Abstract
Background
The red blood cell (RBC) endothelial nitric oxide synthase (eNOS) has been shown to regulate intrinsic erythrocyte rheological properties, such as membrane deformability, suggesting that a functional eNOS could be important in RBC viability and function during storage. This study examines the correlation between RBC eNOS deficiency and the propensity of RBCs to hemolyze under selected stress conditions including prolonged hypothermic storage.
Experimental design
Fresh or stored RBCs from normal and eNOS knock out (KO) mice or from healthy human volunteers were subjected to selected hemolytic stress conditions including mechanical stress hemolysis, osmotic stress hemolysis, oxidation stress hemolysis, and evaluated during standard storage in CPDA-1 solutions.
Results
Fresh RBCs from normal and eNOS KO mice demonstrated comparable susceptibility to hemolysis triggered by mechanical stress (mechanical fragility index = 6.5±0.5 in eNOS KO versus 6.4±0.4 for controls; n=8–9), osmotic stress, and oxidative stress. Additionally, RBCs from both mouse groups exhibited similar hemolytic profile at the end of 14-day hypothermic storage, analogous to 42 days of human RBC storage. Storage of human RBCs (28 days in CPDA-1) in the presence of NOS cofactors (L-arginine and tetrahydro-L-biopterin) or inhibitor (L-NMMA) did not affect cell recovery or hemolytic response to the selected stressors.
Conclusion
These studies suggest that RBC eNOS does not modulate susceptibility to hemolysis in response to selected stress conditions or prolonged hypothermic storage. Other strategies to increase NO bioactivity following prolonged storage utilizing NOS-independent pathways such as the nitrate-nitrite-NO pathway may prove a more promising approach.
Keywords: Hemolysis, red blood cell storage lesion, RBC endothelial nitric oxide synthase (eNOS)
INTRODUCTION
A number of studies have suggested that red blood cells (RBC) express the endothelial nitric oxide synthase isoform 3 (eNOS or type III NOS) and have suggested that this eNOS exerts functional effects including the modulation of RBC rheological properties, the inhibition of platelet activation, and the regulation of systemic levels of nitrite.1–3 These findings have challenged accepted dogma, as RBCs are primarily known for their nitric oxide (NO) scavenging activity via hemoglobin (Hb)-NO interactions.4
Several studies have correlated RBC eNOS activity with rheological properties such as membrane deformability, velocity, and resistance to mechanical stress. Augmenting RBC intracellular levels of NO via NO donors or NOS substrate, L-arginine, has been shown to improve membrane deformability, whereas NOS inhibition appears to compromise deformability and renders the cells more susceptible to shear stress.5–7 Despite growing evidence of altered rheological activity in response to impaired RBC eNOS activity, little is known whether these phenomena irreversibly affect membrane integrity and promote hemolysis.
To date, the RBC hypothermic storage lesion has been shown to compromise NO signaling in vivo and in vitro via several mechanisms. We have recently demonstrated that prolonged hypothermic storage (39 days) of RBC units is correlated with elevated levels of hemolysis and, consequently, enhanced NO scavenging by supernatant Hb (either free or microparticle-encapsulated). Infusions of supernatants from aged RBC units into the rat circulation were capable of inducing hypertension, possibly through interference with NO bioavailability.8 A number of studies in mouse models appear to confirm an NO scavenging effect of red blood cell hemolysis and cell free hemoglobin after transfusion that impairs endothelial function.9,10 Others studies have suggested that storage results in loss of red blood cell NO equivalents, such as S-nitrosylated hemoglobin (SNO-Hb). Isolation of RBCs for banking rapidly depletes SNO-Hb content within 3 hours.11,12
Modulation of RBC eNOS activity offers an alternative mechanism that could potentially contribute to the RBC hypothermic storage lesion and hemolysis and the regulation of intra-cellular NO species.13 Dysfunction of this system could result in decreased membrane deformability and impaired blood flow of transfused RBCs in the microvasculature.1 However, the activity of RBC eNOS during storage and its relevance to hemolysis has never been explored. This study verifies whether eNOS knockout renders RBCs more susceptible to hemolysis under selected stress conditions and during hypothermic storage.
MATERIALS AND METHODS
Reagents
All reagents are of analytical grade. Phosphate buffered saline (PBS), sodium chloride, sodium azide, glycerol, Bis-Tris, hydrogen peroxide (H2O2, 50 % aqueous solution), L-arginine, Brij 35 solution, and Drabkin’s reagent were obtained from Sigma-Aldrich (St. Louis, MO, USA). 2,2′-azobis-2-methyl-propanimidamide, dihydrochloride (AAPH), N5-[imino(methylamino)methyl]-L-ornithine monoacetate (L-NMMA), tetrahydro-L-biopterin (BH4) were purchased from Cayman Chemical (Ann Arbor, MI, USA).
Experimental mice
Males, 13 week old from eNOS knockout (eNOS KO) congenic mutant strain (B6.129P2-Nos3tm1Unc/J)14 and their background controls (C57BL/6J) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Experimental procedures were conducted under regulations and approvals from the Institutional Animal Care and Use Committee, and University of Pittsburgh’s Division of Laboratory Animal Resources.
Preparation of mouse or human RBC suspensions
RBCs were isolated from whole blood samples collected from mice or healthy human volunteers. Human RBCs were also obtained from AS-5 (dextrose-adenine-mannitol-sodium chloride storage solution) packed RBC units stored at 4 °C for 7 ± 3 days. Whole blood or stored RBCs were washed three times (10 min, 3000 g, 4 °C) with PBS (pH 7.4) to remove plasma or additive solution and obtain RBC pellets.
Evaluation of RBC hemolytic propensity
Mouse or human RBCs were subjected to one of four hemolytic assays, which were modified and optimized to allow high throughput screening of RBC specimens. Each assay has been designed to yield considerable effects at the lowest possible HCT levels to overcome issues associated with small volumes of blood specimens obtained from individual mice.
- Mechanical fragility- RBCs were suspended with PBS to a final hematocrit (HCT) of 3.5 % ± 0.5 %. 200 μL aliquots were transferred into a 96-well plate. Mechanical fragility was achieved by adding one stainless steel bead (3/32”, Small Parts, USA) into each well and by shaking the plate for 1.5 h using a titer plate shaker (Lab-Line Instruments, USA). Control samples were rocked in the same manner without the presence of a stainless steel bead. After shaking, each RBC sample was transferred into a V-shaped 96-well plate, which was centrifuged (10 min, 1520 g, 20 °C) on a Universal 320 device (Hettich Zentrifugen, Germany). Aliquots (40 μL) from each sample’s supernatant were collected for measuring the Hb concentration (μmol/L). The mechanical fragility index (MFI) was calculated as follows:15
Whereas Hbrocked is the concentration of free Hb (μmol/L) from RBC supernatants rocked with a bead, Hbcontrol is the concentration of free Hb (μmol/L) from RBC supernatants rocked without a bead, and Hbtotal is the total amount of Hb (μmol/L) of each RBC sample. The assay conditions were optimized as described above to generate an MFI value of about 11 with fresh human RBCs and 6.5 for fresh C57BL/6J RBCs. - Osmotic fragility- RBC osmotic fragility test was performed using a modified Pink Test assay.16 Packed RBCs were suspended with Pink Test solution (a hypotonic Bis-Tris buffer containing 25 mmol/L sodium chloride, 70 mmol/L Bis–Tris buffer, and 135 mmol/L glycerol; pH 6.6) to a final HCT of 1.6 % ± 0.2 %. 200 μL aliquots were then transferred into a 96-well plate, which was sealed and incubated at room temperature for 3 h or 24 h. After the incubation period, each RBC sample was transferred into a V-shaped 96-well plate, which was centrifuged (10 min, 1520 g, 20 °C) on a Universal 320 device. Aliquots (40 μL) from each sample’s supernatant were collected for measuring the Hb concentration (μmol/L). Percent osmotic hemolysis was calculated as follows:
Whereas Hbsupernatant is the concentration of free Hb (μmol/L) from RBC supernatants following Pink Test, and Hbtotal is the total amount of Hb (μmol/L) of each RBC sample. Hydrogen peroxide-induced heme degradation- Hydrogen peroxide attack on RBCs can lead to Hb denaturation and the release of fluorescent heme degradation products that can be detected by fluorospectrophotometer (Ex 460, Em 525).17,18 Packed RBCs were diluted with PBS buffer containing sodium azide (final concentration of 1 mmol/L) to inhibit catalase. The HCT measured 5 % ± 0.5 %. Four 200 μL aliquots from each sample were then transferred into a dark 96-well plate (Nunc, Denmark). One duplicate was treated with hydrogen peroxide (0.5 mmol/L final concentration) and the other with PBS (sample controls). Changes of RBC autofluorescence in response to the peroxide were recorded every 3 min over 48 min. Control RBC autofluorescence was subtracted from each peroxide-treated sample.
- AAPH-induced oxidative hemolysis- Thermal (37 °C) decomposition of AAPH generates peroxyl radicals and, consequently, lipid peroxidation-mediated hemolysis.19 Packed RBCs were diluted with PBS to a final HCT of 4.5 % ± 0.3 %. 180 μL aliquots were transferred into a 96-well plate. AAPH oxidation was achieved by adding 20 μL of AAPH stock solution (500 mmol/L) into each well (50 mmol/L final AAPH concentration). Control samples were treated with 20 μL PBS. The plates were gently rocked and incubated at 37 °C for 3 h (mouse model) or 24 h (human model). The incubation times were determined according to each species’ RBC hemolytic propensity. After the incubation period, each RBC sample was transferred into a V-shaped 96-well plate, which was centrifuged (10 min, 1520 g, 20 °C) on a Universal 320 device. Aliquots (40 μL) from each sample’s supernatant were collected for measuring the Hb concentration (μmol/L). Oxidative hemolysis was calculated as follows:
Evaluation of RBC hemolytic propensity in stored RBCs
Mouse RBCs- Packed RBCs from eNOS KO or control mice were suspended 1:1 with PBS containing citrate-phosphate-dextrose-adenine (CPDA-1) storage solution (14 % final CPDA-1 concentration). Aliquots (100 μL) were taken from each sample to determine pre-storage hemolysis. Samples (300 μL) were stored at 4 °C in 0.5 ml microtubes for 14 days, after which RBCs were assayed for storage hemolysis, mechanical fragility, osmotic fragility, and hydrogen peroxide oxidation.
Human RBCs- Human RBCs were stored in the presence or absence of NOS cofactors (L-arginine and BH4) or inhibitor (L-NMMA). Fresh packed RBCs obtained from three healthy donors were suspended 3:2 with PBS containing CPDA-1 storage solution (14 % final CPDA-1 concentration). Each RBC suspension was divided into three aliquots. One aliquot was supplemented with a combination of L-arginine and BH4 (10 μmol/L and 100 μmol/L final concentration, respectively). The second aliquot was treated with L-NMMA (1 mmol/L final concentration), and the third aliquot was treated with PBS (control), which was used as the drug vehicle for all treatments. Sample HCTs averaged 47 % ± 5.6 %. HCT levels were measured by micro-hematocrit centrifuge (LWS-M24, LW Scientific, USA). Samples (650 μL) were stored at 4 °C in 1.5 ml microtubes for 28 days, after which RBCs were assayed for storage hemolysis, mechanical fragility, osmotic fragility, and hydrogen peroxide oxidation.
Storage hemolysis
Percent hemolysis of human RBCs stored in CPDA-1 for 28 days or mouse RBCs stored in CPDA-1 for 14 days was determined by comparing the supernatant Hb to total Hb concentrations and correcting for the hematocrit level of each sample. Hb micromolar concentrations were determined using Drabkin’s method.20,21
Effect of L-arginine supplementation on RBC membrane integrity
Washed human RBCs obtained from AS-5 units were subjected to mechanical or osmotic fragility test in the presence of L-arginine at 0, 2.5, 10, 100 and 1000 μmol/L. MFI and percent osmotic hemolysis were determined as described under “Evaluation of RBC hemolytic propensity”.
Statistical analyses
Nonparametric statistical analyses were performed using commercial software (GraphPad Prism version 5, GraphPad Software. CA, USA). The differences between two independent groups were analysed using Mann-Whitney U test, while Kruskal-Wallis ANOVA was used to examine the differences among multiple groups. Results are shown as the mean ± SD. Probabilities less than 0.05 were considered significant.
RESULTS
RBC eNOS knockout does not affect susceptibility to hemolysis under mechanical, osmotic or oxidative stress conditions
The correlation between eNOS knockout and hemolysis was examined by comparing the hemolytic propensity of freshly drawn RBCs from eNOS KO mice to that of normal controls. RBCs from both mouse groups exhibited similar hemolytic response to each of the tested stress conditions. The RBC mechanical fragility indexes (MFIs) measured 6.5 ± 0.5 in eNOS KO versus 6.4 ± 0.4 in controls (p= 0.7724, n=8–9, Fig. 1A). Similarly, eNOS KO did not modify hemolytic response to osmotic stress (Pink test), as both mouse groups exhibited similar hemolysis levels after 3 h and 24 h (31 % ± 3.9 % versus 28 % ± 2.2 % at 3 h, and 50 % ± 6.5 % versus 51 % ± 2.5 % at 24 h, eNOS KO versus controls, respectively, n=8–9; Fig. 1B). In regard to oxidative stress, RBCs from both mouse groups demonstrated similar response to hydrogen peroxide oxidation (Fig. 1C) or AAPH-induced hemolysis (80 % ± 6.0 % versus 76 % ± 6.3 %, eNOS KO versus controls, respectively, n=5; Fig. 1D).
Figure 1.
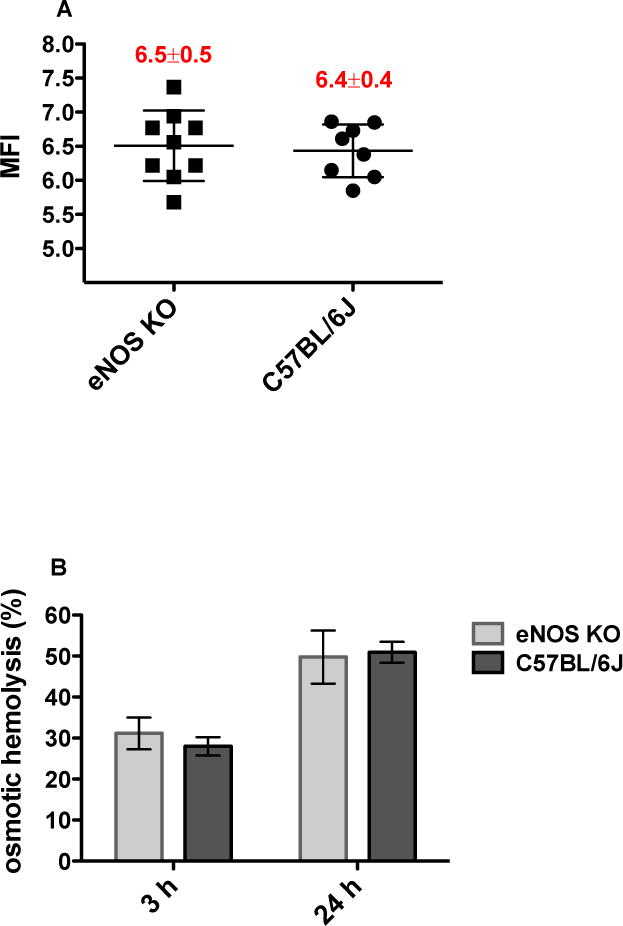

Hemolytic response of eNOS KO RBCs versus C57BL/6J RBCs (normal controls): A. RBC mechanical fragility index (MFI) following 1.5 h rocking in the presence or absence of 3/32” stainless steel bead; B. RBC osmotic hemolysis (%) following 3 h or 24 h incubation in Pink Test buffer at 20 °C; C. Formation of fluorescent heme degradation products following 48 min incubation with 0.5 mmol/L H2O2; D. RBC oxidative hemolysis (%) following 3 h incubation at 37 °C in the presence or absence of 50 mmol/L 2,2′-azobis(2- methylpropionamidine) dihydrochloride (AAPH); Mean±SD, n=8–9 per strain.
eNOS knockout does not affect mouse RBC recovery following 14 days of hypothermic storage
In order to verify whether eNOS depletion contributes to the RBC storage lesion, mouse RBCs from eNOS KO and wild type controls underwent 14 days of hypothermic storage after which cell recovery was evaluated. Under the tested conditions, eNOS KO did not affect the levels of storage hemolysis (Fig. 2A), which were highly comparable to that of the controls (3.1 % ± 1.0 % versus 2.8 % ± 0.4 %, eNOS KO versus controls, respectively, n=8–9) suggesting similar response to storage-induced membrane injury in both groups. RBC storage was correlated with increased susceptibility to mechanical, osmotic and oxidative stress in both mouse groups. However, the overall increase in hemolytic propensity seemed independent of the RBC eNOS, as similar levels of MFI (8.2 ± 2.4 versus 8.1 ± 2.3, eNOS KO versus controls, respectively, n=8–9; Fig. 2B), osmotic hemolysis (61% ± 7.4 % versus 59 % ± 11 %, eNOS KO versus controls, respectively, n=8–9; Fig. 2C), and heme degradation products (Fig. 2D) were observed in RBCs from both mouse groups.
Figure 2.
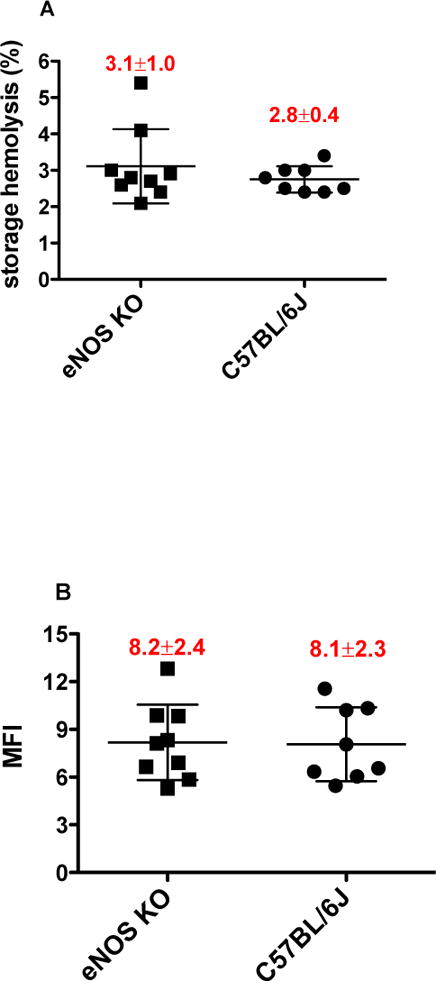

Hemolytic response of mouse RBCs stored with CPDA-1 for 14 days: RBCs were collected from eNOS KO strain or from its background control (C57BL/6J). A. RBC storage hemolysis (%); B. RBC mechanical fragility index (MFI) following 1.5 h rocking in the presence or absence of 3/32″ stainless steel bead; C. RBC osmotic hemolysis (%) in fresh versus stored RBCs following 3 h incubation in Pink Test buffer at 20 °C; D. Formation of fluorescent heme degradation products in fresh versus stored RBCs following 48 min incubation with 0.5 mmol/L H2O2. Mean±SD, n=8–9 per strain.
Supplementing human RBCs with eNOS substrate, L-arginine, has little effect on hemolytic response to mechanical or osmotic stress
L-arginine has been shown to improve RBC deformability via the putative activation of eNOS and the release of intracellular NO.3 To further characterize the correlation between eNOS activity and membrane integrity, human RBCs were treated with L-arginine at selected concentrations (0, 2.5, 10, 100 or 1000 μmol/L) and exposed to mechanical or osmotic stress. L-arginine treatment at 2.5 μmol/L or 10 μmol/L has reduced MFI levels by about 12 % compared with untreated controls (Fig. 3A, n=8). These differences, however, were not significantly different (p= 0.1613, Kruskal-Wallis test). Additionally, L-arginine had no effect on membrane osmotic fragility, as no significant differences in osmotic hemolysis (p=0.9246, Kruskal-Wallis test) were observed between L-arginine-treated RBCs and controls (Fig. 3B, n=8).
Figure 3.

Evaluation of RBC mechanical and osmotic fragility in L-arginine-treated RBCs: Human RBCs were subjected to mechanical or osmotic fragility test in the presence of L-arginine at 0, 2.5, 10, 100 and 1000 μmol/L. A. RBC mechanical fragility index (MFI) following 1.5 h rocking in the presence or absence of 3/32” stainless steel bead; B. RBC osmotic hemolysis (%). Mean±SD, n=8.
Storage of human RBCs with eNOS cofactors or inhibitor does not modify cell recovery or hemolytic response to various stress conditions
In order to confirm our observations with stored mouse RBCs, human RBCs were stored for 4 weeks in the presence of eNOS cofactors, L-arginine (10 μmol/L) and BH4 (100 μmol/L), or inhibitor, L-NMMA (1 mmo/L). At the end of the storage period, these treatments showed no significant effect (p=0.3679, Kruskal-Wallis test) on storage hemolysis levels, which were similar to that of untreated control RBCs (Fig. 4A). Similarly, no significant differences between treated and untreated RBCs were observed in response to mechanical stress (Fig. 4B) or osmotic stress (Fig. 4C).
Figure 4.


Hemolytic response of human RBCs stored with CPDA-1 for 28 days in the presence or absence of eNOS cofactors or inhibitor: RBCs were supplemented with the cofactors L-arginine (10 μmol/L) and tetrahydrobiopterin (BH4, 100 μmol/L) or with the eNOS inhibitor N5-[imino(methylamino)methyl]-L-ornithine, monoacetate (L-NMMA, 1 mmol/L). A. RBC storage hemolysis (%); B. RBC mechanical fragility index (MFI) following 1.5 h rocking in the presence or absence of 3/32” stainless steel bead; C. RBC osmotic hemolysis (%) following 24 h incubation in Pink Test buffer at 20 °C. Mean±SD, n=3.
The tested eNOS cofactors or inhibitor failed to modify oxidative response of stored RBCs to hydrogen peroxide attack (Fig. 5A) demonstrated by near identical increase in the formation rate of heme degradation products. In regard to AAPH oxidation (Fig. 5B), L-arginine and BH4-treated RBCs demonstrated higher, although not significant, levels of hemolysis compared with L-NMMA-treated RBCs or untreated RBCs (43 % ± 6.9 % versus 35 % ± 1.6 % and 38 % ± 4.2 %, respectively, n=3, p=0.679; Fig 5B).
Figure 5.

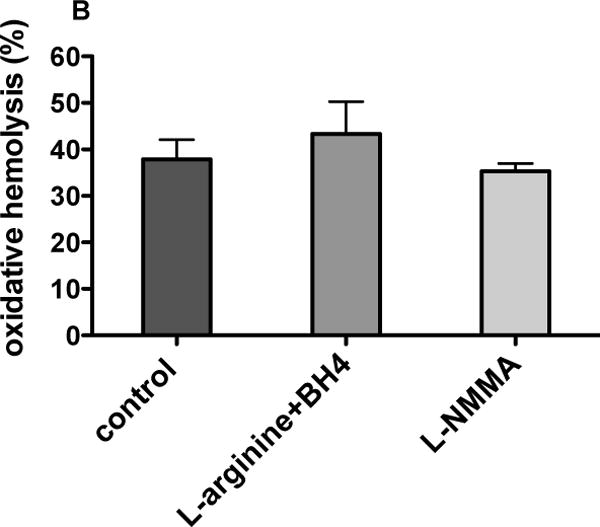
Oxidative response of human RBCs stored with CPDA-1 for 28 days in the presence or absence of eNOS cofactors or inhibitor: RBCs were supplemented with the cofactors L-arginine (10 μmol/L) and tetrahydrobiopterin (BH4, 100 μmol/L) or with the eNOS inhibitor N5-[imino(methylamino)methyl]-L-ornithine, monoacetate (L-NMMA, 1 mmol/L). A. Formation of fluorescent heme degradation products following 48 min incubation with 0.5 mmol/L H2O2; B. RBC oxidative hemolysis (%) following 24 h incubation at 37 °C in the presence or absence of 50 mmol/L 2,2′-azobis(2- methylpropionamidine) dihydrochloride (AAPH). Mean±SD, n=3.
DISCUSSION
Since its initial characterization,22 the RBC eNOS has been shown to modulate membrane deformability in vitro3,5 and in vivo7 suggesting a possible role for this enzyme in regulating blood flow within the microvasculature.1 Reduced RBC membrane deformability and the consequent changes in rheological properties are recognized contributors to the pathophysiology of several hemolytic anemias23 including sickle cell disease,24 as well as the RBC storage lesion.25 We therefore hypothesized that RBC eNOS deficiency would affect membrane integrity via reduced deformability and increased rigidity, which will render the cells more susceptible to hemolysis.
The use of RBCs from eNOS KO mouse strain allowed us to investigate the consequences of eNOS deficiency on cell integrity and hemolysis. We initially characterized the hemolytic propensity of freshly drawn RBCs from eNOS KO and normal (wild type control) mouse strains (Fig. 1) to eliminate the possibility of storage-induced eNOS depletion in normal RBCs. Our data suggest that eNOS KO does not modify hemolytic propensity in terms of mechanical or osmotic fragility (Fig. 1A and 1B), neither does it affect RBC oxidative response to hydrogen peroxide or AAPH treatment (Fig. 1C and 1D).
Previous studies have shown that RBCs generate NO under mechanical stress,26 and that NOS inhibition by L-NG-Nitroarginine methyl ester (L-NAME) compromises membrane deformability.5 Based on these observations, we expected eNOS KO to exacerbate hemolytic response under mechanical or osmotic stress. The disagreement between our observations and the aforementioned studies may originate from different experimental designs and the manner of mechanical stress applied to the RBCs. Our output for mechanical stress (MFI) is based on hemolysis whereas RBC elongation index (EI) and changes in membrane deformability are primarily used by other research groups.5–7 Additionally, our method of exposing RBCs to mechanical stress (i.e. shaking in the presence of stainless steel beads) may have been too vigorous to detect minor changes in RBC membrane properties including deformability.
Enriching RBCs with NOS substrate, L-arginine had little effect on membrane response to mechanical stress (Fig. 3A) and no affect on osmotic fragility (Fig. 3B). Our observations are in agreement with similar studies showing that supplementation of L-arginine at millimolar concentrations does not affect membrane deformability in vitro.5,7 Furthermore, addition of radiolabelled arginine (L-[guanidine-15N2]-arginine) to fresh human RBC suspensions failed to generate NO suggesting little NOS activity in RBCs.27 Taken together, RBC enrichment with L-arginine alone may not be sufficient to induce eNOS activity. Therefore, our subsequent studies of human RBC storage combined L-arginine treatment with the NOS cofactor, BH4.
The relationship between RBC eNOS deficiency and the RBC storage lesion has not been studied, in part due to challenges involved in monitoring eNOS activity in the RBC milieu, such as Hb interference and a massive complex of membrane proteins.3 Our RBC storage data from eNOS KO mice (Fig. 2) and human (Figs. 4 and 5) indicate that the RBC eNOS has little functional effect on the RBC storage lesion. The rates of storage-induced hemolysis in eNOS KO RBCs were no different than normal controls. Furthermore, both eNOS KO RBCs and normal RBCs demonstrated similar elevated levels of MFI, osmotic hemolysis, and heme degradation products at the end of the storage period (Fig. 2B–D, respectively). Likewise, storing human RBCs with NOS cofactors (L-arginine and BH4) or inhibitor (L-NMMA) resulted in similar levels of hemolysis (Fig. 4A), MFI (Fig. 4B), osmotic fragility (Fig. 4C), as well as similar oxidative response to H2O2 or AAPH treatments (Fig. 5).
Similar studies of nitric oxide metabolism in stored RBCs have correlated storage with rapid and remarkable loss of total Hb-bound NO and SNO-Hb levels.11,12 Investigation of NO metabolism in density-fractionated RBCs, which distinguishes between younger and older RBCs, indicated that RBC senescence is correlated with compromised NO synthesis, as well as the ability to response to external NO signalling.28 In regard to eNOS, prolonged RBC storage could induce protein uncoupling, which can result in impaired NOS functionality leading to the release of superoxide in place of nitric oxide. Other scenarios are oxidation of NOS cofactors such as BH4, as well as depletion of cellular arginine.13
CONCLUSION
This is the first study to examine the correlation between RBC eNOS deficiency and storage related hemolysis. Our data suggest that RBC eNOS KO does not modify hemolytic propensity under selected stress conditions or prolonged hypothermic storage. Furthermore, adding eNOS cofactors to RBC storage media has no apparent benefits in terms of cell recovery. These studies suggest that a functional RBC eNOS is not required for adequate RBC storage and that interventions that aim to increase eNOS function are unlikely to improve this process. Our findings are supported by a recent study, which utilized highly sensitive gas chromatography-mass spectrometry (GC-MS) methods to evaluate eNOS activity in human RBCs.27 The authors concluded that human RBCs have little, if any, eNOS activity, thereby challenging previous reports of NOS-mediated NO production by RBCs. Alternative strategies to increase NO bioactivity following prolonged storage utilizing NOS-independent pathways such as the nitrate-nitrite-NO pathway may prove a more promising approach.29–32
Acknowledgments
This work was supported by NIH grant HL098032, the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania
Footnotes
Conflict of interest: The authors declare no conflict of interest relevant to this paper
References
- 1.Chen K, Popel AS. Nitric oxide production pathways in erythrocytes and plasma. Biorheology. 2009;46:107–19. doi: 10.3233/BIR-2009-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozuyaman B, Grau M, Kelm M, Merx MW, Kleinbongard P. RBC NOS: regulatory mechanisms and therapeutic aspects. Trends Mol Med. 2008;14:314–22. doi: 10.1016/j.molmed.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozuyaman B, Schnurch HG, Godecke A, Weber AA, Robenek M, Robenek H, Bloch W, Rosen P, Kelm M. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–51. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 4.Rifkind JM, Nagababu E, Ramasamy S. Nitric oxide redox reactions and red cell biology. Antioxid Redox Signal. 2006;8:1193–203. doi: 10.1089/ars.2006.8.1193. [DOI] [PubMed] [Google Scholar]
- 5.Bor-Kucukatay M, Wenby RB, Meiselman HJ, Baskurt OK. Effects of nitric oxide on red blood cell deformability. Am J Physiol Heart Circ Physiol. 2003;284:H1577–84. doi: 10.1152/ajpheart.00665.2002. [DOI] [PubMed] [Google Scholar]
- 6.Uyuklu M, Meiselman HJ, Baskurt OK. Role of hemoglobin oxygenation in the modulation of red blood cell mechanical properties by nitric oxide. Nitric Oxide. 2009;21:20–6. doi: 10.1016/j.niox.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horn P, Cortese-Krott MM, Keymel S, Kumara I, Burghoff S, Schrader J, Kelm M, Kleinbongard P. Nitric oxide influences red blood cell velocity independently of changes in the vascular tone. Free Radic Res. 2011;45:653–61. doi: 10.3109/10715762.2011.574288. [DOI] [PubMed] [Google Scholar]
- 8.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled Nitric Oxide Enables Artificial Blood Transfusion Without Hypertension. Circulation. 2008;117:1982–90. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C, Wu MX, Bloch KD, Zapol WM. Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiology. 2010;112:586–94. doi: 10.1097/ALN.0b013e3181cd7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: A mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104:17058–62. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–51. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–81. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raval JS, Waters JH, Seltsam A, Scharberg EA, Richter E, Daly AR, Kameneva MV, Yazer MH. The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang. 2010;99:325–31. doi: 10.1111/j.1423-0410.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- 16.Vettore L, Zanella A, Molaro GL, De Matteis MC, Pavesi M, Mariani M. A New Test for the Laboratory Diagnosis of Spherocytosis. Acta Haematol. 1984;72:258–63. doi: 10.1159/000206398. [DOI] [PubMed] [Google Scholar]
- 17.Nagababu E, Chrest FJ, Rifkind JM. The origin of red cell fluorescence caused by hydrogen peroxide treatment. Free Radic Biol Med. 2000;29:659–63. doi: 10.1016/s0891-5849(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 18.Nagababu E, Rifkind JM. Formation of fluorescent heme degradation products during the oxidation of hemoglobin by hydrogen peroxide. Biochem Biophys Res Commun. 1998;247:592–6. doi: 10.1006/bbrc.1998.8846. [DOI] [PubMed] [Google Scholar]
- 19.Takebayashi J, Kaji H, Ichiyama K, Makino K, Gohda E, Yamamoto I, Tai A. Inhibition of free radical-induced erythrocyte hemolysis by 2-O-substituted ascorbic acid derivatives. Free Radical Bio Med. 2007;43:1156–64. doi: 10.1016/j.freeradbiomed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Sowemimo-Coker SO. Red blood cell hemolysis during processing. Transfus Med Rev. 2002;16:46–60. doi: 10.1053/tmrv.2002.29404. [DOI] [PubMed] [Google Scholar]
- 21.Zwart A, van Assendelft OW, Bull BS, England JM, Lewis SM, Zijlstra WG. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobinocyanide standard (4th edition) J Clin Pathol. 1996;49:271–4. doi: 10.1136/jcp.49.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LY, Mehta JL. Evidence for the presence of L-arginine-nitric oxide pathway in human red blood cells: relevance in the effects of red blood cells on platelet function. J Cardiovasc Pharmacol. 1998;32:57–61. doi: 10.1097/00005344-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Stuart J, Nash GB. Red cell deformability and haematological disorders. Blood Rev. 1990;4:141–7. doi: 10.1016/0268-960x(90)90041-p. [DOI] [PubMed] [Google Scholar]
- 24.Ballas SK. Sickle cell anemia with few painful crises is characterized by decreased red cell deformability and increased number of dense cells. Am J Hematol. 1991;36:122–30. doi: 10.1002/ajh.2830360211. [DOI] [PubMed] [Google Scholar]
- 25.Scott KL, Lecak J, Acker JP. Biopreservation of Red Blood Cells: Past, Present, and Future. Transfus Med Rev. 2005;19:127–42. doi: 10.1016/j.tmrv.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Ulker P, Meiselman HJ, Baskurt OK. Nitric oxide generation in red blood cells induced by mechanical stress. Clin Hemorheol Microcirc. 2010;45:169–75. doi: 10.3233/CH-2010-1293. [DOI] [PubMed] [Google Scholar]
- 27.Böhmer A, Beckmann B, Sandmann J, Tsikas D. Doubts concerning functional endothelial nitric oxide synthase in human erythrocytes. Blood. 2012;119:1322–3. doi: 10.1182/blood-2011-11-393124. [DOI] [PubMed] [Google Scholar]
- 28.Bor-Kucukatay M, Meiselman HJ, Baskurt OK. Modulation of density-fractionated RBC deformability by nitric oxide. Clin Hemorheol Microcirc. 2005;33:363–7. [PubMed] [Google Scholar]
- 29.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 30.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A. 2000;97:11482–7. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 32.Minneci PC, Deans KJ, Zhi H, Yuen PST, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–17. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]


