Abstract
Context
Cardiovascular disease (CVD) risk increases beginning at systolic blood pressure levels of 115 mm Hg. Use of antihypertensive medications among patients with a history of CVD or diabetes and without hypertension has been debated.
Objective
To evaluate the effect of antihypertensive treatment on secondary prevention of CVD events and all-cause mortality among persons without clinically defined hypertension.
Data Sources
Meta-analysis with systematic search of MEDLINE (1950 to week 3 of January 2011), EMBASE, and the Cochrane Collaboration Central Register of Controlled Clinical Trials and manual examination of references in selected articles and studies.
Study Selection
From 874 potentially relevant publications, 25 trials that fulfilled the predetermined inclusion and exclusion criteria were included in the meta-analysis.
Data Extraction
Information on participant characteristics, trial design and duration, treatment drug, dose, control, and clinical events were extracted using a standardized protocol. Outcomes included stroke, myocardial infarction (MI), congestive heart failure (CHF), composite CVD outcomes, CVD mortality, and all-cause mortality.
Results
Compared with controls, participants receiving antihypertensive medications had a pooled relative risk of 0.77 (95% confidence interval [CI], 0.61 to 0.98) for stroke, 0.80 (95% CI, 0.69 to 0.93) for MI, 0.71 (95% CI, 0.65 to 0.77) for CHF, 0.85 (95% CI, 0.80 to 0.90) for composite CVD events, 0.83 (95% CI, 0.69 to 0.99) for CVD mortality, and 0.87 (95% CI, 0.80 to 0.95) for all-cause mortality from random-effects models. The corresponding absolute risk reductions per 1000 persons were −7.7 (95% CI, −15.2 to −0.3) for stroke, −13.3 (95% CI, −28.4 to 1.7) for MI, −43.6 (95% CI, −65.2 to −22.0) for CHF events, −27.1 (95% CI, −40.3 to −13.9) for composite CVD events, −15.4 (95% CI, −32.5 to 1.7) for CVD mortality, and −13.7 (95% CI, −24.6 to −2.8) for all-cause mortality. Results did not differ according to trial characteristics or subgroups defined by clinical history.
Conclusions
Among patients with clinical history of CVD but without hypertension, antihypertensive treatment was associated with decreased risk of stroke, CHF, composite CVD events, and all-cause mortality. Additional randomized trial data are necessary to assess these outcomes in patients without CVD clinical recommendations.
Cardiovascular disease (CVD) is the leading cause of death in the United States and globally, representing 30% of all deaths worldwide.1 Prospective cohort studies have established a strong, graded, and independent positive association between blood pressure levels and risk of CVD, stroke, and premature death.2,3 Increased CVD risk begins at systolic blood pressure levels as low as 115 mm Hg, with 54% of stroke and 46% of ischemic heart disease events occurring in persons with blood pressures in this range.4 In persons with prehypertension, 90% have at least 1 risk factor above optimal levels for heart disease or stroke, and 68% have at least 1 clinically high-risk factor for heart disease or stroke.5
Among adults 35 years and older, more than 17% of those with normal blood pressure and 37% of those with blood pressure in the prehypertensive range (130–139 mm Hg systolic, 86–89 mm Hg diastolic) progress to overt hypertension within 4 years without changes in lifestyle or pharmacological intervention.6 In adults 55 years and older, lifetime risk of developing hypertension is greater than 90%.7 Recent national surveys report that more than 30% of the general adult population in the United States, Korea, and China has prehypertension.8–10
Clinical trials have documented that lowering blood pressure reduces cardiovascular mortality among patients with hypertension.3,11 Several randomized controlled trials of blood pressure lowering for the prevention of CVD have demonstrated benefit among persons with prehypertension or normal blood pressures,12,13 while others have not shown benefit.14,15 Given these conflicting results, a meta-analysis of randomized controlled trials that examine antihypertensive treatment among persons with blood pressures in the prehypertensive or normal range for the primary or secondary prevention of CVD may help clarify this issue. The objective of this meta-analysis is to evaluate the association between antihypertensive treatment and secondary prevention of CVD events and all-cause mortality among persons without clinically defined hypertension (≥140 mm Hg systolic or ≥90 mm Hg diastolic and/or use of antihypertensive medications or history of hypertension).
METHODS
Study Selection
We searched online databases including MEDLINE (1950 to week 3 of January 2011), EMBASE, and the Cochrane Collaboration Central Register of Controlled Clinical Trials using the following terms as Medical Subject Headings and keywords: hypertension or blood pressure or normal blood pressure or prehypertension or prehypertension or prehypertensive or normotensive and antihypertensive agents, and cardiovascular disease. No language restrictions were applied. Searches were limited to randomized clinical trials in human participants 19 years or older. A manual examination of references in selected articles was also performed.
The titles and abstracts of 874 potentially relevant references were identified through the literature search and reviewed independently by 3 investigators (A.M.T., T.H., C.L.E.) to determine whether they met eligibility criteria for inclusion. Discrepancies regarding whether to include or exclude a study were resolved by consensus with other investigators (J.H., L.A.B.).
Studies were eligible for inclusion if they were randomized controlled trials of antihypertensive treatment among persons with blood pressure less than 140 mm Hg systolic or less than 90 mm Hg diastolic for the prevention of CVD events (fatal or nonfatal stroke, fatal or nonfatal myocardial infarction [MI], congestive heart failure [CHF], or CVD mortality). For studies that produced multiple publications, data from the most recent or most complete publication were included in the analysis.
Studies were excluded if CVD events were not reported by hypertension status in studies that included participants with and without hypertension; the study population did not include persons with blood pressure in the normal or prehypertensive ranges; the study population did not include persons with preexisting CVD or CVD equivalents, such as diabetes; antihypertensive treatment was not part of the intervention; treatment allocation was not random; a measure of variance (P value or confidence interval [CI]) was not reported or could not be calculated from the information provided; participants were younger than 18 years; or there were differences between intervention and control groups other than antihypertensive treatment.
Data Abstraction
All data were independently abstracted by 3 investigators (A.M.T., T.H., C.L.E.) using a standardized data collection form. Discrepancies were resolved through discussion with other investigators (J.H., L.A.B.) and through reference to the original articles. We attempted to contact study authors for additional information when necessary. Trial characteristics abstracted included design of the randomized controlled trial, type of control, number of treatment groups, description of treatment regimens, description of inclusion and exclusion criteria, numbers of fatal and nonfatal events, definition of participants without hypertension, and demographic characteristics of study populations at baseline. The outcomes recorded included incidence of stroke, MI, CHF events, composite CVD events (as defined by the study), CVD mortality, and all-cause mortality.
The definition of nonhypertensive varied in each study; however, all studies included in this analysis had populations with blood pressure less than 140 mm Hg systolic, less than 90 mm Hg diastolic, or no clinical history of hypertension at baseline. The study-specific definitions of persons without hypertension and outcomes included in this analysis are provided in eTable 1 and eTable 2, available at http://www.jama.com.
Quality Assessment
Two authors (A.M.T., T.H.) independently evaluated quality of each study using an established tool.16 Nine domains were assessed: randomization, concealment of treatment allocation, similarity of groups at baseline, eligibility criteria, blinding of outcome assessor, patient and care provider, point estimates, and intention-to-treat analysis. Disagreement was resolved through consensus and discussion.
Statistical Analysis
For studies that provided an effect estimate such as a relative risk (RR) or hazard ratio, the study-provided effect estimate was directly used in the pooled meta-analysis calculations. For studies that published number of events but did not publish an effect estimate, this information was used to calculate the RR of each outcome for the intervention compared with the placebo group. We logarithmically transformed the RR and corresponding standard error to stabilize the variance and normalize the distribution. We calculated the overall pooled-effect estimates using inverse-variance weighting to calculate both fixed-effects and DerSimonian and Laird random-effects models.17 The Q test was used to assess the presence of heterogeneity and the I2 index to quantify the extent of heterogeneity.18,19 Fixed- and random-effects models yielded similar findings, but we detected between-study heterogeneity for several outcomes; therefore, results from the random-effects models are presented. Absolute risk reductions for individual studies were calculated as the difference in event rates between treatment and control groups based on the reported or estimated number of events for each outcome. Pooled absolute risk reductions were calculated using inverse-variance weighted DerSimonian and Laird random-effects models.
To assess for publication bias, we constructed funnel plots for each outcome in which the ln(RR) was plotted against its standard error. The Begg rank correlation test was used to examine the asymmetry of the funnel plot,20 and the Egger weighted linear regression test was used to examine the association between mean effect estimate and its variance.21 Prestated subgroup analyses were conducted to assess the influence of the presence or absence of co-morbid conditions at baseline and class of antihypertensive treatment. We then conducted sensitivity analyses to examine the robustness of the results and restricted analyses by antihypertensive medication use at baseline, definition of persons without hypertension, trial size, duration of follow-up, and year of publication. Additionally, we conducted sensitivity analyses whereby each study was excluded in turn to evaluate the relative influence of each trial on the pooled estimates. P<.05 was considered statistically significant, and all tests were 2-sided. All analyses were conducted in STATA version 9.2 (StataCorp, College Station, Texas).
RESULTS
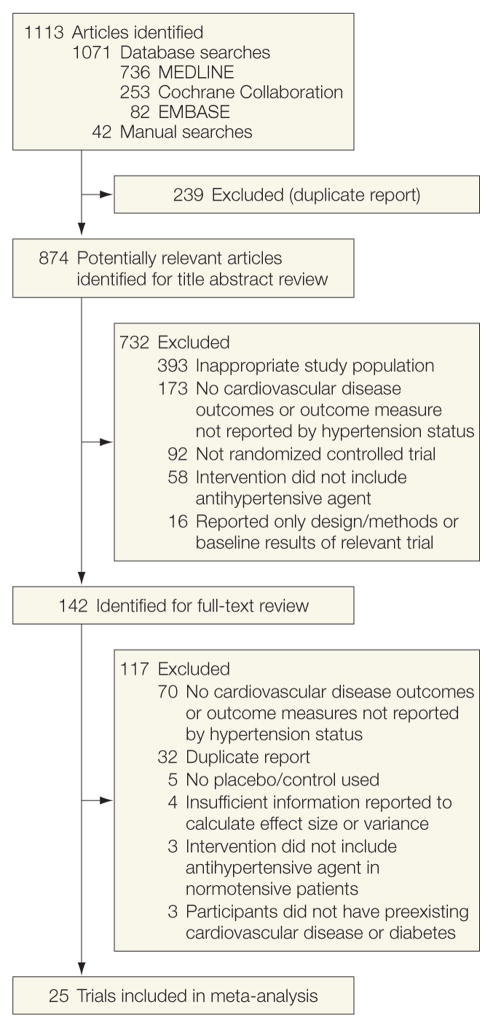
Of 874 potentially relevant studies identified in the initial literature search, 25 were included in the meta-analysis (Figure 1). Table 1 describes the characteristics of trials included in the meta-analysis. The class and dose of medication administered in the antihypertensive treatment group varied between studies, but for most studies it progressively increased to a defined target dose. Study duration ranged from a mean length of 1.5 to 63 months. Entry criteria also varied between studies; however, all studies required a history of CVD; clinical evidence of recent MI, CHF, coronary artery disease, or stroke; or CVD equivalent such as type 2 diabetes.
Figure 1.
Selection Process for Studies Included in the Meta-analysis.
Table 1.
Characteristics of Randomized Clinical Trials of Antihypertensive Medications Included in the Meta-analysis (N = 25)a
| Trialb | Treatment Regimen | Participant Population | Duration of Follow-up, Mean (Range), Mo | ||
|---|---|---|---|---|---|
| Medication | Drug Class | Dose/Titration | |||
| MIS,22 1975 | Practolol | β-Blocker | 100 mg/d | 7–28 d post-MI | 14 (1–36) |
| MPI,23 1980 | Propranolol | β-Blocker | 40 mg ×3/d | 2–14 d post-MI (anterior infarction) | 5.6 (1–9) |
| BHAT,24 1982 | Propranolol | β-Blocker | 180 mg/d or 240 mg/d | 5–21 d post-MI | 25.1 (1–36) |
| ASPS,25 1983 | Pindolol | β-Blocker | 15 mg/d | 0–21 d post-MI (with electrical and/or mechanical complications) | 24 (1–24) |
| CONSENSUS II,26,27 1992 | Enalapril | ACEI | 20 mg/d | Presented within 24 h of onset of acute MI | 6 (1–20)c |
| SOLVD,28–31 1995 | Enalapril | ACEI | 2.5 or 5 mg ×2/d titrated to 10 mg ×2/d | CHF and LVEF ≤35% | 40 (15–62)d |
| USCHF,32 1996 | Carvedilol | β-Blocker | 12.5 mg ×2/d increased to 25 or 50 mg ×2/d | LVEF ≤35%, not receiving CCB, α- or β-adrenergic agonists or antagonists, or class IC or III antiarrhythmic agents | 6.5 (0–15)e |
| TRACE,33,34 1997 | Trandolapril | ACEI | 1 mg, titrated to 4 mg/d | LVEF ≤35%, 3–7 d post-MI | 26 (24–50) |
| AIRE,35,36 1999 | Ramipril | ACEI | 1.25, 2.5, or 5.0 mg ×2/d | Transient or persistent CHF, 2–9 d post-MI | 15 (6–20) |
| SMILE,37–39 1999 | Zofenopril calcium | ACEI | 7.5 mg ×2/d titrated to 30 mg/d | Presented within 24 h of onset of MI | 1.5 (0–1.5)f |
| MERIT-HF,40 2000 | Metoprolol CR/XL | β-Blocker | 25 or 12.5 mg/d to 200 mg | Symptomatic CHF for at least 3 mo, LVEF ≤40% | 12 (0–18) |
| CCS-1,41,42 2001 | Captopril | ACEI | 6.25 mg initial dose + 12.5 mg 2 h later, 12.5 mg ×3/d thereafter | Post-MI (acute MI in past 36 h) | 23.4 (6.5–40.3) |
| HOPE,43–46 2001 | Ramipril | ACEI | 2.5 mg initial dose progressively increased to 10 mg/d | History of CAD, stroke or PAD, or diabetes plus 1 additional risk factor | 54 (0–60) |
| ABCD,14,47 2002 | Nisoldipine or enalapril | CCB or ACEI | Nisoldipine 10 mg titrated to 60 mg/d or enalapril 5 mg/d titrated to 40 mg/d | Type 2 diabetes, DBP 80–89 mm Hg, not receiving antihypertensive medications | 63.6 (0–63.6) |
| CAMELOT,12 2004 | Amlodipine | CCB | 10 mg/d | LVEF ≥40%, CAD >20% stenosis by coronary angiography, and DBP <100 mm Hg | 24 (0–24) |
| COPERNICUS,48,49 2004 | Carvedilol | β-Blocker | 3.125 mg titrated to 25 mg ×2/d | LVEF <25% despite conventional therapies, dyspnea or fatigue at rest or with minimal exertion | 10.4 (0–28.7) |
| DIABHYCAR,50 2004 | Ramipril | ACEI | 1.25 mg/d | Type 2 diabetes, persistent microalbuminuria or proteinuria, serum creatinine ≤150 μmol/L, no MI in past 3 mo | 47 (36–72)e |
| PEACE,51–53 2004 | Trandolapril | ACEI | 2 mg/d increased to 4 mg/d | History of major CVD (if MI, at least 3 mo prior), LVEF >40% | 57.6 (0–84)e |
| SAVE,54,55 2004 | Captopril | ACEI | 12.5 mg titrated to target dose of 25 mg ×3/d, maximum 50 mg ×3/d | 3–16 d post-MI with LVEF ≤40% | 42 (24–60) |
| PROGRESS,56–58 2006 | Perindopril + indapamide | ACEI +diuretic | 4 mg perindopril + 2.5 mg indapamide daily (2.0 mg indapamide in Japan) | History of stroke or TIA within previous 5 y | 46.8 (0–54) |
| ADVANCE,59,60 2007 | Perindopril + indapamide | ACEI + diuretic | 2 mg/d perindopril + 0.625 mg indapamide; 4mg/d perindopril + 1.25 mg/d indapamide after 3 mo | Type 2 diabetes, ≥1 CVD risk factor, or history of microvascular or macrovascular disease | 51.6 (0–60) |
| PRoFESS,61,62 2008 | Temisartan | ARB | 80 mg/d | Stroke within previous 90 d if ≥55 y; stroke within previous 120 d if 50–54 y | 30 (18–52) |
| TRANSCEND,15 2008 | Temisartan | ARB | 80 mg/d | History of CAD, PVD, stroke, or diabetes with end-organ damage; intolerance to ACEIs | 56 (IQR, 51–64)e |
| EUROPA,13,63,64 2009 | Perindopril | ACEI | 8 mg/d | Documented CAD (MI >3 mo prior to enrollment) in men or women, history of angina, and confirmed ischemia on stress testing in men | 50.4 (0–60) |
| PATS,65 2009 | Indapamide | Diuretic | 2.5 mg/d | History of stroke or TIA (qualifying cerebrovascular event ≥4 weeks prior to enrollment) | 24 (0–45) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CCB, calcium channel blocker; CHF, congestive heart failure; CVD, cardiovascular disease; DBP, diastolic blood pressure; IQR, interquartile range; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PAD, peripheral artery disease; PVD, peripheral vascular disease; TIA, transient ischemic attack.
All trials were double-blinded with the exception of the ABCD Normotensive Trial, which was single-blinded. Placebo control was used in all studies.
ABCD indicates Appropriate Blood Pressure Control in Diabetes–Normotensive Study; ADVANCE, Action in Diabetes and Vascular Disease: PreterAx and Diamicro N-MR Controlled Evaluation; AIRE, Acute Infarction Ramipril Efficacy; ASPS, Australian and Swedish Pindolol Study; BHAT, β-Blocker Heart Attack Trial Research Group; CAMELOT, Comparison of Amlodipine vs Enalapril to Limit Occurances of Thrombosis; CCS-1, Chinese Cardiac Study; CONSENSUS II, Cooperative New Scandinavian Enalapril Survival Study II; COPERNI-CUS, Carvedilol Prospective Randomized Cumulative Survival; DIABHYCAR, Noninsulin-Dependent Diabetes, Hypertension, Microalbuminuria or Proteinuria, Cardiovascular Events, and Ramipril Study; EUROPA, European trial on Reduction of Cardiac Events With Perindopril in Patients With Stable Coronary Artery Disease: HOPE, Heart Outcomes Prevention Evaluation; MERIT-HF, Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure; MIS, Multicenter International Study; MPI, Multicenter Post-Infarction Study; PATS, Post-Stroke Antihypertensive Treatment Study; PEACE, Prevention of Events With Angiotensin Converting Enzyme Inhibition Trial; PRoFESS, Prevention Regimen for Effectively Avoiding Second Strokes; PROGRESS, Perindopril Protection Against Recurrent Stroke Study; SAVE, Survival and Ventricular Enlargement Trial; SMILE, Survival of Myocardial Infarction Long-term Evaluation Study; SOLVD, Studies of Left Ventricular Dysfunction; TRACE, Trandolapril Cardiac Event Study; TRANSCEND, Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease; USCHF, US Carvedilol Heart Failure Study Group.
Patients (N=2952) underwent follow-up for 180 days, observation period ranged from 41 to 180 days.26
Mean follow-up time was 37.4 (range, 14.6–62.0) months and 41.4 (range, 22–55) months for the SOLVD Prevention and Treatment trials, respectively. Participants from both trials were included in the analysis of nonhypertensive participants.
Median follow-up time reported.
Double-blind treatment period was 6 weeks; maintenance treatment using conventional therapy was continued for 48 additional weeks. Six-week outcomes are evaluated in this meta-analysis.37
The 25 studies included in the meta-analysis incorporated data from 64 162 participants without hypertension (Table 2). The mean age of participants in the studies ranged from 55.0 to 68.0 years, and 76% of study participants were men. Clinical history of MI, CHF, diabetes, stroke, and coronary artery disease at baseline varied between studies.
Table 2.
Baseline Characteristics of Participants Without Hypertension in Randomized Controlled Trials of Antihypertension Medications
| Trial | No.a | Age, Mean (SD), y | No. (%)b
|
BP or Cutpoint for Nonhypertensive, Mean, mm Hgc
|
Clinical History, No. (%)b
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Nonwhite Race | Systolic | Diastolic | MI | CHF | Diabetes | Stroke | CAD | |||
| MIS,22 1975d | 1334 | 55.0 (NR) | 1154 (86.5) | NR | NR | <78.0 | 1334 (100) | 0 | 40 (3.0) | NR | NR |
|
| |||||||||||
| MPI,23 1980d | 292 | 54.9 (NR) | 247 (84.5) | NR | NR | <79.7 | 292 (100) | 5 (1.5) | 11 (3.6) | NR | NR |
|
| |||||||||||
| BHAT,24 1982d | 2715 | 54.8 (NR) | 2294 (84.5) | 304 (11.2) | NR | ≤76.0 | 2715 (100) | 250 (9.2) | 313 (11.5) | NR | NR |
|
| |||||||||||
| ASPS,25 1983d | 390 | 58.0 (NR) | 324 (83.0) | NR | NAe | NAe | 390 (100) | NR | 27 (6.8) | NR | NR |
|
| |||||||||||
| CONSENSUS II,26,27 1992d | 4437 | 65.8 (NR) | 3262 (73.5) | NR | NAe | NAe | 4437 (100) | 271 (6.1) | 497 (11.2) | NR | NR |
|
| |||||||||||
| SOLVD,28–31 1995d | 4145 | 59.1 (NR) | 3673 (88.6) | 485 (11.7) | NAe | NAe | 3312 (79.9) | 4145 (100) | 634 (15.3) | NR | NR |
|
| |||||||||||
| USCHF,32 1996d | 547 | 58.0 (12.2) | 419 (76.6) | NR | 115.0 | 73.0 | NR | 547 (100) | NR | NR | 261 (47.6) |
|
| |||||||||||
| TRACE,33,34 1997 | 1349 | 68.0 (NR) | 1000 (74.1) | NR | 119.0 | 75.0 | 1349 (100) | 285 (21.1) | 157 (11.6) | NR | NR |
|
| |||||||||||
| AIRE,35,36 1999 | 1432 | 64.4 (11.0) | 1117 (78.0) | NR | NAe | NAe | 1432 (100) | 1432 (100) | 145 (10.1) | NR | NR |
|
| |||||||||||
| SMILE,37–39 1999 | 876 | 63.3 (10.0) | 701 (80.0) | NR | 132.1 | 81.8 | 876 (100) | 0 | 158 (18.0) | NR | NR |
|
| |||||||||||
| MERIT-HF,40 2000d | 2235 | 63.8 (NR) | 1732 (77.5) | 135 (6.0) | NAe | NAe | 1078 (48.2) | 2235 (100) | 552 (24.7) | NR | NR |
|
| |||||||||||
| CCS-1,41,42 2001d | 4760 | 63.5 (10.8) | 3627 (76.2) | 4760 (100) | <140.0 | NR | 4760 (100) | 929 (19.5) | 405 (8.5) | NR | NR |
|
| |||||||||||
| HOPE,43–46 2001d | 4673 | 66.0 (7.0) | 3425 (73.3) | NR | <138.0 | NR | 2458 (52.6) | 0 | 4712 (38.5) | 510 (10.9) | 3739 (80.0) |
|
| |||||||||||
| ABCD,14,47 2002 | 480 | 59.1 (0.6) | 262 (54.5) | NR | 136.4 | 84.4 | NR | 10 (2.0) | 480 (100) | 17 (3.5) | NR |
|
| |||||||||||
| CAMELOT,12 2004d | 699 | 57.3 (9.6) | 522 (74.7) | 76 (10.8) | <129.5 | NR | 263 (37.6) | 0 | 130 (18.6) | 28 (3.9) | 699 (100) |
|
| |||||||||||
| COPERNICUS,48,49 2004f | 1336 | 62.4 (12.4) | 1082 (81.0) | NR | 85–125 | 71.6 | NR | 1336 (100) | NR | NR | NR |
|
| |||||||||||
| DIABHYCAR,50 2004d | 2177 | 65.1 (8.4) | 1516 (69.9) | NR | <140.0 | <90.0 | 131 (6.0) | 0 | 2177 (100) | 92 (4.2) | NR |
|
| |||||||||||
| PEACE,51 2004d | 6050 | 64.0 (8.0) | 4961 (82.0) | 454 (7.5) | <140.0 | <90.0 | 3328 (55.0) | 0 | 1029 (17.0) | 394 (6.5) | 6050 (100) |
|
| |||||||||||
| SAVE,54,55 2004 | 1325 | 58.0 (10.9) | 1141 (86.1) | 116 (8.7) | 108.0 | 68.0 | 1325 (100) | 0 | 223 (16.8) | NR | NR |
|
| |||||||||||
| PROGRESS56–58 2006f | 2137 | 61.7 (10.0) | 1554 (72.7) | 962 (45.0) | 127.4 | 78.9 | 349 (16.3) | NR | 229 (10.7) | NR | 2137 (100)g |
|
| |||||||||||
| ADVANCE,59,60 2007d | 1939 | 66.0 (6.5) | 1106 (57.0) | NR | <140.0 | <90.0 | 233 (12.0) | NR | 1939 (100) | 1939 (9.0) | NR |
|
| |||||||||||
| PRoFESS,61,62 2008d | 6822 | 66.2 (8.6) | 4366 (64.0) | 2900 (42.5) | ≤135.0 | NR | NR | NR | 1924 (28.2) | 6822 (100) | NR |
|
| |||||||||||
| TRANSCEND,15 2008d | 1955 | 66.9 (7.4) | 1115 (57.0) | 761 (38.9) | ≤133.0 | NR | 906 (46.3) | 0 | 698 (35.7) | 1459 (74.6) | 430 (22.0) |
|
| |||||||||||
| EUROPA,13,63,64 2009d | 9154 | 60.0 (9.0) | 7818 (85.4) | NR | <140.0 | NR | 5923 (64.7) | 0 | 1126 (12.3) | 302 (3.3) | 9154 (100) |
|
| |||||||||||
| PATS,65 2009 | 903 | 60.2 (6.5) | 651 (72.0) | 903 (100) | <140.0 | <90.0 | NR | NR | NR | 903 (100)h | NR |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; DBP, diastolic blood pressure; MI, myocardial infarction; NA, not applicable; NR, not reported; SBP, systolic blood pressure.
Number of participants meeting the definition of normotensive or prehypertensive and included in the analyses.
In studies in which the number is not overtly given, the number of men is estimated from the total proportion of men and the number of participants without hypertension included in the analysis assuming that the proportion remained consistent between those with and without hypertension.
Mean blood pressures or cutpoint indicating those without hypertension for participants included in the data analysis. Five studies included subgroup analyses of participants with no history of hypertension; however, SBP or DBP were not provided for these participants. Mean of SBP or DBP is provided for studies in which this could be determined in the population without hypertension. For studies that defined the nonhypertensive participants using a cutpoint, this has been denoted in the blood pressure data as an inequality. For studies in which the population without hypertension was defined as having no clinical history of hypertension, this has been denoted as no history of hypertension.
Proportion at baseline includes data from normotensive and hypertensive study participants.
No history of hypertension; see footnote “c.”
Weighted average or proportion, data pooled from multiple normotensive and prehypertensive categories.
Within previous 5 years.
All participants had a history of either transient ischemic attack or stroke.
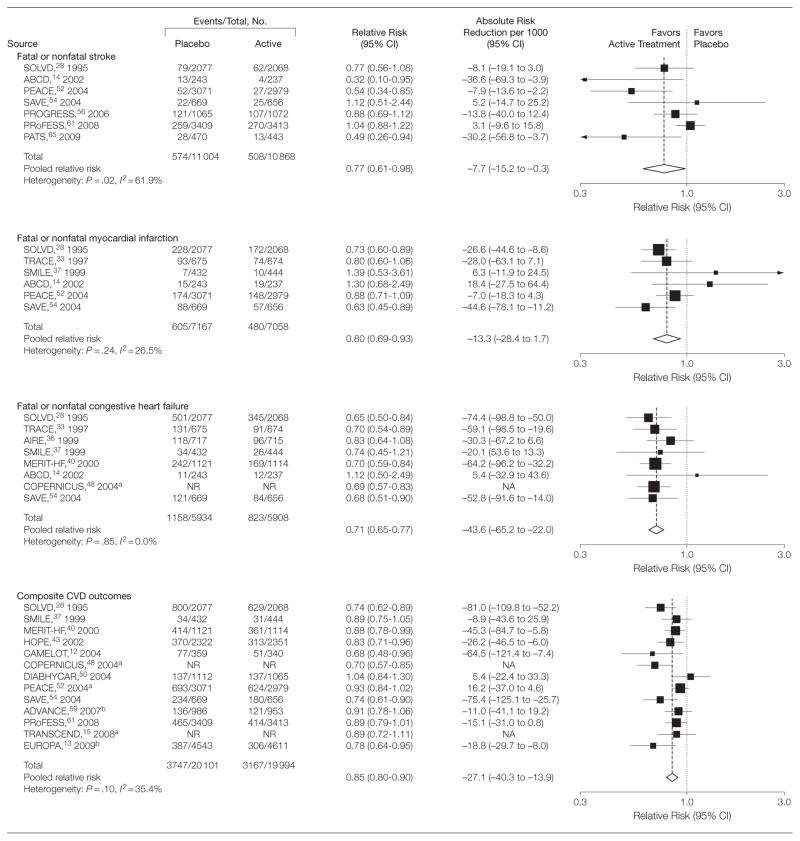
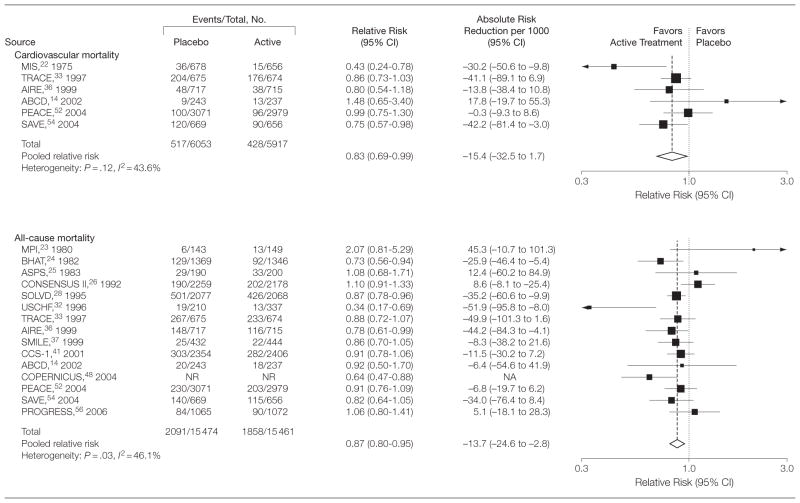
Pooled overall RRs and absolute risk reductions per 1000 persons are presented in Figure 2 and Figure 3 for all study outcomes. There was a 23% reduction in risk of stroke (RR, 0.77 [95% CI, 0.61 to 0.98]), 20% reduction in risk of MI (RR, 0.80 [95% CI, 0.69 to 0.93]), 29% reduction in risk of CHF events (RR, 0.71 [95% CI, 0.65 to 0.77]), 15% reduction in risk of composite CVD events (RR, 0.85 [95% CI, 0.80 to 0.90]), 17% reduction in risk for CVD mortality (RR, 0.83 [95% CI, 0.69 to 0.99]), and a 13% reduction in risk for all-cause mortality (RR, 0.87 [95% CI, 0.80 to 0.95]). The absolute risk reduction per 1000 persons was −7.7 (95% CI, −15.2 to −0.3) for stroke, −13.3 (95% CI, −28.4 to 1.7) for MI, −43.6 (95% CI, −65.2 to −22.0) for CHF events, −27.1 (95% CI, −40.3 to −13.9) for composite CVD events, −15.4 (95% CI, −32.5 to 1.7) for CVD mortality, and −13.7 (95% CI, −24.6 to −2.8) for all-cause mortality.
Figure 2.
Pooled Relative Risks and Absolute Risk Reductions for Fatal or Nonfatal Stroke, Myocardial Infarction, and Congestive Heart Failure and Composite Cardiovascular Disease Outcomes
CI indicates confidence interval; CVD, cardiovascular disease; NA, not applicable; NR, not reported. Sizes of data markers indicate the weight of each study in the analysis. For expansions of study names, see Table 1 footnote.
aNumber of events could not be calculated from information provided.
bNumber of events was estimated from information provided.
Figure 3.
Pooled Relative Risks and Absolute Risk Reductions for Cardiovascular and All-Cause Mortality
CI indicates confidence interval; CVD, cardiovascular disease; NA, not applicable; NR, not reported. Sizes of data markers indicate the weight of each study in the analysis. For expansions of study names, see Table 1 footnote.
I2 values were calculated to quantify heterogeneity between studies. The I2 values were 26.5% (P =.24) and 0.0% (P =.85) for MI and CHF events, indicating low heterogeneity between studies. Moderate heterogeneity was detected for stroke events (I2= 61.9% [P =.02 from Q test]), composite CVD events (I2=35.4% [P =.10]), CVD mortality (I2= 43.6% [P = .12]), and all-cause mortality (I2=46.1% [P =.03]).
We found no evidence of publication bias as indicated by Begg rank correlation test for any outcome examined. However, possible publication bias was detected for stroke (P =.04) using Egger linear regression tests. Applying the trim and fill adjustment method produced no change in the overall effect estimate for stroke. Exclusion of any single study did not change the significance of the pooled estimates for CHF events, composite CVD outcomes, and all-cause mortality. After individual exclusion of the SOLVD, ABCD, PEACE, PROGRESS, or PATS studies, treatment with antihypertensive medications no longer showed a statistically significant benefit for the outcome of stroke. After exclusion of the SOLVD study, antihypertensive treatment for the prevention of MI no longer showed statistically significant benefit. For the prevention of CVD mortality, the benefit of antihypertensive treatment among persons without hypertension was no longer statistically significant after omission of TRACE, AIRE, or SAVE.
We conducted sensitivity analyses to examine the robustness of the results for the composite CVD outcome and all-cause mortality (eTable 3). Sensitivity analyses were not conducted for the outcomes of stroke, MI, CHF, and CVD mortality because of the small number of studies and events. Results did not differ according to any of these criteria. On a 9-point scale, our quality assessment scores ranged from 7.0 to 9.0 for all studies included. The median score was 9.0 points, and these studies were considered to be excellent quality. There was no difference in the association of antihypertensive treatment and composite CVD outcome or all-cause mortality after exclusion of studies that scored fewer than 9 points (MIS and BHAT received 8 points each; MPI, ASPS, and ABCD received 7 points each).
Additionally, we conducted subgroup analyses to examine whether the association of antihypertensive treatment differed among persons with clinical history of MI or coronary artery disease, those with preexisting CHF, and those with history of diabetes or class of antihypertensive medication (eTable 4). There was little change in the overall effect estimates by clinical history for any of the outcomes, with the exception of diabetes. For prevention of composite CVD outcomes and all-cause mortality, no statistically significant benefit of antihypertensive treatment was reported in trials conducted exclusively in patients with diabetes; however, these results should be interpreted cautiously because of the limited number of trials.
Blood pressure change from baseline to follow-up was available for non-hypertensive participants in 3 studies.14,37,54 The blood pressure difference between the treatment and placebo groups at the end of the intervention period was significantly different only for those in the ABCD normotensive study.14
COMMENT
This meta-analysis is unique in that, to our knowledge, it is the first to focus on the association of antihypertensive medication use and secondary prevention of CVD events and all-cause mortality among persons without clinically defined hypertension. Our results show that persons with a history of CVD but with blood pressures in the normal and prehypertensive ranges can obtain significant benefit from antihypertensive treatments. The overall pooled results for antihypertensive treatment compared with control showed a significant reduction in risk for fatal or nonfatal stroke, CHF events, composite CVD events, an all-cause mortality. For fatal and nonfatal MI and for CVD mortality, the pooled relative risk reduction was significant but the pooled absolute risk reduction did not achieve statistical significance. This discrepancy reflects the increased variance of the absolute measures compared with the variance of the relative measures. Results for the outcomes studied were consistent across subgroups and did not differ significantly by trial characteristics.
Risk for CVD increases monotonically at all blood pressure levels in the normotensive and prehypertensive range.2,3 Although prehypertension affects nearly 70 million adults in the United States and is associated with an increased risk of CVD similar to that seen for those with hypertension, the use of antihypertensive treatment among persons with blood pressures less than 140/90 mm Hg has been debated.66–72 According to the current algorithm for treatment of hypertension in persons with compelling indications (CHF, post-MI, high coronary disease risk, and recurrent stroke prevention), pharmacological treatment is indicated for those whose blood pressure is not controlled to less than 140/90 mm Hg with lifestyle intervention alone.3 Hypertension precedes the development of CHF in the majority of patients and increases risk for MI and CHF.3
The results of this meta-analysis suggest that persons with these compelling indications but without hypertension may also benefit from reduced morbidity and mortality attributable to CVD events when treated with antihypertensive medications. In persons 40 years and older with prehypertension, more than 90% have at least 1 above-optimal risk factor, and more than 68% have at least 1 clinically high risk factor for heart disease or stroke.5 Although pharmacological treatment for all individuals in this population would not be economically feasible, a more reasonable strategy might be to identify groups within the prehypertensive population who would obtain the greatest benefit from early pharmacological intervention.
For patients with diabetes, the current algorithm for treatment of hypertension indicates pharmacological treatment for those whose blood pressure is not controlled to less than 130/80 mm Hg with lifestyle intervention alone.3 Recent findings reported from the ACCORD BP (Action to Control Cardiovascular Risk in Diabetes Blood Pressure) trial conducted in patients with diabetes demonstrated no reduction in the rate of fatal or nonfatal CVD events when systolic blood pressure was controlled to less than 120 mm Hg compared with less than 140 mm Hg.73 The ACCORD BP trial included participants with systolic blood pressures of 130 to 180 mm Hg who were taking 3 or fewer antihypertensive medications at baseline. The results of our meta-analysis show that for the prevention of composite CVD outcomes and all-cause mortality, no benefit of antihypertensive treatment was seen in trials conducted in patients with diabetes and without hypertension. Our findings should be interpreted with caution because of the small number of studies in such patients.
We identified only 2 studies of antihypertensive treatment conducted in populations with blood pressures less than 140/90 mm Hg and without a history of CVD or diabetes.74,75 The primary objective of both trials was to examine the prevention of hypertension in persons with blood pressure in the prehypertensive range, but CVD events were also examined. Although both studies were small and had relatively few events, there was an indication of possible benefit overall. Additional studies are needed to determine if any benefit of antihypertensive treatment would be obtained in populations without hypertension or clinical history of CVD.
We were able to identify no evidence among populations with specific risk factors such as elevated lipid levels, history of smoking, or chronic kidney disease. Additionally, few studies included racial and ethnic minorities or reported results according to race/ethnicity. Because of the increased risk for CVD events in the presence of these risk factors, additional studies should be conducted to determine if there is benefit of treating prehypertension at levels less than 140/90 mm Hg in populations with these risk factors. Although antihypertensive agents, including β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers are generally well tolerated, deleterious adverse effects are not uncommon and can be serious.
The primary strength of this meta-analysis was its inclusion of only randomized controlled trials, which are less subject to bias and confounding than observational studies. Additionally, study characteristics were very similar at baseline, lending confidence to the findings.
The primary limitation of this meta-analysis was the dearth of studies reporting the outcomes of interest for normotensive and prehypertensive participants. Few studies included in this meta-analysis presented the results by baseline blood pressure levels and treatment regimen; therefore, it was not possible to determine the dose-response relationship between baseline blood pressure and risk of first occurrence or recurrence of CVD events among persons with blood pressure less than 140/90 mm Hg. Additional studies should be conducted to examine the baseline blood pressure level at which antihypertensive treatment should begin in persons with CVD or CVD equivalents such as diabetes.
Moreover, this meta-analysis is not a mechanistic study; thus, we cannot determine whether the benefit associated with use of antihypertensive treatment was attributable to blood pressure lowering or to other tissue or neurohormonal mechanisms. Additionally, it is possible that misclassification of participants may have occurred owing to variations in methods of blood pressure measurement across studies included in the meta-analysis; however, less stringent methods of measurement may overdiagnose hypertension among participants. Because of the small number of studies included, potential publication bias and the influence of heterogeneity between studies cannot be ruled out.
Although we calculated the effect estimate from available data when it was not provided in the published data, it is possible that confounding occurred owing to differential loss to follow-up by treatment group. In addition, the statistical methods resulted in a discrepancy for the findings of 2 outcomes (MI and CVD mortality), perhaps reflecting the increased variance of the absolute measures compared with the variance of the relative measures, which may be compounded by the effect of pooling. Lastly, the total numbers of events were unavailable in some studies; therefore, the counts of events were estimated from the effect estimate and other information provided in the text of publications.13,59 It was not possible to estimate the total number of events in the COPERNICUS or TRANSCEND studies from the information provided in the text.15,48 A collaborative meta-analysis pooling individual-patient data could serve to eliminate many of these limitations.
CONCLUSION
Prehypertension affects nearly 30% of the adult population and carries an elevated risk for CVD incidence and mortality. To our knowledge, this meta-analysis is the first to examine the association between antihypertensive medications and CVD morbidity and mortality as well as all-cause mortality in individuals without hypertension. Among patients with clinical history of CVD but without hypertension, antihypertensive treatment was associated with decreased risk of stroke, CHF, composite CVD events, and all-cause mortality. Additional randomized trial data are necessary to assess these outcomes in patients without CVD clinical recommendations.
Supplementary Material
Acknowledgments
Funding/Support: Ms Thompson is supported by a grant from the Research Enhancement Fund of Tulane University. Dr He is supported by research grants R01 HL087263 and R01 HL090682 from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health. Dr Bazzano is supported by grant K08 HL091108 from the NHLBI.
Role of the Sponsor: The funding organizations and sponsors had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Online-Only Material: eTables 1–4 are available at http://www.jama.com.
Author Contributions: Ms Thompson and Dr Bazzano had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Reynolds, He, Bazzano.
Acquisition of data: Thompson, Hu, Eshelbrenner, Bazzano.
Analysis and interpretation of data: Thompson, Hu, He, Bazzano.
Drafting of the manuscript: Thompson, Hu, Bazzano.
Critical revision of the manuscript for important intellectual content: Thompson, Hu, Eshelbrenner, Reynolds, He, Bazzano.
Statistical analysis: Thompson, Eshelbrenner, Bazzano.
Obtained funding: He, Bazzano.
Administrative, technical, or material support: Hu, Eshelbrenner, Bazzano.
Study supervision: He, Bazzano.
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 4.Lawes CM, Vander Hoorn S, Rodgers A International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371 (9623):1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 5.Greenlund KJ, Croft JB, Mensah GA. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999–2000. Arch Intern Med. 2004;164(19):2113–2118. doi: 10.1001/archinte.164.19.2113. [DOI] [PubMed] [Google Scholar]
- 6.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framing-ham Heart Study: a cohort study. Lancet. 2001;358(9294):1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 7.Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287(8):1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Wang QJ. The prevalence of prehypertension and hypertension among US adults according to the new Joint National Committee guidelines: new challenges of the old problem. Arch Intern Med. 2004;164(19):2126–2134. doi: 10.1001/archinte.164.19.2126. [DOI] [PubMed] [Google Scholar]
- 9.Choi KM, Park HS, Han JH, et al. Prevalence of prehypertension and hypertension in a Korean population: Korean National Health and Nutrition Survey 2001. J Hypertens. 2006;24(8):1515–1521. doi: 10.1097/01.hjh.0000239286.02389.0f. [DOI] [PubMed] [Google Scholar]
- 10.Gu D, Reynolds K, Wu X, et al. InterASIA Collaborative Group. The International Collaborative Study of Cardiovascular Disease in ASIA. Prevalence, awareness, treatment, and control of hypertension in China. Hypertension. 2002;40(6):920–927. doi: 10.1161/01.hyp.0000040263.94619.d5. [DOI] [PubMed] [Google Scholar]
- 11.Whelton PK, He J. Blood pressure reduction. In: Buring JE, Manson JE, Ridker PM, editors. Clinical Trials in Cardiovascular Disease. Philadelphia, PA: WB Saunders; 2004. pp. 282–296. [Google Scholar]
- 12.Nissen SE, Tuzcu EM, Libby P, et al. CAMELOT Investigators. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004;292(18):2217–2225. doi: 10.1001/jama.292.18.2217. [DOI] [PubMed] [Google Scholar]
- 13.Remme WJ, Deckers JW, Fox KM, Ferrari R, Bertrand M, Simoons ML EUROPA Investigators. Secondary prevention of coronary disease with ACE inhibition—does blood pressure reduction with perindopril explain the benefits in EUROPA? Cardiovasc Drugs Ther. 2009;23(2):161–170. doi: 10.1007/s10557-008-6143-6. [DOI] [PubMed] [Google Scholar]
- 14.Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61(3):1086–1097. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Teo K, Anderson C, et al. Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372(9644):1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 16.Verhagen AP, de Vet HCW, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21 (11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Improvement in prognosis of myocardial infarction by long-term beta-adrenoreceptor blockade using practolol: a multicentre international study. Br Med J. 1975;3(5986):735–740. doi: 10.1136/bmj.3.5986.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baber NS, Evans DW, Howitt G, et al. Multicentre post-infarction trial of propranolol in 49 hospitals in the United Kingdom, Italy, and Yugoslavia. Br Heart J. 1980;44(1):96–100. doi: 10.1136/hrt.44.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beta-Blocker Heart Attack Trial Research Group. A randomized trial of propranolol in patients with acute myocardial infarction, I: mortality results. JAMA. 1982;247(12):1707–1714. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 25.Australian and Swedish Pindolol Study Group. . The effect of pindolol on the two years mortality after complicated myocardial infarction. Eur Heart J. 1983;4(6):367–375. [PubMed] [Google Scholar]
- 26.Swedberg K, Held P, Kjekshus J, Rasmussen K, Rydén L, Wedel H. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction: results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II) N Engl J Med. 1992;327(10):678–684. doi: 10.1056/NEJM199209033271002. [DOI] [PubMed] [Google Scholar]
- 27.CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316(23):1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 28.Kostis JB. The effect of enalapril on mortal and morbid events in patients with hypertension and left ventricular dysfunction. Am J Hypertens. 1995;8(9):909–914. doi: 10.1016/0895-7061(95)00156-j. [DOI] [PubMed] [Google Scholar]
- 29.SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 30.SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327(10):685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 31.SOLVD Investigators. Studies of left ventricular dysfunction (SOLVD)—rationale, design and methods: two trials that evaluate the effect of enalapril in patients with reduced ejection fraction. Am J Cardiol. 1990;66(3):315–322. doi: 10.1016/0002-9149(90)90842-o. [DOI] [PubMed] [Google Scholar]
- 32.Packer M, Bristow MR, Cohn JN, et al. U.S. Carvedilol Heart Failure Study Group. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334(21):1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 33.Gustafsson F, Torp-Pedersen C, Køber, Hildebrandt P TRACE Study Group. Effect of angiotensin converting enzyme inhibition after acute myocardial infarction in patients with arterial hypertension. J Hypertens. 1997;15(7):793–798 L. doi: 10.1097/00004872-199715070-00012. [DOI] [PubMed] [Google Scholar]
- 34.Køber L, Torp-Pedersen C, Carlsen JE, et al. Trandolapril Cardiac Evaluation (TRACE) Study Group. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1995;333(25):1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 35.Cleland JG, Erhardt L, Hall AS, Winter C, Ball SG. Validation of primary and secondary outcomes and classification of mode of death among patients with clinical evidence of heart failure after a myocardial infarction: a report from the Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. J Cardiovasc Pharmacol. 1993;22(suppl 9):S22–S27. [PubMed] [Google Scholar]
- 36.Spargias K, Ball S, Hall A. The prognostic significance of a history of systemic hypertension in patients randomised to either placebo or ramipril following acute myocardial infarction: evidence from the AIRE study. J Hum Hypertens. 1999;13(8):511–516. doi: 10.1038/sj.jhh.1000883. [DOI] [PubMed] [Google Scholar]
- 37.Borghi C, Bacchelli S, Esposti DD, Bignamini A, Magnani B, Ambrosioni E SMILE Study Investigators. Effects of the administration of an angiotensin-converting enzyme inhibitor during the acute phase of myocardial infarction in patients with arterial hypertension. Am J Hypertens. 1999;12(7):665–672. doi: 10.1016/s0895-7061(99)00042-4. [DOI] [PubMed] [Google Scholar]
- 38.Ambrosioni E, Borghi C, Magnani B. Survival of Myocardial Infarction Long-term Evaluation (SMILE) study: rationale, design, organization, and outcome definitions. Control Clin Trials. 1994;15(3):201–210. doi: 10.1016/0197-2456(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 39.Ambrosioni E, Borghi C, Magnani B Survival of Myocardial Infarction Long-term Evaluation (SMILE) Study Investigators. The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. N Engl J Med. 1995;332(2):80–85. doi: 10.1056/NEJM199501123320203. [DOI] [PubMed] [Google Scholar]
- 40.Hjalmarson A, Goldstein S, Fagerberg B, et al. MERIT-HF Study Group. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF) JAMA. 2000;283(10):1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 41.Liu L Chinese Cardiac Study (CCS-I) Collaborative Group. Long-term mortality in patients with myocardial infarction: impact of early treatment with captopril for 4 weeks. Chin Med J (Engl) 2001;114 (2):115–118. [PubMed] [Google Scholar]
- 42.Chinese Cardiac Study (CCS-1) Collaborative Group. Oral captopril versus placebo among 14,962 patients with suspected acute myocardial infarction: a multicenter, randomized, double-blind, placebo controlled clinical trial. Chin Med J (Engl) 1997;110 (11):834–838. [PubMed] [Google Scholar]
- 43.Sleight P, Yusuf S, Pogue J, Tsuyuki R, Diaz R, Probstfield J Heart Outcomes Prevention Evaluation (HOPE) Study. Blood-pressure reduction and cardiovascular risk in HOPE study. Lancet. 2001;358 (9299):2130–2131. doi: 10.1016/S0140-6736(01)07186-0. [DOI] [PubMed] [Google Scholar]
- 44.Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355(9200):253–259. [PubMed] [Google Scholar]
- 45.HOPE Study Investigators. The HOPE (Heart Outcomes Prevention Evaluation) study: the design of a large, simple randomized trial of an angiotensin-converting enzyme inhibitor (ramipril) and vitamin E in patients at high risk of cardiovascular events. Can J Cardiol. 1996;12(2):127–137. [PubMed] [Google Scholar]
- 46.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 47.Estacio RO, Savage S, Nagel NJ, Schrier RW. Baseline characteristics of participants in the Appropriate Blood pressure Control in Diabetes trial. Control Clin Trials. 1996;17(3):242–257. doi: 10.1016/0197-2456(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 48.Rouleau JL, Roecker EB, Tendera M, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group. Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure: the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. J Am Coll Cardiol. 2004;43(8):1423–1429. doi: 10.1016/j.jacc.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 49.Packer M, Coats AJS, Fowler MB, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 50.Marre M, Lievre M, Chatellier G, Mann JF, Passa P, Ménard J DIABHYCAR Study Investigators. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study) BMJ. 2004;328(7438):495. doi: 10.1136/bmj.37970.629537.0D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braunwald E, Domanski MJ, Fowler SE, et al. PEACE Trial Investigators. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351(20):2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Heart, Lung, and Blood Institute; Biologic Specimen and Data Repository Information Coordinating Center. PEACE study formal data request. Vol. 383. National Heart, Lung, and Blood Institute; [Accessed July 21, 2010]. Web site. https://biolincc.nhlbi.nih.gov/login/?next=/requests/data-formal-request/383/.2010. [Google Scholar]
- 53.Pfeffer MA, Domanski M, Rosenberg Y, et al. Prevention of events with angiotensin-converting enzyme inhibition (the PEACE study design): prevention of Events with Angiotensin-Converting Enzyme Inhibition. Am J Cardiol. 1998;82(3A):25H–30H. doi: 10.1016/s0002-9149(98)00488-3. [DOI] [PubMed] [Google Scholar]
- 54.Kenchaiah S, Davis BR, Braunwald E, et al. Survival and Ventricular Enlargement Trial. Antecedent hypertension and the effect of captopril on the risk of adverse cardiovascular outcomes after acute myocardial infarction with left ventricular systolic dysfunction: insights from the Survival and Ventricular Enlargement Trial. Am Heart J. 2004;148(2):356–364. doi: 10.1016/j.ahj.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 55.Pfeffer MA, Braunwald E, Moyé LA, et al. SAVE Investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trial. N Engl J Med. 1992;327(10):669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 56.Arima H, Chalmers J, Woodward M, et al. PROGRESS Collaborative Group. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens. 2006;24(6):1201–1208. doi: 10.1097/01.hjh.0000226212.34055.86. [DOI] [PubMed] [Google Scholar]
- 57.PROGRESS Management Committee. Blood pressure lowering for the secondary prevention of stroke: rationale and design for PROGRESS. J Hypertens Suppl. 1996;14(2):S41–S45. [PubMed] [Google Scholar]
- 58.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358(9287):1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 59.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 60.Rationale and design of the ADVANCE study: a randomised trial of blood pressure lowering and intensive glucose control in high-risk individuals with type 2 diabetes mellitus: Action in Diabetes and Vascular disease: PreterAx and DiamicroN modified-release Controlled evaluation. J Hypertens Suppl. 2001;19(4):S21–S28. [PubMed] [Google Scholar]
- 61.Yusuf S, Diener HC, Sacco RL, et al. PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359 (12):1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diener HC, Sacco R, Yusuf S Steering Committee, PRoFESS Study Group. Rationale, design and baseline data of a randomized, double-blind, controlled trial comparing two antithrombotic regimens (a fixed-dose combination of extended-release dipyridamole plus ASA with clopidogrel) and telmisartan versus placebo in patients with strokes: the Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS) Cerebrovasc Dis. 2007;23(5–6):368–380. doi: 10.1159/000100105. [DOI] [PubMed] [Google Scholar]
- 63.Gomma AH, Fox KM. The EUROPA trial: design, baseline demography and status of the substudies. Cardiovasc Drugs Ther. 2001;15(2):169–179. doi: 10.1023/a:1011131130922. [DOI] [PubMed] [Google Scholar]
- 64.Fox KM EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362(9386):782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 65.Liu L, Wang Z, Gong L, et al. Blood pressure reduction for the secondary prevention of stroke: a Chinese trial and a systematic review of the literature. Hypertens Res. 2009;32(11):1032–1040. doi: 10.1038/hr.2009.139. [DOI] [PubMed] [Google Scholar]
- 66.Yusuf S. Unresolved issues in the management of hypertension. Hypertension. 2010;55(4):832–834. doi: 10.1161/HYPERTENSIONAHA.109.142349. [DOI] [PubMed] [Google Scholar]
- 67.Pimenta E, Oparil S. Medscape. Prehypertension: epidemiology, consequences and treatment. Nat Rev Nephrol. 2010;6(1):21–30. doi: 10.1038/nrneph.2009.191. [DOI] [PubMed] [Google Scholar]
- 68.Papadopoulos DP, Makris TK, Papademetriou V. Is it time to treat prehypertension? Hypertens Res. 2008;31(9):1681–1686. doi: 10.1291/hypres.31.1681. [DOI] [PubMed] [Google Scholar]
- 69.Egan BM, Julius S. Prehypertension: risk stratification and management considerations. Curr Hypertens Rep. 2008;10(5):359–366. doi: 10.1007/s11906-008-0068-0. [DOI] [PubMed] [Google Scholar]
- 70.McInnes GT. Drug treatment of prehypertension: not now, not ever? Blood Press. 2009;18 (6):304–307. doi: 10.3109/08037050903416436. [DOI] [PubMed] [Google Scholar]
- 71.Kiely AE, Kwatra SG, Kwatra MM. Treating prehypertension: medically sound and economically viable. Blood Press. 2009;18(6):300–303. doi: 10.3109/08037050903444024. [DOI] [PubMed] [Google Scholar]
- 72.Mitka M. Experts ponder treating prehypertension. JAMA. 2006;295(18):2125–2126. doi: 10.1001/jama.295.18.2125. [DOI] [PubMed] [Google Scholar]
- 73.Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lüders S, Schrader J, Berger J, et al. PHARAO Study Group. The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens. 2008;26(7):1487–1496. doi: 10.1097/HJH.0b013e3282ff8864. [DOI] [PubMed] [Google Scholar]
- 75.Julius S, Nesbitt SD, Egan BM, et al. Trial of Preventing Hypertension (TROPHY) Study Investigators. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354 (16):1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.