Abstract
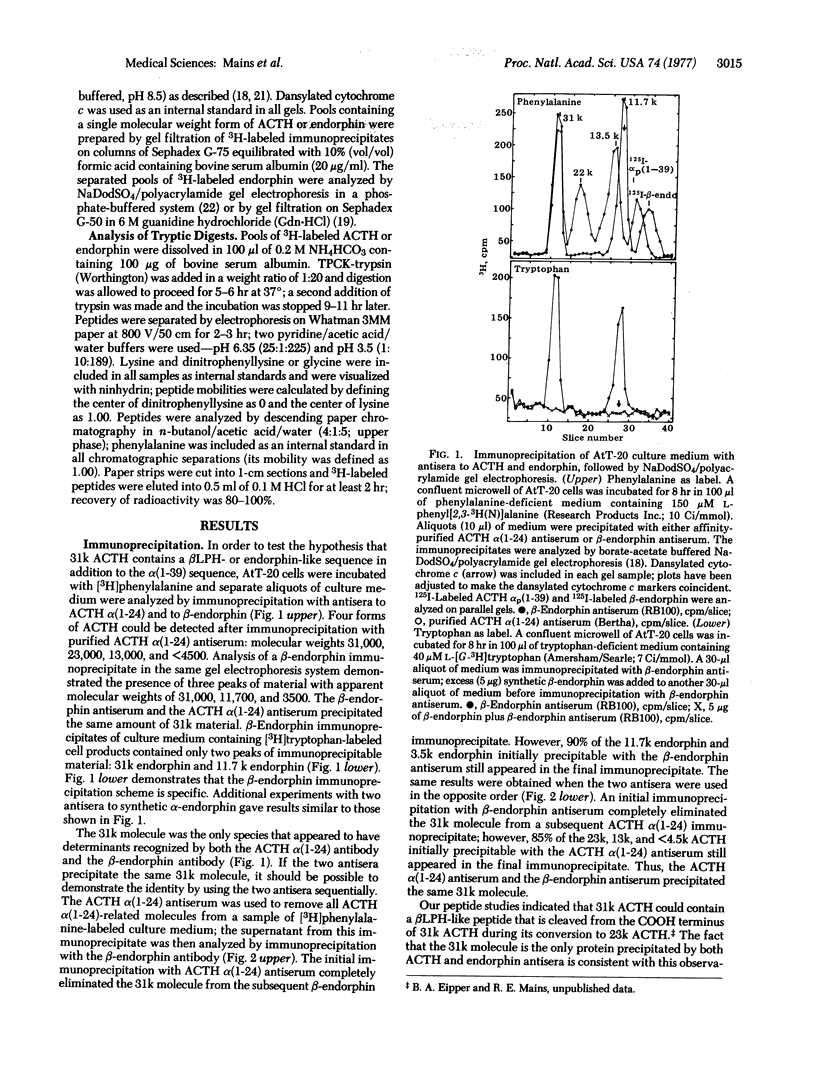
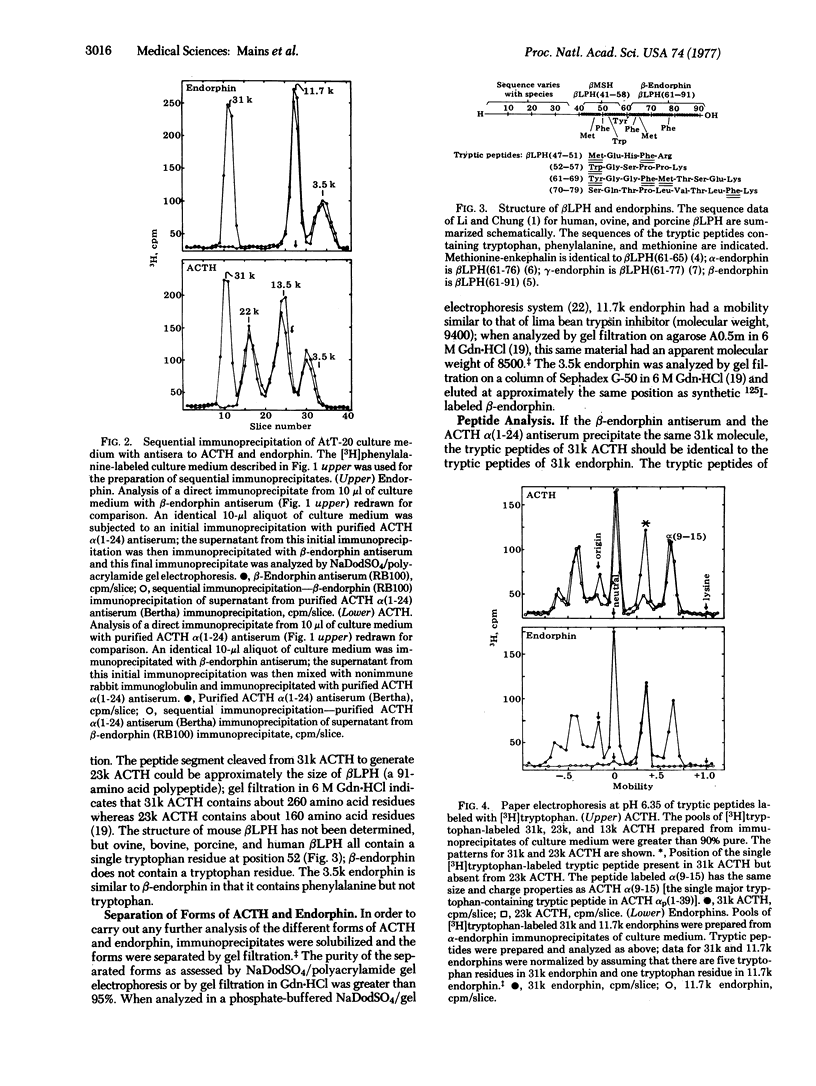
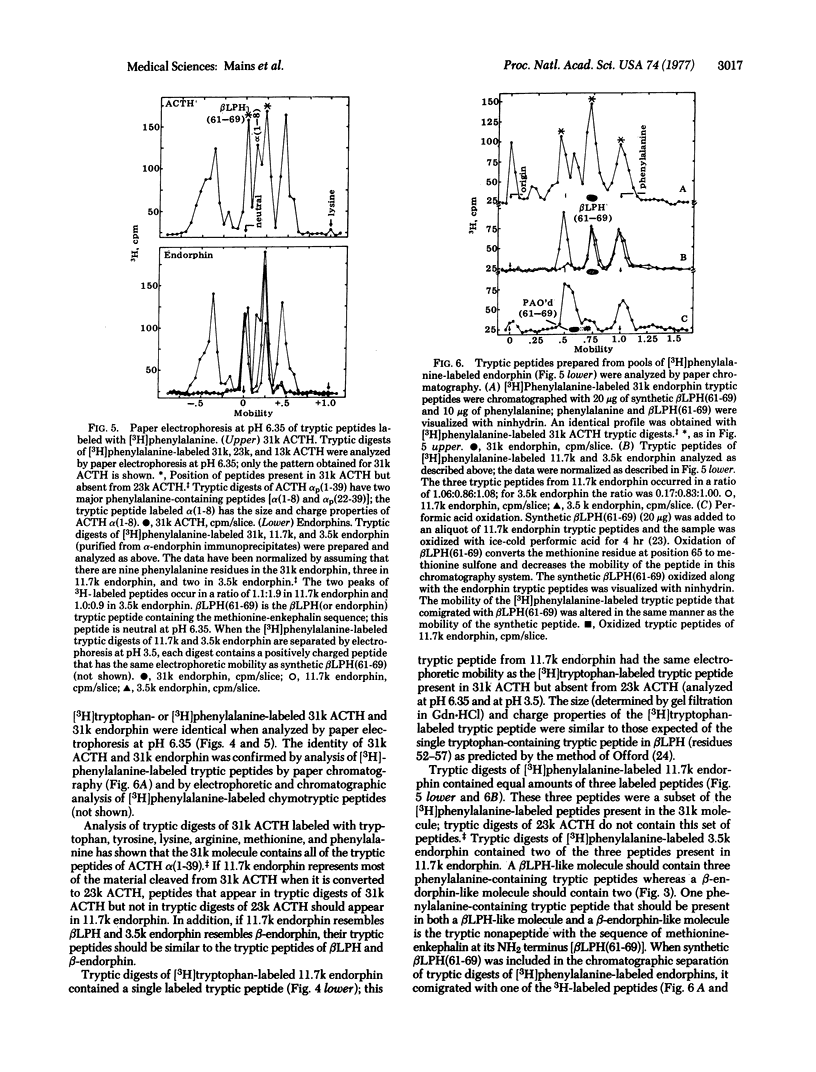
Double-antibody immunoprecipitation procedures with antisera to endorphins and to corticotropin (ACTH) were used to study the biosynthesis of these peptides in a mouse pituitary tumor cell line. Cultures were incubated with a 3H-labeled amino acid, and aliquots of culture medium were immunoprecipitated. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis of [3H]phenylalanine-labeled immunoprecipitates prepared with endorphin antisera resolved three forms of endorphin with apparent molecular weights of 31,000, 11,700, and 3500; immunoprecipitates prepared with the ACTH antiserum contained four forms of ACTH with apparent molecular weights of 31,000, 23,000, 13,000 and <4500. Sequential immunoprecipitation of culture medium with the ACTH antiserum and then with the endorphin antiserum (or the reverse order) indicated that both antisera precipitated the same 31,000 dalton molecule. Purified pools of the different forms of ACTH and endorphin were prepared by immunoprecipitation and gel filtration. The tryptic peptides found in [3H]phenylalanine- or [3H]tryptophan-labeled 31,000 dalton ACTH were identical to the tryptic peptides found in digests of 31,000 dalton endorphin labeled with the same amino acid. A tryptic peptide similar to the lipotropin tryptic peptide [βLPH(61-69)] that contains the opiate-active methionine-enkephalin sequence could be identified in 31,000 dalton ACTH and in all the different forms of endorphin. Most of the peptide cleaved from 31,000 dalton ACTH when it is converted to 23,000 dalton ACTH could be precipitated by endorphin antisera; this 11,700 dalton endorphin molecule is similar to the pituitary hormone βLPH in size and structure. The 3500 dalton endorphin is similar to β-endorphin in size and structure. The culture medium from the AtT-20 mouse pituitary tumor cells contained approximately equimolar amounts of ACTH-related peptides and endorphin-related peptides.
Keywords: pituitary tumor cells, β-lipotropin, β-melanotropin, peptide analysis, immunoprecipitation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Nicholson W. E., Liddle G. W., Orth D. N., Island D. P. Normal and abnormal regulation of beta-msh in man. J Clin Invest. 1969 Aug;48(8):1580–1585. doi: 10.1172/JCI106123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom F., Battenberg E., Rossier J., Ling N., Leppaluoto J., Vargo T. M., Guillemin R. Endorphins are located in the intermediate and anterior lobes of the pituitary gland, not in the neurohypophysis. Life Sci. 1977 Jan 1;20(1):43–47. doi: 10.1016/0024-3205(77)90126-6. [DOI] [PubMed] [Google Scholar]
- Chrétien M., Gilardeau C., Seidah N., Lis M. Purification and partial chemical characterization of human pituitary lipolytic hormone. Can J Biochem. 1976 Sep;54(9):778–782. doi: 10.1139/o76-111. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois P., Vargues-Regairaz H., Dubois M. P. Human foetal anterior pituitary immunofluorescent evidence for corticotropin and melanotropin activities. Z Zellforsch Mikrosk Anat. 1973 Nov 23;145(1):131–143. doi: 10.1007/BF00307194. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Guenzi D. High molecular weight forms of adrenocorticotropic hormone are glycoproteins. J Biol Chem. 1976 Jul 10;251(13):4121–4126. [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. High molecular weight forms of adrenocorticotropic hormone in the mouse pituitary and in a mouse pituitary tumor cell line. Biochemistry. 1975 Aug 26;14(17):3836–3844. doi: 10.1021/bi00688a016. [DOI] [PubMed] [Google Scholar]
- Gilkes J. J., Bloomfield G. A., Scott A. P., Lowry P. J., Ratcliffe J. G., Landon J., Rees L. H. Development and validation of a radioimmunoassay for peptides related to beta-melanocyte-stimulating hormone in human plasma: the lipotropins. J Clin Endocrinol Metab. 1975 Mar;40(3):450–457. doi: 10.1210/jcem-40-3-450. [DOI] [PubMed] [Google Scholar]
- Guillemin R., Ling N., Burgus R. Endorphines, peptides, d'origine hypothalamique et neurohypophysaire à activité morphinomimétique. Isolement et structure moléculaire de l'alpha-endorphine. C R Acad Sci Hebd Seances Acad Sci D. 1976 Feb 23;282(8):783–785. [PubMed] [Google Scholar]
- Hirata Y., Matsukura S., Imura H., Nakamura M., Tanaka A. Size heterogeneity of beta-MSH in ectopic ACTH-producing tumors: presence of beta-LPH-like peptide. J Clin Endocrinol Metab. 1976 Jan;42(1):33–40. doi: 10.1210/jcem-42-1-33. [DOI] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Li C. H., Chung D. Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1145–1148. doi: 10.1073/pnas.73.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. H., Chung D. Primary structure of human beta-lipotropin. Nature. 1976 Apr 15;260(5552):622–624. doi: 10.1038/260622a0. [DOI] [PubMed] [Google Scholar]
- Ling N., Burgus R., Guillemin R. Isolation, primary structure, and synthesis of alpha-endorphin and gamma-endorphin, two peptides of hypothalamic-hypophysial origin with morphinomimetic activity. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3942–3946. doi: 10.1073/pnas.73.11.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry P. J., Rees L. H., Tomlin S., Gillies G., Landon J. Chemical characterization of ectopic ACTH purified from a malignant thymic carcinoid tumor. J Clin Endocrinol Metab. 1976 Oct;43(4):831–835. doi: 10.1210/jcem-43-4-831. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976 Jul 10;251(13):4115–4120. [PubMed] [Google Scholar]
- Moriarty G. C. Adenohypophysis: ultrastructural cytochemistry. A review. J Histochem Cytochem. 1973 Oct;21(10):855–894. doi: 10.1177/21.10.855. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Orth D. N., Nicholson W. E., Mitchell W. M., Island D. P., Shapiro M., Byyny R. L. ACTH and MSH production by a single cloned mouse pituitary tumor cell line. Endocrinology. 1973 Feb;92(2):385–393. doi: 10.1210/endo-92-2-385. [DOI] [PubMed] [Google Scholar]
- Phifer R. F., Orth D. N., Spicer S. S. Specific demonstration of the human hypophyseal adrenocortico-melanotropic (ACTH-MSH) cell. J Clin Endocrinol Metab. 1974 Oct;39(4):684–692. doi: 10.1210/jcem-39-4-684. [DOI] [PubMed] [Google Scholar]
- Scott A. P., Lowry P. J., Van Wimersma Greidanus T. B. Incorporation of 14C-labelled amino acids into corticotrophin-like intermediate lobe peptide and alpha-melanocyte-stimulating hormone by the rat pituitary neurointermediate lobe in vitro, and the identification of four new pars intermedia peptides. J Endocrinol. 1976 Aug;70(2):197–205. doi: 10.1677/joe.0.0700197. [DOI] [PubMed] [Google Scholar]
- Shapiro M., Nicholson W. E., Orth D. N., Mitchell W. M., Island D. P., Liddle G. W. Preliminary characterization of the pituitary melanocyte stimulating hormones of several vertebrate species. Endocrinology. 1972 Jan;90(1):249–256. doi: 10.1210/endo-90-1-249. [DOI] [PubMed] [Google Scholar]



