1. Introduction
Human disease caused by highly pathogenic avian influenza (HPAI) H5N1 virus is associated with fulminant viral pneumonia and mortality rates in excess of 60% [1]. Cytokine dysregulation is thought to contribute to its pathogenesis [2,3]. We previously found delayed onset of apoptosis in H5N1 infected human macrophages and, therefore, a longer survival time of the target cells for prolonged virus replication and cytokine and chemokine secretion, which may contribute to the pathogenesis of H5N1 disease in humans [4]. As bronchial and alveolar epithelial cells are target cells of influenza virus because of their proximal physiological location and interaction with macrophages, we further investigated if the differential onset of apoptosis could be found in influenza H5N1 and seasonal influenza H1N1 infected human respiratory epithelia. We dissected the apoptotic pathways triggered by influenza virus infection.
2. Materials and methods
Seasonal influenza H1N1 virus (A/HK/54/98), a low pathogenic avian influenza H9N2 lineage isolated from poultry (A/Quail/HK/G1/97), and two virus isolates of highly pathogenic avian influenza (HPAI) A subtype (A/HK/483/97 and A/VN/1203/04) were included. Primary human bronchial and alveolar epithelial cells were infected with influenza viruses at MOI of 2 and the cell monolayer was collected at 24, 30, and 48 h post infection for TUNEL assay, and supernatant were collected for LDH assay. Fragments of human lung tissues were cut into multiple 2–3 mm fragments within 2 h of collection and infected with influenza A viruses at a titer of 106 TCID50/ml. These tissues fragments were infected for 1 h and incubated for 48 h at 37°C. One part of the infected tissue was fixed in formalin and processed for immunohistochemistry for influenza antigen, and the other part was homogenized and underwent RNA extraction. Apoptosis cDNA superarray platform (SABioscience) was employed to conduct apoptosis pathway analysis.
3. Results
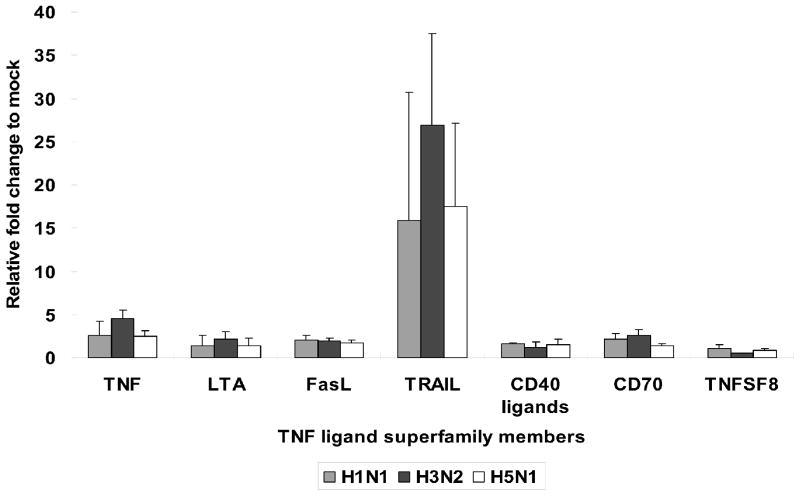
In bronchial epithelial cells, seasonal influenza H1N1 virus induced a high percentage of apoptotic cells by TUNEL assay at 24, 30, and 48 h post infection with a peak of approximately 50% apoptotic cells at 30 h maintained through 48 h post infection. The percentage of apoptotic cells induced by influenza H9N2 and H5N1 viruses remained low (below 10%) at 24 h and 30 h post infection with a minor increase at 48 h post infection (up to approximately 20%) (Figure 1). A similar observation of delayed onset of apoptosis was found in influenza H5N1 and H9N2 infected alveolar epithelial cells. Besides, cDNA array data of ex vivo infected human lung showed that both influenza H5N1 and H1N1 virus induced TRAIL expression compared with mock-infected tissue (approximately 15 folds) at 48 h post infection, but influenza H3N2 virus infected lung induced significantly more TRAIL (27 folds compared to mock infected cells), albeit with a limited viral replication (Figure 2). Influenza H3N2 virus infected lung also elicited more TNF-alpha and FasR transcription than either H5N1 or H1N1. These observations can account for the greater apoptotic response in influenza H3N2 virus infected lung. As little impact on the expression of intrinsic pathway components was observed, it seems that the apoptotic response to influenza virus infection in lung was mainly through the extrinsic pathways. No significant changes in the expression of anti-apoptotic protein gene was found, except for a moderate induction of BIRC3 by influenza H3N2 virus, which may act to modulate the apoptotic response.
Figure 1.
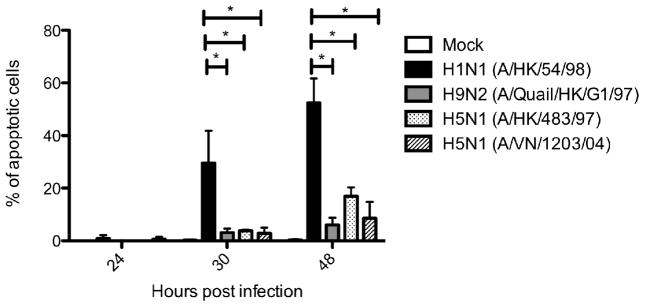
Differential time of onset of apoptosis in primary human bronchial epithelial cells infected by different influenza viruses. Bronchial epithelial cells were infected with H1N1 (A/HK/54/98), H9N2 (A/Quail/HK/G1/97), H5N1 (A/HK/483/97 and A/VN/1203/04) virus at an M.O.I. of 2 or treated with mock reagents. The percentage of apoptotic cells were determined by TUNEL assay at 24, 30, and 48 h post infection. The chart showed the mean and the standard deviation pooled from two independent experiments. Asterisk indicates significant difference with p < 0.001.
Figure 2.
Transcription of Tumor Necrosis Factor (TNF) ligand in lung infected by different influenza viruses. Ex vivo lung tissues were infected with H1N1 (A/HK/54/98), H3N2 (A/HK/1174/99), and H5N1 (A/Vietnam/3046/04) virus with virus titer at 1x106 TCID50/ml or treated with mock reagents. Total RNA was extracted for first-strand cDNA sysnthesis. The gene expression of key apoptotic genes were profiled by RT-PCR-based RT2 Profiler Apoptosis PCR Arrays (SUPERARRAYS BIOSCIENCE). The chart showed the mean and the standard deviation pooled from three independent experiments.
4. Discussion
The delayed onset of apoptosis by HPAI H5N1 and low pathogenic avian influenza H9N2 virus infected respiratory epithelial cells may be a mechanism for the influenza viruses to have more prolonged replication within the human respiratory tract, and this may contribute to the pathogenesis of human disease.
References
- 1.Claas EC, de Jong JC, van Beek R, et al. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine. 1998;16:977–978. doi: 10.1016/s0264-410x(98)00005-x. [DOI] [PubMed] [Google Scholar]
- 2.Cheung CY, Poon LL, Lau AS, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 3.Chan MC, Cheung CY, Chui WH, et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok CK, Lee DC, Cheung CY, et al. Differential onset of apoptosis in influenza A virus H5N1- and H1N1-infected human blood macrophages. J Gen Virol. 2007;88:1275–1280. doi: 10.1099/vir.0.82423-0. [DOI] [PubMed] [Google Scholar]