive disease of 5% would be considered to be successful, and the study was powered to have a 95% probability of rejecting the null hypothesis. The planned sample size was 400 patients. Two interim analyses for futility were scheduled after accrual of 225 and 800 patient-years, with a final analysis conducted after accrual of 1600 total patient-years of follow-up. If 39 or fewer events of invasive disease were observed at the time of the final analysis, the regimen would be considered to be effective. Overall one-sided type I and type II errors of 0.05 were controlled in a group-sequential design by means of a Pocock-style error-spending function for beta that is constrained by the exact Poisson distribution.
Estimation of the survival and of cumulative probability functions for survival free from invasive disease was performed by means of the Kaplan–Meier product-limit method; confidence boundaries were calculated on the log-scale with the use of Greenwood’s formula for the variance. Planned subgroup analyses were performed in strata defined according to tumor size (≤1 cm or >1 cm) and hormone-receptor status (with a positive status defined as staining of 1% or more of either the estrogen receptor or the progesterone receptor on immunohistochemical analysis). The analyses of the incidence of adverse events, including planned analyses of cardiac toxicity and neurotoxicity of grade 3 or higher, were reported with 95% confidence intervals from the binomial distribution. In an amendment to the protocol, the follow-up period was extended to 10 years for all patients. Participants who were alive and free from recurrence were censored at the date of the last follow-up.
RESULTS
PATIENT-YEARS OF FOLLOW-UP
A total of 410 patients were enrolled in the study between October 9, 2007, and September 3, 2010 (see Table S1 in the Supplementary Appendix, available at NEJM.org); 406 of these patients began the protocol therapy (Fig. 1). The data and safety monitoring board at the Dana–Farber/Harvard Cancer Center reviewed data on toxicity on a semi-annual basis. The first interim analysis was performed at 167 patient-years, before the target accrual was reached, and the subsequent interim analysis was performed after the accrual of 841 patient-years. On the basis of the strength of the data at the second analysis, the study team asked the board to consider early release of the data from a third interim analysis, which was performed after accrual of 1316 patient-years. The board released the data to the study team, and the results were initially presented after accrual of 1435 patient-years. The results shown here are from all data available as of April 21, 2014, including 1605 patient-years of follow-up, and represent the final analysis.
Figure 1.
Enrollment and Follow-up.
PATIENTS
The median age of patients in the study was 55 years (range, 24 to 85). A total of 272 patients (67.0%) had hormone-receptor–positive disease. Among these patients, 62.2% had tumors that measured 1 cm or less in the greatest dimension, and 68.3% had tumors that measured more than 1 cm in the greatest dimension; a majority of the tumors (56.2%) were high-grade. In the total study population, 49.5% of the patients had tumors that measured 1 cm or less (stages T1mic [≤0.1 cm], T1a [>0.1 to ≤0.5 cm], and T1b [>0.5 to ≤1.0 cm]); 8.9% of the patients had tumors that measured between 2 and 3 cm (stage T2). Six patients (1.5%) had nodal micrometastases (Table 1).
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | Patients (N = 406) |
|---|---|
| no. (%) | |
| Age group | |
| <50 yr | 132 (32.5) |
| 50–59 yr | 137 (33.7) |
| 60–69 yr | 96 (23.6) |
| ≥70 yr | 41 (10.1) |
| Sex | |
| Female | 405 (99.8) |
| Male | 1 (0.2) |
| Race† | |
| White | 351 (86.5) |
| Black | 28 (6.9) |
| Asian | 11 (2.7) |
| Other | 16 (3.9) |
| Primary tumor | |
| Size | |
| T1mic: ≤0.1 cm | 9 (2.2) |
| T1a: >0.1 to ≤0.5 cm | 68 (16.7) |
| T1b: >0.5 to ≤1.0 cm | 124 (30.5) |
| T1c: >1.0 to ≤2.0 cm | 169 (41.6) |
| T2: >2.0 to ≤3.0 cm | 36 (8.9) |
| Nodal status | |
| N0 | 400 (98.5) |
| N1mic | 6 (1.5) |
| Histologic grade | |
| I: well-differentiated | 44 (10.8) |
| II: moderately differentiated | 131 (32.3) |
| III: poorly differentiated | 228 (56.2) |
| Unknown | 3 (0.7) |
| HER2-positive status | 406 (100) |
| Estrogen-receptor status | |
| Positive | 260 (64.0) |
| Negative | 141 (34.7) |
| Borderline | 5 (1.2) |
| Progesterone-receptor status | |
| Positive | 201 (49.9) |
| Negative | 196 (48.3) |
| Borderline | 8 (2.0) |
| Unknown | 1 (0.2) |
| Hormone-receptor status | |
| Positive | 272 (67.0) |
| Negative | 134 (33.0) |
Percentages may not total 100 because of rounding. HER2 denotes human epidermal growth factor receptor type 2, N0 no regional lymph-node involvement, and N1mic lymph-node involvement with tumor larger than 0.2 mm in diameter but smaller than 2 mm.
Race was self-reported.
Among the 406 patients who began the protocol therapy, 356 (87.7%) completed all 52 weeks of therapy; 24 discontinued therapy because of protocol-specified toxic effects, and 6 patients discontinued because of other toxic effects. By the time of the final analysis, 29 patients had left the study: 2 patients died from causes unrelated to breast cancer (0.5%), 17 patients withdrew consent (4.2%), and 10 were lost to follow-up (2.5%) (Fig. 1). The median follow-up time was 4.0 years; the maximum follow-up period was 6.2 years.
EFFICACY
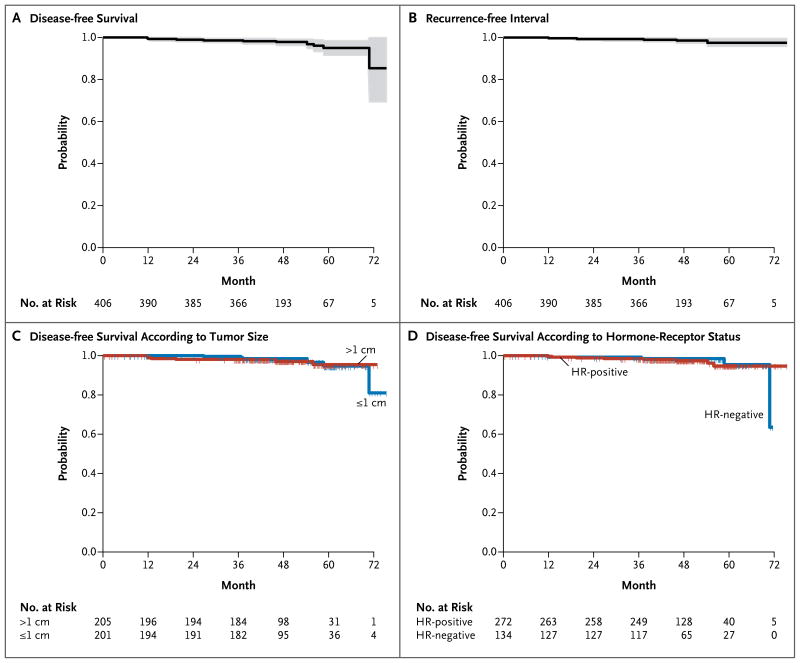
A total of 12 patients had invasive disease events or died (Table 2): 2 had distant metastases (one of which was HER2-negative), 4 had local or regional recurrences, 4 had contralateral breast cancer (3 of whom had HER2-negative breast cancer), 1 died from a rapidly progressive primary ovarian cancer, and 1 died from a stroke after participating in the study for 71 months. Four patients had an event that was not considered to be a relapse of invasive disease (second, nonbreast primary cancers in the bladder, thyroid, or lung), and data for these patients were censored at the date of diagnosis of the second cancer; 4 patients received a diagnosis of ductal carcinoma in situ (an event not categorized as a relapse of invasive disease) and continue to be followed for relapse of invasive disease. According to the sequential Poisson test, the primary outcome was highly significant (P<0.001). The Kaplan–Meier plot and the 95% confidence interval for survival free from invasive disease (Fig. 2A) show a 3-year rate of 98.7% (95% confidence interval [CI], 97.6 to 99.8). Patient outcomes even exceeded the 95% 3-year rate of survival free from invasive disease that defined success in powering the study to reject the null hypothesis (uncorrected Poisson model, P<0.001).
Table 2.
Events Observed for the Primary End Point of Disease-free Survival.
| Event | Patients (N = 406) | Time to Event |
|---|---|---|
| no. (%) | mo | |
| Any recurrence or death | 12 (3.0) | |
| Local or regional recurrence* | ||
| Ipsilateral axilla, HER2-positive | 3 (0.7) | 12, 20, 54 |
| Ipsilateral breast, HER2-positive | 1 (0.2) | 37 |
| New contralateral primary breast cancer | ||
| HER2-positive | 1 (0.2) | 56 |
| HER2-negative | 3 (0.7) | 12, 37, 59 |
| Distant recurrence* | ||
| Skeletal tissue, HER2-positive | 1 (0.2) | 27 |
| Soft tissue, HER2-negative | 1 (0.2) | 46 |
| Death | ||
| Breast-cancer–related | 0 | |
| Not breast-cancer–related | 2 (0.5) | 13, 71 |
These events were included in the calculation of the recurrence-free interval.
Figure 2. Probabilities of Disease-free Survival and Recurrence-free Interval.
Panel A shows the probability of disease-free survival in the intention-to-treat population, and Panel B the recurrence-free interval in the intention-to-treat population (unlike recurrence-free survival, the recurrence-free interval did not include death from cancer other than breast cancer). The shading in Panels A and B denotes the 95% confidence intervals. Panel C shows the probability of disease-free survival according to tumor size, and Panel D the probability of disease-free survival according to hormone-receptor (HR; estrogen receptor or progesterone receptor) status. Tick marks represent the time of censoring for patients who were recurrence-free.
The duration of the recurrence-free interval (which, unlike recurrence-free survival, did not include death from cancer other than breast cancer) according to standardized efficacy end points15 was considered to be an exploratory end point. Six patients had a local or regional recurrence or a distant recurrence or died from breast cancer (Table 2). The 3-year rate of recurrence-free survival was 99.2% (95% CI, 98.4 to 100) (Fig. 2B). Survival curves in subgroups defined according to tumor size (≤1 cm vs. >1 cm) and hormone-receptor status (positive vs. negative) (Fig. 2C and 2D) showed that the rate of recurrence in these subgroups was lower than anticipated (the lower boundaries of the 95% confidence interval for survival free of invasive disease at 3 years in the subgroups exceeded 96.0%).
ADVERSE EVENTS
During 12 weeks of combined therapy, 13 patients (3.2%; 95% CI, 1.7 to 5.4) reported at least one grade 3 episode of neuropathy. No grade 4 neurotoxic effects were reported (0%; 95% CI, 0 to 0.9). Two patients (0.5%; 95% CI, 0.1 to 1.8) had grade 3 systolic dysfunction of the left ventricle (symptomatic congestive heart failure) during active therapy — at 6 months and 11 months — and both recovered after the discontinuation of trastuzumab. A clinically significant asymptomatic decline in ejection fraction (as defined in the protocol) that led to an interruption in treatment with trastuzumab occurred in 13 patients (3.2%; 95% CI, 1.7 to 5.4); in 2 of these patients, the ejection fraction did not normalize, and the patients were unable to complete the remaining year of trastuzumab therapy. A total of 7 patients had grade 3 or grade 4 allergic reactions to the study treatment, and only 1 of these patients was able to complete treatment. Alopecia was expected in the vast majority of patients; data regarding its incidence were not collected. Other specified toxic effects reported during the 52 weeks of protocol therapy are summarized in Table 3.
Table 3.
Most Common Adverse Events Occurring during Protocol Therapy.
| Event | Maximum Grade | Total | ||
|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | ||
| number of patients (percent) | ||||
| Fatigue | 81 (20.0) | 9 (2.2) | 0 | 90 (22.2) |
| Diarrhea | 47 (11.6) | 6 (1.5) | 0 | 53 (13.1) |
| Neuropathy | 39 (9.6) | 14 (3.4) | 0 | 53 (13.1) |
| Neutropenia | 26 (6.4) | 15 (3.7) | 2 (0.5) | 43 (10.6) |
| Hyperglycemia | 35 (8.6) | 7 (1.7) | 0 | 42 (10.3) |
| Leukopenia | 28 (6.9) | 10 (2.5) | 0 | 38 (9.4) |
| Allergic reaction | 28 (6.9) | 6 (1.5) | 1 (0.2) | 35 (8.6) |
| Elevated alanine amino-transferase level | 23 (5.7) | 7 (1.7) | 0 | 30 (7.4) |
| Anemia | 28 (6.9) | 1 (0.2) | 0 | 29 (7.1) |
DISCUSSION
Two controversies affect the management of stage I HER2-positive breast cancer. The first involves defining the threshold for initiating systemic therapy. Guidelines from the National Comprehensive Cancer Network (NCCN) suggest that adjuvant chemotherapy with trastuzumab should be considered in patients with small, node-negative tumors, including patients with T1bN0 tumors (with N0 denoting no regional lymph-node involvement), but the NCCN acknowledges that such patients are generally not included in randomized trials of adjuvant therapy.16 The second controversy relates to the determination of the safest and most effective regimen, bearing in mind the potential for considerable toxic effects with chemotherapy and trastuzumab and the generally favorable outcomes in women with very small tumors. Many physicians recommend treatment regimens such as doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab (ACTH) or docetaxel, carboplatin, and trastuzumab (TCH). These regimens are associated with substantial toxic effects and are commonly used in patients who have a much higher risk of disease recurrence, according to the results of randomized trials.5–8 These studies enrolled a limited number of patients with stage I breast cancer. In the joint analysis, only 5.7% of patients had node-negative disease.8 Both the Herceptin Adjuvant (HERA) study (2 years vs. 1 year of adjuvant trastuzumab for HER2-positive breast cancer) and the Breast Cancer International Research Group 006 (BCIRG-006) study included greater numbers of node-negative patients, including a limited number of patients with stage I disease.6,7 Only the BCIRG-006 study allowed the enrollment of patients with node-negative tumors measuring less than 1 cm in the greatest dimension.
In our trial, we administered adjuvant therapy prospectively only in patients with small, node-negative, HER2-positive tumors and used a treatment regimen similar to ACTH, but omitting doxorubicin and cyclophosphamide. The results suggest a low risk of cancer recurrence (less than 2% at 3 years) with a regimen in which the rate of serious toxic effects was low (with an incidence of heart failure that was only 0.5%).
The results must be considered in the context of several studies that have examined the risk of disease recurrence in patients who have not received trastuzumab or, in most cases, chemotherapy. The limitations of these studies are clear; they included a modest number of patients, and there were biases inherent to their retrospective designs. The largest of the studies focused on 520 patients in the NCCN database who had small HER2-positive cancers (tumors up to 1 cm in the greatest dimension).10 The 5-year rate of survival free from distant recurrence was 94% for patients with T1bN0 hormone-receptor–negative tumors, 93% for T1aN0 hormone-receptor–negative tumors, and 94 to 96% for patients with T1a–bN0 hormone-receptor–positive disease. A study from the M.D. Anderson Cancer Center suggests that among 98 patients with T1a–bN0 HER2-positive tumors, the 5-year rate of recurrence-free survival was 77.1%, and the 5-year rate of survival free from distant recurrence was 86.4%.11 In a study of 117 node-negative, HER2-positive tumors measuring up to 2 cm in the greatest dimension in a tumor registry in British Columbia, Canada, the 10-year rate of relapse-free survival was 68.3% among patients with hormone-receptor–negative tumors and 77.5% among patients with hormone-receptor–positive tumors.17 Although recurrence rates vary across these studies, the rates range from approximately 5 to 30%, with distant recurrences occurring in as many as 20% of patients with tumors measuring up to 1 cm in the greatest diameter. The studies consistently suggest that the risk of recurrence, at least in the first 5 years, is higher in the hormone-receptor–negative group than in the hormone-receptor–positive group.
The median follow-up for patients in our trial is only 4.0 years, but the benefits that were observed in the initial published reports of the randomized trials of adjuvant trastuzumab-based chemotherapy were maintained with additional follow-up. The study population in our trial had a higher proportion of hormone-receptor–positive tumors (67%) than did the populations in the pivotal adjuvant trastuzumab studies (51 to 54%),5–7 although the proportion of hormone-receptor–positive tumors in our trial is consistent with that reported in some studies involving women with small, node-negative tumors. The higher frequency of hormone-receptor–positive tumors in our study could have implications for late recurrence, and all patients will be followed for 10 years to facilitate a comprehensive description of patient outcomes. Because the influence of chemotherapy on the risk of recurrence is generally most notable during the first several years after diagnosis,18 it seems unlikely that a different chemotherapy regimen administered with trastuzumab would affect the risk of late recurrence.
We recognize that a prospective, randomized trial would have been the best option. However, we did not believe that such a design would have been feasible given the accumulating evidence from retrospective studies. Patients and their providers may have been unlikely to enroll in a trial that included a group in which patients would not receive trastuzumab. Some clinicians and investigators might have argued for a trial of trastuzumab alone versus trastuzumab plus chemotherapy, but there are limited data indicating that trastuzumab alone is an effective approach.19–21 Instead, we opted for a regimen of trastuzumab plus chemotherapy that would be associated with fewer toxic effects than the established regimens for patients with a higher risk of recurrence.
The regimen we used in this study was associated with patient outcomes that were better than expected on the basis of historical data. However, the study does not provide data to support the use of trastuzumab-based chemotherapy in all patients with small HER2-positive tumors, and there will be many patients with T1a disease and some with T1b disease who will decide with their physicians to avoid the toxic effects of a trastuzumab-based regimen.
Supplementary Material
Acknowledgments
Supported by Genentech.
We thank all the patients who participated in this trial and all the investigators at the participating centers; Rebecca Gelmon for her assistance with the study design and the development of the statistical analysis plan; and Jennifer Savoie, Michelle Demeo, Emily Morley, Kathryn Josephs, and Jessica Sohl for their assistance with data management.
APPENDIX
The authors’ affiliations are as follows: the Departments of Medical Oncology (S.M.T., B.A.O., A.H.P., I.E.K., H.J.B., E.P.W.) and Biostatistics and Computation Biology (W.T.B., H.G.), Dana–Farber Cancer Institute, and Department of Hematology–Oncology, Massachusetts General Hospital (B.M.) — both in Boston; Breast Cancer Medicine Service, Department of Medicine, Solid Tumor Division, Memorial Sloan Kettering Cancer Center, and Department of Medicine, Weill Cornell Medical Center, New York (C.T.D., C.A.H.), and Department of Medical Oncology, Hofstra North Shore–LIJ School of Medicine, New Hyde Park (I.S.) — all in New York; Sarah Cannon Cancer Center, Department of Medical Oncology, Nashville (D.A.Y.); Department of Medicine, Division of Medical Oncology, Duke Cancer Institute, Durham (P.K.M.), and Department of Medical Oncology, University of North Carolina, Chapel Hill (L.A.C.) — both in North Carolina; Cardinal Bernardin Cancer Center, Department of Medicine, Division of Hematology–Oncology, Loyola University Chicago Stritch School of Medicine, Maywood, IL (K.S.A.); Comprehensive Cancer Center, Department of Medicine, Division of Oncology, University of California, San Francisco, San Francisco (H.S.R.); Department of Medical Oncology, Washington University in St. Louis, St. Louis (M.E.); and Johns Hopkins Kimmel Cancer Center, Department of Oncology, Baltimore (A.C.W.).
Footnotes
The authors’ affiliations are listed in the Appendix.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Seshadri R, Firgaira FA, Horsfall DJ, McCaul K, Setlur V, Kitchen P. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. J Clin Oncol. 1993;11:1936–42. doi: 10.1200/JCO.1993.11.10.1936. [DOI] [PubMed] [Google Scholar]
- 4.Press MF, Pike MC, Chazin VR, et al. Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res. 1993;53:4960–70. [PubMed] [Google Scholar]
- 5.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 6.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 7.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romond E, Suman VJ, Jeong J-H, et al. Trastuzumab plus adjuvant chemotherapy for HER2-positive breast cancer: final planned joint analysis of overall survival (OS) from NSABP B-31 and NCCTG N9831. Presented at the San Antonio Breast Cancer Symposium; San Antonio, TX. December 4–8, 2012; abstract. [Google Scholar]
- 9.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 Years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382:1021–8. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 10.Vaz Duarte Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol. 2014;32:2142–50. doi: 10.1200/JCO.2013.53.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–6. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McArthur HL, Mahoney KM, Morris PG, et al. Adjuvant trastuzumab with chemotherapy is effective in women with small, node-negative, HER2-positive breast cancer. Cancer. 2011;117:5461–8. doi: 10.1002/cncr.26171. [DOI] [PubMed] [Google Scholar]
- 13.Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27:5693–9. doi: 10.1200/JCO.2009.22.0962. [DOI] [PubMed] [Google Scholar]
- 14.Fehrenbacher L, Capra AM, Quesenberry CP, et al. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: a cohort from an integrated health care delivery system. J Clin Oncol. 2014;32:2151–8. doi: 10.1200/JCO.2013.52.0858. [DOI] [PubMed] [Google Scholar]
- 15.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–32. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network website. ( http://www.nccn.org)
- 17.Chia S, Norris B, Speers C, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26:5697–704. doi: 10.1200/JCO.2007.15.8659. [DOI] [PubMed] [Google Scholar]
- 18.Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–44. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–44. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 20.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 21.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.