Abstract
The evolution of hearing in cetaceans is a matter of current interest given that odontocetes (toothed whales) are sensitive to high frequency sounds and mysticetes (baleen whales) are sensitive to low and potentially infrasonic noises. Earlier diverging stem cetaceans (archaeocetes) were hypothesized to have had either low or high frequency sensitivity. Through CT scanning, the morphology of the bony labyrinth of the basilosaurid archaeocete Zygorhiza kochii is described and compared to novel information from the inner ears of mysticetes, which are less known than the inner ears of odontocetes. Further comparisons are made with published information for other cetaceans. The anatomy of the cochlea of Zygorhiza is in line with mysticetes and supports the hypothesis that Zygorhiza was sensitive to low frequency noises. Morphological features that support the low frequency hypothesis and are shared by Zygorhiza and mysticetes include a long cochlear canal with a high number of turns, steeply graded curvature of the cochlear spiral in which the apical turn is coiled tighter than the basal turn, thin walls separating successive turns that overlap in vestibular view, and reduction of the secondary bony lamina. Additional morphology of the vestibular system indicates that Zygorhiza was more sensitive to head rotations than extant mysticetes are, which likely indicates higher agility in the ancestral taxon.
Keywords: archaeocete, bony labyrinth, Cetacea, cochlea, Mysticeti, Zygorhiza
Introduction
The evolution of whales from terrestrial to fully aquatic lifestyles is one of the most significant and famous transitions in vertebrate history. Those animals were faced with physiological challenges, especially when considering the special senses that had been evolving for hundreds of millions of years to function on land. The sense of hearing in stem cetaceans is of particular interest, given the physiological differences in the extant biota – the toothed whales (Odontoceti) are sensitive to high frequency and ultrasonic sound vibrations (Hall & Johnson, 1972; Ridgway et al. 1981; Brill et al. 2001; Hemilä et al. 2001; Ketten, 2004; Au et al. 2007; Nachtigall et al. 2007; Popov et al. 2007), whereas baleen whales (Mysticeti) are likely sensitive to lower frequency and potentially infrasonic noises based on behavioral models (Houser et al. 2001; Erbe, 2002; Parks et al. 2007). There is a great deal of interest in studying the evolution of hearing in cetaceans and two general hypotheses have been proposed for the attainment of the different auditory capabilities between the extant clades of whales. The first hypothesis is that low frequency sensitivity is ancestral for Neoceti (Odontoceti plus Mysticeti) and perhaps ancestral for Cetacea as a whole. Under that hypothesis, low frequency sensitivity is retained in mysticetes from their archaeocete ancestor with subsequent development of high frequency sensitivity in odontocetes. The low frequency hypothesis is supported by structures of the middle and inner ear (e.g. Fleischer, 1976; Thewissen et al. 1996; Nummela et al. 2004, 2007; Uhen, 2004). A second hypothesis is that high frequency sound reception is ancestral for Neoceti and retained in odontocetes with subsequent development of low frequency sensitivity in mysticetes. This intriguing hypothesis is supported by some data from the cochlea (Ketten, 1992b) as well as mandibular anatomy and asymmetry in archaeocete and odontocete skulls (e.g. Bianucci & Gingerich, 2011; Fahlke et al. 2011).
Crucial to the testing of these hypotheses is the auditory capability of archaeocetes and other stem cetaceans. Numerous attempts have been made to reconstruct the hearing physiology of archaeocetes, primarily through comparative anatomy of the middle ear region as preserved on and around the petrosal bone and tympanic bulla (Lancaster, 1990; Nummela et al. 1999, 2004, 2007), the hypothesized sound transmission pathway through the lower jaw (e.g. Roth, 1978; Thewissen et al. 1996; Nummela et al. 2007; Steeman, 2009), cranial endocasts in the region of cranial nerves VII (facial) and VIII (vestibulocochlear) (Edinger, 1955), and cranial asymmetry as related to directional hearing (Fahlke et al. 2011). However, few studies have examined the functional unit of the auditory portion of the inner ear in early whales as contained within fluid-filled chambers that contribute to the cochlea in mammals (exceptions include Fleischer, 1976; Uhen, 2004).
The functional unit of the cochlea is the spiral organ of hearing (organ of Corti), which extends the length of the cochlea upon the basilar membrane. Several quantifiable features of the basilar membrane are thought to be correlated with hearing physiology in mammals, including the length, thickness, and width of the basilar membrane (e.g. Békésy, 1970; Wever et al. 1971b; Fleischer, 1976; Echteler et al. 1994; Ketten, 1994, 1997; Wartzok & Ketten, 1999). The morphology of the basilar membrane is difficult to study in extinct animals because the soft-tissue structure is not preserved in the fossil record. However, the bony supports for the membrane, which are the primary and secondary bony laminae, are readily preserved in fossils as spiral ridges along the axial (inner) and radial (outer) walls of the cochlear canal, respectively (e.g. Fleischer, 1976; Court, 1992; Meng & Fox, 1995; Geisler & Luo, 1996; Ruf et al. 2009; Macrini et al. 2010; Ekdale & Rowe, 2011). Thus, many of those features thought to relate to hearing can be measured from a fossil, which offers the opportunity for documenting important transformations in the anatomy of the auditory system of extinct and extant cetaceans. Other features that are not directly related to basilar membrane morphology include the number of cochlear turns and gross anatomy of the cochlear spiral (e.g. West, 1985; Ketten & Wartzok, 1990; Manoussaki et al. 2006, 2008).
In this paper, the anatomy of the bony labyrinth of the inner ear, including the cochlea and semicircular canals, is described for the extinct archaeocete Zygorhiza kochii. Zygorhiza is a basilosaurid archaeocete (Uhen, 2004) and commonly is used in phylogenetic analyses of cetaceans given its close relationship with Neoceti (e.g. Geisler & Sanders, 2003; Deméré et al. 2008; Ekdale et al. 2011; Geisler et al. 2011; Marx, 2011; Bisconti, 2012; Fordyce & Marx, 2013). The descriptions are supplemented with novel observations of the inner ears of extinct and extant mysticetes, for which little information is known compared with odontocetes, as well as comparisons with published information for other cetaceans. Imaging of the internal cavities of the bony labyrinths was accomplished through high resolution X-ray computed tomography (CT) coupled with digital segmentation of inner ear cavities. Through detailed description of structures assumed to be associated with hearing sensitivity in the cochlea of Zygorhiza as compared with the ear regions of Neoceti, a more accurate interpretation of early cetacean physiology can be reconstructed.
Materials and methods
An isolated right petrosal of Zygorhiza kochii (USNM PAL 214433) was CT-scanned at the University of Texas CT facility in Austin, TX (UTCT). Scanning parameters for the novel data considered here, including mysticetes, are listed in Supporting Information Table S1. Digital segmentation and inner ear endocast extractions were performed in the visualization software avizo Standard Edition 8.0.0® (Visualization Sciences Group, an FEI Company, 2013). The bony channel for the cochlear aqueduct was included within the segment of the cochlea following the method of Ekdale (2013).
Because the inner ears of odontocetes are better known and described in the literature relative to mysticetes, novel anatomical data of the bony labyrinths of eight extant and extinct mysticete species were CT-scanned and examined for comparison with Zygorhiza (Table S1): Balaena mysticetus (bowhead), Balaenoptera acutorostrata (minke), two specimens of Eschrichtius robustus (neonate and adult gray), Eubalaena glacialis (right), Megaptera novaeangliae (humpback), and three fossil specimens from California including ‘Megaptera’ miocaena [Temblor Formation (Miocene)], an undescribed extinct balaenopterid [San Diego Formation (Pliocene)], and an undescribed extinct eschrichtiid [San Diego Formation (Pliocene)]. The phylogenetic position of ‘Megaptera’ miocaena is unresolved, and the taxon does not share a recent ancestry with extant Megaptera novaeangliae to the exclusion of other baleen whales, but almost certainly is a member of the balaenopterid lineage (Deméré et al. 2005; Bisconti, 2010; Fordyce & Marx, 2013). Further comparisons were made with published descriptions of extinct and extant taxa, including both odontocetes and mysticetes (e.g. Yamada & Yoshizaki, 1959; Fleischer, 1976; Ketten & Wartzok, 1990; Ketten, 1992a, 2000; Luo & Eastman, 1995; Geisler & Luo, 1996; Luo & Marsh, 1996; Uhen, 2004). In particular, data from Ekdale (2013) for extant Tursiops truncatus and an additional specimen of extinct Balaenopteridae [Yorktown Formation (Pliocene) of North Carolina] were used in figures and tables for comparison. Anatomical terminology for the bony labyrinth largely follows Fleischer (1976) and Ekdale (2013). Measurement protocols follow Fleischer (1976), Geisler & Luo (1996), Ekdale (2010: fig. 2; Ekdale, 2013: fig. 3), and Ekdale & Rowe (2011: fig. 1). All measurements of the inner ear were made with the avizo software.
Fig. 2.
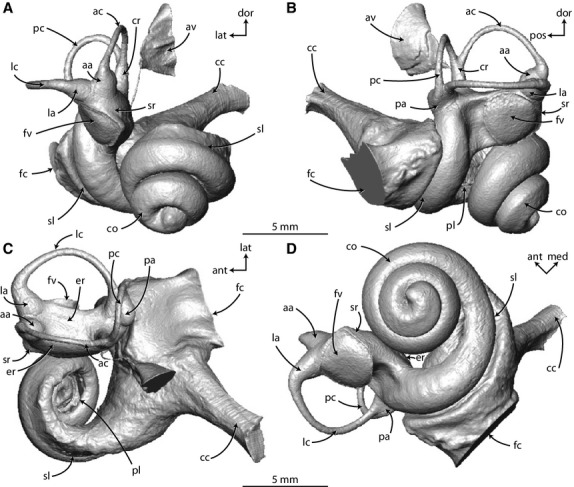
Digital endocast of right bony labyrinth of Zygorhiza kochii rendered from CT data in (A) anterior, (B) lateral, (C) dorsal, and (D) vestibular view. Anatomical abbreviations are listed at the end of the Materials and methods section.
Fig. 3.
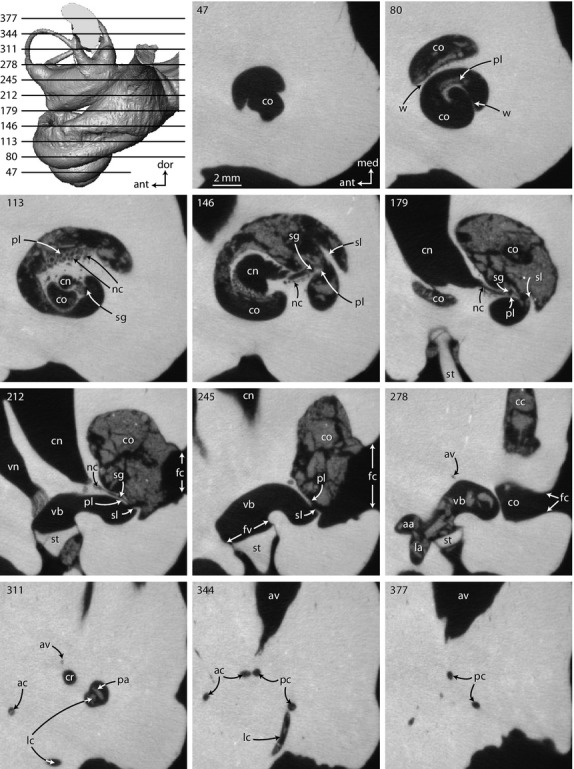
Original CT slices through right inner ear of Zygorhiza kochii in which osseous and other internal structures of the cochlea can be observed that are otherwise unavailable in the digital endocast. Numbers refer to specific CT slices. Anatomical abbreviations are listed at the end of the Materials and methods section.
Fig. 1.
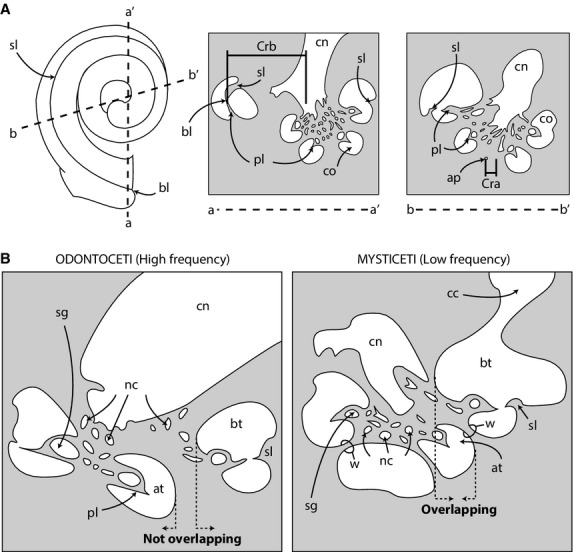
Graphical representations of measurement methods. (A) Schematic of cochlea (left) with cross-sections taken along lines a-a′ and b-b′. Cross-sections depict basal and apical radii used to calculate grade ratio of cochlea (method modified from Manoussaki et al. 2008). Shaded areas indicate bone and white areas indicate lumens of canals. (B) Cross-sections through cochleae of an odontocete (left) and mysticete (right) illustrating the positional relationships among basal and apical turns. Anatomical abbreviations are listed at the end of the Materials and methods section.
Quantitative and qualitative features that have been identified and described for numerous extinct mammals (Court, 1992; Meng & Fox, 1995; Ruf et al. 2009; Ekdale & Rowe, 2011; Luo et al. 2011), including whales (Fleischer, 1976; Luo & Eastman, 1995; Geisler & Luo, 1996; Luo & Marsh, 1996; Uhen, 2004; Ekdale, 2013), are cochlear and vestibular volumes, length of the cochlea, shape and nature of coiling of the cochlear spiral, distance between the bony laminae (‘laminar gap’, sensu Geisler & Luo, 1996; equivalent to ‘basilar gap’ of Fleischer, 1976), and extent of the cochlear canal for which the secondary bony lamina is present. The product of the length of the cochlear canal, which approximates the length of the basilar membrane, and the number of turns completed by the cochlea are thought to be negatively correlated to hearing in that a large product indicates a lower frequency threshold (West, 1985; Manoussaki et al. 2008). The length of the cochlear canal was measured using the SplineProbe tool in the avizo software and the degree of coiling followed the method utilized by Geisler & Luo (1996) and Ekdale (2013).
The shape of the cochlear spiral is described quantitatively by several ratios, including the graded curvature between basal and apical turns, basal ratio, axial pitch, and cochlear slope, as well as the positional relationship between the basal and more apical turns of the cochlea (modified from observations by Fleischer, 1976). The graded curvature of the cochlear spiral is expressed as the ratio between the radii of the basal turn of the cochlea over the apical turn (Manoussaki et al. 2006, 2008). In general, those authors found that mammals with lower auditory thresholds (i.e. lower frequency limits of hearing) possess cochleae with higher ratios (steeper grades). The radii of the turns were taken in two dimensions along re-sliced planes of the original CT data (modified from Manoussaki et al. 2008) (Fig. 1A). The data were re-sliced within the avizo software using the Slice tool. The basal radius was measured from the proximal ends of the primary and secondary bony spiral laminae to the center of the modiolus (axis of spiral rotation that contains the canal for cochlear branches of the cranial nerve VIII). The measurement was taken parallel to a line connecting the proximal ends of the bony spiral laminae and the outermost wall of opposing arm of the basal turn (Fig. 1A). The apical radius was measured from the apical tip of the cochlea to the center of the modiolus, on a plane parallel with the basal radius and perpendicular to the axis of rotation of the cochlear spiral.
The limit of low frequency hearing sensitivity of Zygorhiza and the mysticetes examined in this study was estimated using the equation derived by Manoussaki et al. (2008) using audiograms from mostly terrestrial mammals, although two marine species were examined as well (dolphin and sea lion). Behavioral audiograms in water for marine mammals were measured at 120 dB re 1 μPa and audiograms in air were taken at 60 dB re 20 μPa (Manoussaki et al. 2008). The equation was applied to extinct mammals by Macrini et al. (2013) and Orliac et al. (2012) (f = low frequency hearing limit at 60 dB re 20 μPa in air and 120 dB re 1 μPa under water; ρ = graded ratio of basal radius over apical radius):
The basal ratio was measured as the aspect ratio of cochlear spiral height over width (Ketten & Wartzok, 1990). The height and width of the cochlear spiral were measured following the method of Ekdale (2010: fig. 2). The axial pitch of the cochlea was measured as the ratio of spiral height over the number of turns completed by the cochlea and the cochlear slope was calculated as the height of the spiral over cochlear length over the number of turns (Ketten & Wartzok, 1990). The basal ratio, axial pitch, and cochlear slope appear to be related to different auditory classifications of extant whales (‘types’ I, II, and M of Ketten & Wartzok, 1990).
The positional relationships between the basal and more apical turns of the cochlea were observed qualitatively from re-sliced images through the axis of rotation of the cochlea. The successive turns were considered to be ‘overlapping’ if the axial wall of the more basal turn was positioned closer to the axis of rotation than the radial wall of the more apical turn (odontocete in Fig. 1B). Conversely, successive turns were considered to be ‘non-overlapping’ if the radial wall of the more apical turn was positioned closer to the axis of rotation than the axial wall of the more basal turn (mysticete in Fig. 1B).
The dimensions of the internal structures of the cochlea were measured with the avizo software following the methods of Fleischer (1976), including the widths of both the primary and secondary bony laminae (when preserved), the laminar gap (distance between the tips of opposing laminae), diameter of the spiral nerve ganglion canal within the primary bony lamina, and the thickness of the wall separating adjacent whorls of the cochlea (see Fleischer, 1976: fig. 2; Ekdale & Rowe, 2011: fig. 1C). The initial measurement was taken immediately internal to the fenestra cochleae, and each subsequent measurement was taken at every quarter turn along the cochlear spiral. The dimensions of the inner ear elements were used to graphically reconstruct the cochlear elements following the methods of Guild (1921) that were modified by Schuknecht (1953) and Wever et al. (1971a,b). Such graphical reconstructions have been used to illustrate the relationships among cochlear structures in several extinct and extant mammals and can be used to quickly observe dimensional changes across the cochlea (Wever et al. 1971b; Fleischer, 1976; Ramprashad et al. 1979; Luo & Eastman, 1995; Geisler & Luo, 1996; Luo & Marsh, 1996; Ekdale & Rowe, 2011).
Although the laminar gap is a common dimension that has been used to reconstruct the hearing abilities of many extinct mammals (e.g. Fleischer, 1976; Luo & Eastman, 1995; Geisler & Luo, 1996; Luo & Marsh, 1996; Ekdale & Rowe, 2011), the secondary bony lamina is extremely delicate and the entire structure often is lost in even the best-preserved recent specimens. The laminar gap may not be determined accurately for some fossil specimens, but the radial base of the secondary bony lamina is almost always better preserved than the axial edge. The extent of the secondary bony lamina through the cochlear spiral (spiral length of the lamina) can be determined for more specimens than either laminar widths or laminar gaps using the presence of the radial base of the bony lamina. Low ratios of secondary bony lamina spiral length over basilar membrane (or cochlear canal) length may correlate with low frequency limits (Ketten, 1992a).
Finally, one qualitative feature that might relate to hearing bandwidths is a radial expansion of the scala tympani, or the ‘tympanal recess’ of Fleischer (1976). Fleischer attempted to quantify the structure in part by calculating the ratio in cross-sectional area between the scalae tympani and vestibuli, two chambers within the bony cochlear canal that are separated by the basilar membrane. As mentioned above, the membrane is not available in fossil specimens and preservation of the primary and secondary laminae often is inadequate to accurately estimate the boundary between the two scalae. Therefore, this particular metric was not evaluated quantitatively here, although it may indeed prove to be informative using recent specimens. However, the tympanal recess is easily observed qualitatively on digital endocasts, and its presence might relate to low frequency sensitivity (Fleischer, 1976).
Although the discussion of the inner ear focuses on the cochlea and structures associated with hearing sensitivity, a description of the vestibule and semicircular canals is included to allow comparisons between the vestibular systems of Zygorhiza and other mammals from the published literature (e.g. Spoor et al. 2002, 2007; Silcox et al. 2009; Macrini et al. 2010, 2013; Billet et al. 2012, 2013; Orliac et al. 2012; Ekdale, 2013). Specific measurements of the vestibular system include the semicircular canal arc radii of curvature, lengths of the unampullated portions of the semicircular canals, and angles between the planes of the three semicircular canals (see Ekdale, 2010, 2013 and Ekdale & Rowe, 2011 for further discussion of semicircular canal measurement methods).
Morphology of the vestibular system has been used to estimate the sensitivity of the semicircular canals, and in turn agility. The most common method is based on the arc radius of curvature of the semicircular canals (Spoor et al. 2007; Silcox et al. 2009; Macrini et al. 2010, 2013; Orliac et al. 2012). However, the correlations are based on terrestrial mammals and depend on body mass, which is not always available for extinct vertebrates. Furthermore, the semicircular canals of cetaceans are significantly reduced with respect to body mass (e.g. Spoor et al. 2002) and the correlations may be inappropriate for whales (Spoor et al. 2007).
A second method that is independent of body mass and more appropriate for cetaceans measures the variance or deviation of ipsilateral (same side) semicircular canal pairs from orthogonality or 90° (90VAR of Malinzak et al. 2012; Berlin et al. 2013). Because this second method is independent of body mass, it may be more appropriate for fossil specimens and isolated petrosals for which body mass estimates are difficult to determine. The deviation from orthogonality is calculated as the absolute value of the difference of the angle between semicircular canal planes (anterior-lateral, anterior-posterior, lateral-posterior) from 90. The average deviation of the semicircular canal system is calculated by dividing the sum of the deviations of the individual canal pairs by three. In general, there is a negative relationship between the deviation and sensitivity of the canals such that an animal with more orthogonal canal pairs (low deviation) would have a vestibular system that is more sensitive to head rotations (Malinzak et al. 2012).
Anatomical abbreviations used in figures
aa – anterior ampulla; ac – anterior semicircular canal; ant – anterior direction; ap – apical tip of cochlea; at – apical turn of cochlear spiral; av – bony channel for endolymphatic duct (vestibular aqueduct); bl – basal end of bony laminae (represents origin of basilar membrane); bt – basal turn of cochlear spiral; cc – canaliculus cochleae for membranous perilymphatic duct; cn – canal for cranial nerve VIII within modiolus; co –cochlea; cr – common crus; Cra – radius of apical turn of cochlea; Crb – radius of basal turn of cochlea; dor – dorsal direction; er – elliptical recess of vestibule; fc – fenestra cochleae; fv – fenestra vestibuli; la – lateral ampulla; lat – lateral direction; lc – lateral semicircular canal; lg – laminar gap (distance between primary and secondary bony laminae); med – medial direction; nc – nerve canals for branches of cranial nerve VIII; pa – posterior ampulla; pc – posterior semicircular canal; pl – primary bony lamina; pos – posterior direction; sg – canal for spiral ganglion within primary bony lamina; sl – secondary bony lamina; sr – spherical recess; st – stapes; vb – vestibule; vn – canal for vestibular branch of cranial nerve VIII; w – wall separating successive turns of cochlea.
Morphological descriptions
Bony labyrinth (inner ear) of Zygorhiza kochii
The cochlea of Zygorhiza contributes approximately 84% of the total labyrinthine volume (Supporting Information Table S2; Fig. 2). A large cochlea relative to the vestibular system is characteristic of extinct and extant whales (Yamada & Yoshizaki, 1959; Geisler & Luo, 1996; Spoor et al. 2002; Ekdale, 2013). The cochlea completes approximately two and a half turns (Fig. 2D), and the large fenestra cochleae is situated at the basal end of the cochlea near the posterior region of the bony labyrinth (fc in Figs 2 and 3, slices 212–278). Unlike the condition in several extinct and extant odontocetes (Yamada & Yoshizaki, 1959; Luo & Eastman, 1995; Luo & Marsh, 1996; Ekdale, 2013), the cochlea does not curve outward to form a ‘cochlear hook’ that ends at the fenestra cochleae. The scala tympani (tympanal or towards the root of the modiolus with respect to the primary and secondary bony laminae) is inflated axial to the fenestra cochleae and cochlear canaliculus (Fig. 2). However, there is not an expanded and distinct tympanal recess as is observed in some extant and extinct mysticetes (Fleischer, 1976; Geisler & Luo, 1996; Ekdale, 2012, 2013). The bony canaliculus cochleae for the membranous perilymphatic duct is wide and oval in cross-section, and it extends from the scala tympani just apical to the fenestra cochleae and ultimately opens onto the external surface of the petrosal (cc in Figs 2 and 3, slice 278).
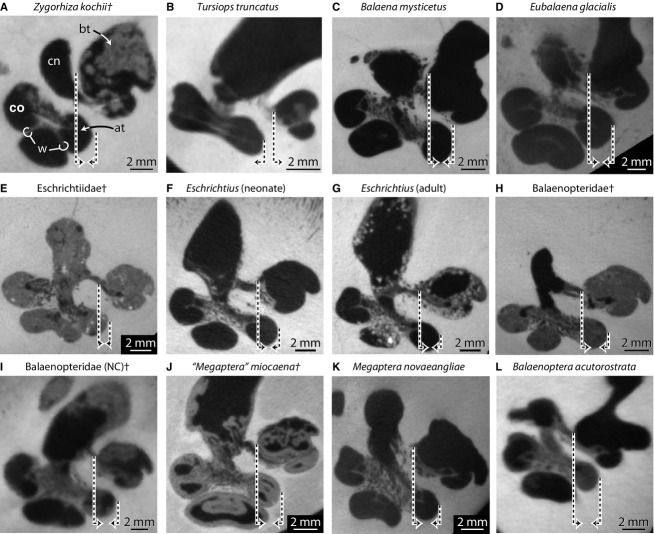
The cochlear spiral is equiangular in that the basalmost quarter of the cochlea is visibly separated from the apical turns when the endocast is in vestibular view (down axis of rotation from apex to base), and the third turn sits upon the second (Fig. 2D). In this sense, the apical turns are overlapping with respect to the more basal turns (Fig. 4A). The basal ratio of the cochlea is 0.52, indicating that the axis of the spiral is roughly half as high as the diameter of the basal turn (Table S2). The axial pitch of the cochlea is 2.62, and the slope 0.08. The grade of the cochlea (ρ; basal radius over apical radius) is 10.0, which is large compared with many terrestrial mammals (ranging from 1.7 in mouse and 8.9 in cow as reported by Manoussaki et al. 2008). The wall of bone between the second and third turns is exceptionally thin (Supporting Information Table S3; w in Figs 4A and 5A) and is not always resolved completely in the CT images (Fig. 3, slice 80; each voxel is approximately 24 × 24 × 35 μm, Table S1). The specimen of Zygorhiza examined by Fleischer (1976) also possessed thin walls between the second and third turns.
Fig. 4.
Cross-sections through the cochlea of (A) basilosaurid Zygorhiza kochii, (B) odontocete Tursiops truncatus, and (C–L) extinct and extant mysticete cetaceans at first quarter turn. Morphologies of the bony labyrinths of Tursiops truncatus (A) and Balaenopteridae (NC)† (H) are described and illustrated further by Ekdale (2013) and are included here for comparison. Daggers (†) indicate extinct taxa. Dashed lines ending in arrows indicate either the inner wall of the basal turn (long line) or the outer wall of the more apical turn (short line). Arrows directed towards each other (e.g. A) indicate turns that overlap in vestibular view and arrows directed away from each other (e.g. B) indicate turns that do not overlap. Anatomical abbreviations listed at the end of the Materials and methods section.
Fig. 5.
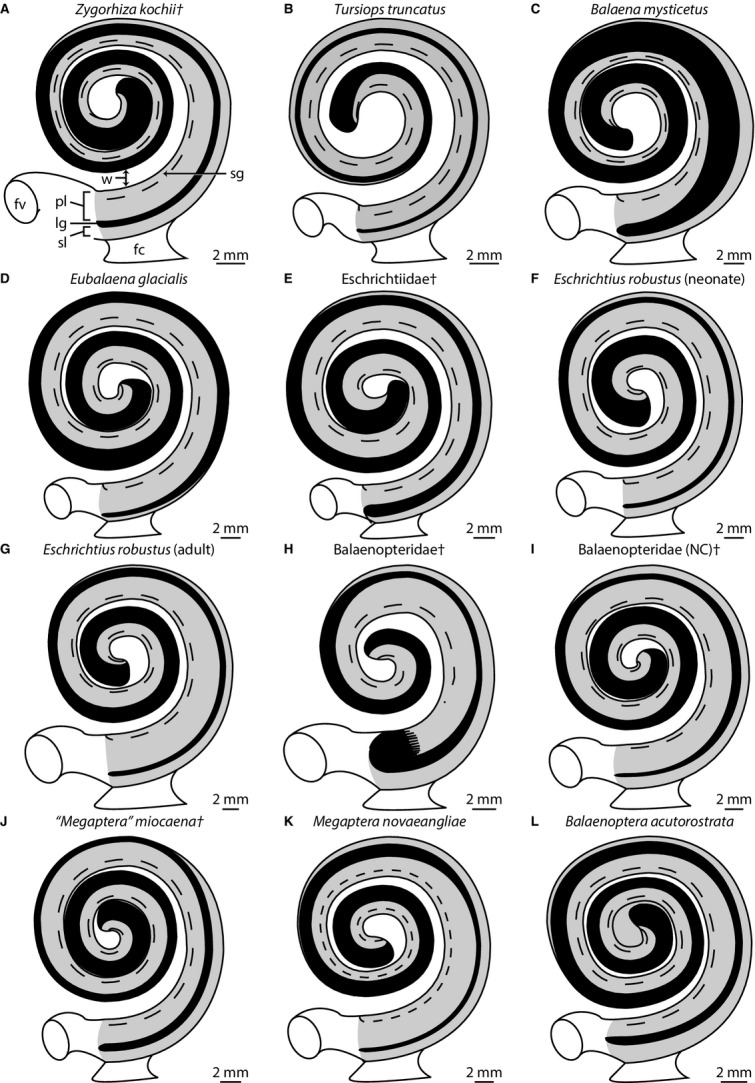
Graphical reconstructions of internal structures of the cochlea of (A) basilosaurid Zygorhiza kochii, (B) odontocete Tursiops truncatus, and (C–L) extinct and extant mysticete cetaceans. Reconstructions follow Guild (1921), Schuknecht (1953), and Wever et al. (1971a,b). Graphical reconstructions such as these illustrate the relationships among internal cochlear structures and can be used to quickly observe dimensional changes across the cochlea. Morphologies of the bony labyrinths of (A) Tursiops truncatus and (H) Balaenopteridae (NC)† are described and illustrated further by Ekdale (2013). They are included here for comparison. Measurements taken from Table S3. Projections do not preserve three dimensional relationships among successive turns. Daggers (†) indicate extinct taxa. Anatomical abbreviations are listed at the end of the Materials and methods section.
Both the axial and radial walls of the cochlear canal are marked with the primary and secondary bony spiral laminae to support the basilar membrane respectively (pl and sl in Fig. 3, slices 80–245). The bony laminae are expressed on the endocast of the bony labyrinth as distinct grooves (Fig. 2). The canal for the spiral nerve ganglion extends almost through the entire second cochlear turn within the primary bony lamina (Table S3; sg in Fig. 3, slices 113–212 and 4A). Small canals lead from the modiolus to the spiral ganglion canal (nc in Fig. 3 slices 113–212). The spiral ganglion canal is widest at the base of the cochlea but does not vary much in its diameter along its length (Table S3; sg in Fig. 5).
The primary bony lamina extends nearly to the apical tip of the cochlea, and it decreases in width from the base to the apex (Table S3; pl in Fig. 5). The secondary bony lamina is developed along the radial wall of the cochlea (sl in Fig. 3, slices 146–245) and disappears near the third quarter of the basal turn (Figs 2A,C, and 5; Table S3). Overall, the secondary bony lamina extends for approximately half the length of the cochlear canal (Tables S2 and S3). The laminar gap, or distance between the primary and secondary bony laminae, is narrowest at the base of the cochlea and doubles in width within the third quarter of the basal turn (distal to the terminus of the secondary bony lamina) while continuing to increase in width towards the apex (Table S3; lg in Fig. 5).
The spherical recess of the vestibule, which is connected to the cochlea and opens into the middle ear cavity via the fenestra vestibuli for the footplate of the stapes, is laterally concave and gives the vestibule a curved appearance in ventral view (sr in Fig. 2A,D). Overall, the vestibule is elongate, particularly the elliptical recess (er in Fig. 2C). A very slight transverse constriction deep to the fenestra vestibuli divides the spherical and elliptical recesses (groove between recesses in Fig. 2C). The three ampullae (anterior, lateral, and posterior), common crus, and bony aqueductus vestibuli (for membranous endolymphatic duct) exit at the four dorsal corners of the rectangular elliptical recess (aa, av, cr, la, and pa in Fig. 2C). The aqueductus vestibuli exits the vestibule ventral to the stout common crus (av in Fig. 3, slices 278–311). The aqueductus extends dorsally and curves posteriorly as a slender passage before opening into a pyramidal depression via the endolymphatic foramen on the endocranial surface of the petrosal (Figs 2A,C, and 3, slices 278–377).
The three ampullae for the semicircular canals are teardrop in shape (aa, la, and pa in Fig. 2). The lateral semicircular canal is continuous with a groove along the anterior wall of the posterior ampulla (expressed as a ridge on the endocast; lc in Figs 2C,D, and 3, slice 311). The lateral canal does not empty into the vestibule via its own foramen, nor does it form a secondary common crus with the poster semicircular canal as observed in many terrestrial mammals (Sánchez-Villagra & Schmelzle, 2007; Ekdale & Rowe, 2011; Benoit et al. 2013; Ekdale, 2013). The anterior semicircular canal is the most planar among the three canals, and the posterior canal is the least planar (ac and pc in Fig. 2B). The lateral limb of the posterior semicircular canal curves posteriorly as it enters the posterior ampulla (pa and pc in Fig. 2B,C). The lateral semicircular canal bends ventrally along its midpoint (lc in Fig. 2B). The area enclosed by the posterior semicircular canal arc is closer to a circle than are the areas enclosed by either anterior or lateral canal arcs (Fig. 2C,D). The area enclosed by the lateral semicircular canal arc is particularly elongate, and the arc radius of curvature of the lateral semicircular canal is the largest among the three canal arcs (Supporting Information Table S4). Likewise, the length of the lateral semicircular canal is greater than the length of the other canals. The plane of the posterior semicircular canal forms approximate right angles with the planes of the other canals, but the angle between the anterior and lateral semicircular canal planes is obtuse (Table S4).
Bony labyrinth (inner ear) comparisons with mysticeti
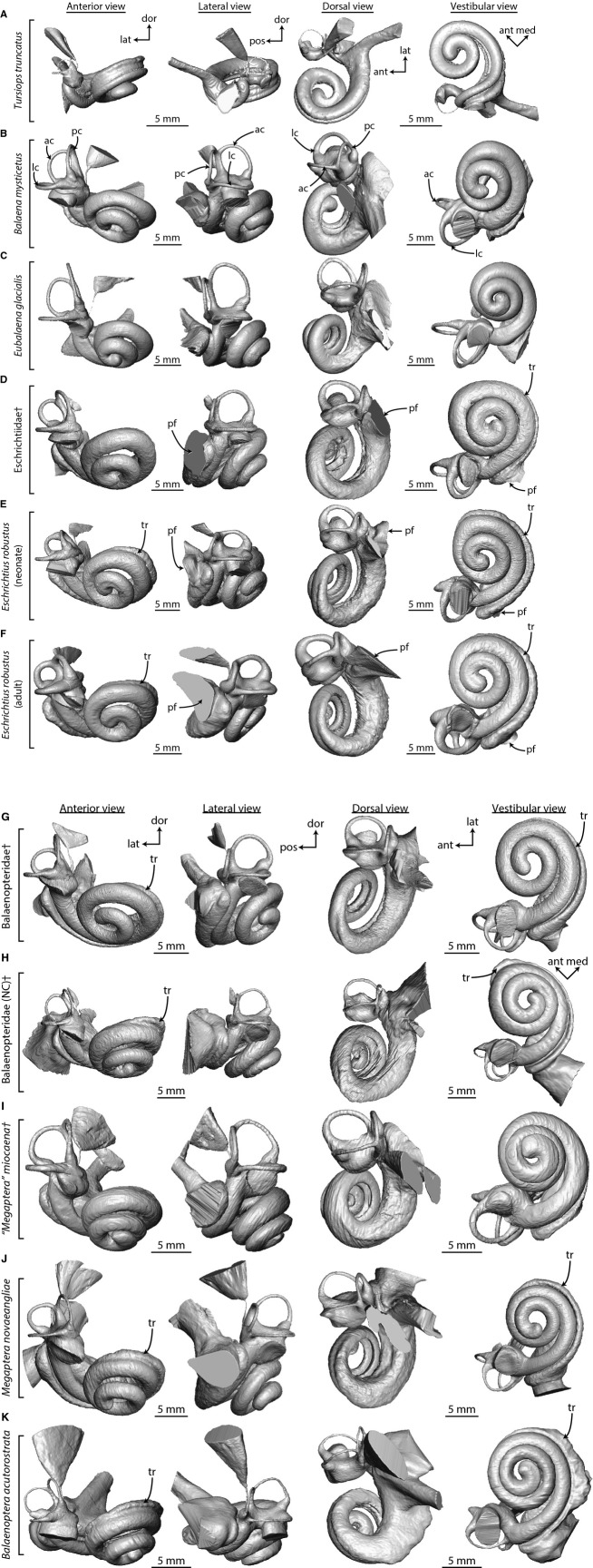
The most taxonomically comprehensive study of the gross anatomy of the bony labyrinth of mysticetes was by Yamada & Yoshizaki (1959). In their descriptions of resin casts of various cetacean inner ear labyrinths, they found it striking that Eubalaena appeared more similar to terrestrial mammals and unlike any other cetacean in that the semicircular canals did not appear to be reduced in the two specimens that they examined. In our comparisons of the mysticete bony labyrinth (Fig. 6), the vestibular system of all cetaceans is reduced with respect to the cochlea when compared with terrestrial mammals, and Eubalaena is no exception (Table S2; Ekdale, 2013). The Eubalaena individual that we examined appears anatomically consistent with other mysticetes and does not have a particularly ‘terrestrial’ morphology (Fig. 6C). The biggest difference between Eubalaena and other mysticetes is the short spiral length of the secondary bony lamina (Table S2), but that varies among terrestrial mammals as well (Ekdale, 2013).
Fig. 6.
Digital endocasts of bony labyrinths of (A) odontocete and (B–K) mysticete cetaceans in anterior, lateral, dorsal, and vestibular view for comparison with the archaeocete Zygorhiza (Fig. 2). Labyrinths for most mysticetes are from the right side of the body, but those from the left side (D, G, H, and J) are reversed for ease of comparison with the labyrinth of Zygorhiza in Fig. 2. Parts (A) and (H) are modified from Ekdale (2013: figs 28 and 30). Anatomical abbreviations are listed at the end of the Materials and methods section. [Note: Figure constructed in two parts].
Perhaps one of the largest structural differences observed among the bony labyrinths of mysticetes is the confluence of the fenestra cochleae and canaliculus cochleae into an undivided perilymphatic foramen in Eschrichtius and the undescribed eschrichtiid fossil (pf, Fig. 6D–F). The two passages are separate in all other specimens examined here, including Zygorhiza (cc and fc in Figs 2 and 6A–C,G–K), although they are narrowly separated in a balaenopterid fossil petrosal from the Pliocene of North Carolina [referred to as Balaenopteridae (NC) in Fig. 6H; Ekdale, 2013: figs 28–29]. A slit-shaped confluence of the fenestra cochleae and canaliculus cochleae has been reported for some immature specimens of the fin whale Balaenoptera physalus, but most fin whale specimens possess separate openings (Ekdale et al. 2011). Another difference is a radial expansion or tympanal recess within the basal turn of the scala tympani in most of the balaenopteroids (tr in Fig. 6D–K). In this condition, the scala tympani is easily observed when the cochlear endocast is in vestibular view (down the central modiolus). Except for the region immediately internal to the fenestra cochleae, the scala tympani is not visible in odontocetes (Fig. 6A), balaenids (Fig. 6B–C), ‘Megaptera’ miocaena (Fig. 6I) or Zygorhiza (Fig. 2D). The tympanic recess of the scala tympani is only partially visible in the vestibular aspect in the extinct eschrichtiid (tr in Fig. 6D).
The smallest bony labyrinth among extant mysticetes is measured for Balaenoptera in terms of anterior-posterior length, although the labyrinth of the extinct ‘Megaptera’ miocaena is the smallest in terms of gross volume (Table S2). The most voluminous labyrinth is calculated for Megaptera novaeangliae, although the labyrinth as a whole is the longest in Eubalaena (Table S2). The bony labyrinth of Zygorhiza is noticeably smaller than that of mysticetes. A positive correlation between body size and bony labyrinth size was discovered previously (e.g. Ekdale, 2013), and Zygorhiza was a smaller animal than any of the mysticetes examined here. A body mass of 3400 kg was estimated by Marino et al. (2000) as compared with the range for extant mysticetes considered here of between 7000 and 110 000 kg (Rice & Wolman, 1971; Silva & Downing, 1995).
The volumetric contribution of the cochlea to the entire bony labyrinth of Zygorhiza (84%) falls within the upper end of the range calculated for mysticetes (Table S2; 77% in Balaena to 84% in Megaptera novaeangliae; a contribution of around 90% was calculated for the North Carolina balaenopterid by Ekdale, 2013). The large cochlea relative to the vestibular system in cetaceans is well documented (Yamada & Yoshizaki, 1959; Fleischer, 1976; Luo & Gingerich, 1999; Luo & Marsh, 1996), and it has been established that the vestibule and semicircular canals have been reduced with respect to terrestrial mammals (Spoor et al. 2002).
The cochlea completes at least two full turns in all mysticetes reported here and elsewhere (Table 1, Table S2; Yamada & Yoshizaki, 1959; Geisler & Luo, 1996), and the cochlea of Zygorhiza (2.5 turns) falls within the mysticete range (2.0 in Balaena and the San Diego Balaenoperidae fossil to 2.7 in ‘Megaptera’ miocaena). However, all of the mysticete cochleae examined here coil to a lesser degree than the extinct mysticete Herpetocetus (Geisler & Luo, 1996). Among mysticetes, the longest and shortest cochlear canals (measured as the spiral length through the midline of the canal lumen) are observed for Eschrichtius (∼ 60 mm) and the extinct balaenopterid from San Diego (∼ 51 mm), respectively. As with volume and labyrinth length, the cochlea of Zygorhiza is smaller than that of any mysticete in terms of spiral length (Table S2).
Table 1.
Comparisons among cochlear ‘types’ (Ketten & Wartzok, 1990; Ketten, 1992b). Calculations for Balaenopteridae (NC) (from Pliocene of North Carolina) and Tursiops truncatus (this study) were based on data from Ekdale (2013)
| Taxon | Type | Turns | Pitch | Basal | Slope | Height | Source |
|---|---|---|---|---|---|---|---|
| Basilosauridae | |||||||
| Zygorhiza kochii | – | 2.50 | 2.60 | 0.50 | 0.08 | 6.50 | This study |
| Odontoceti | |||||||
| Inia geoffrensis | I | 1.50 | 1.50 | 0.27 | – | 2.30 | Ketten (1992b) |
| Phocoena phocoena | I | 1.50 | 0.98 | 0.28 | 0.04 | 1.47 | Ketten & Wartzok (1990) |
| Physeter macrocephalus | I | 1.75 | 1.78 | 0.22 | 0.03 | 3.12 | Ketten & Wartzok (1990) |
| Grampus griseus | II | 2.50 | 2.14 | 0.61 | 0.05 | 5.35 | Ketten & Wartzok (1990) |
| Lagenorhynchus albirostris | II | 2.50 | 2.11 | 0.60 | 0.06 | 5.28 | Ketten & Wartzok (1990) |
| Stenella attenuata | II | 2.50 | 1.75 | 0.51 | 0.05 | 4.36 | Ketten & Wartzok (1990) |
| Tursiops truncatus | II | 2.25 | 2.24 | 0.53 | 0.05 | 5.03 | Ketten & Wartzok (1990) |
| Tursiops truncatus | II | 1.70 | 3.60 | 0.50 | 0.10 | 6.20 | This study |
| Mysticeti | |||||||
| Balaena mysticetus | M | 2.00 | 4.30 | 0.50 | 0.08 | 8.50 | This study |
| Balaenoptera acutorostrata | M | 2.50 | 4.00 | 0.70 | 0.07 | 9.90 | This study |
| Balaenopteridae† | M | 2.00 | 4.10 | 0.50 | 0.08 | 8.10 | This study |
| Balaenopteridae (NC)† | M | 2.40 | 5.00 | 0.50 | 0.08 | 12.10 | This study |
| Eschrichtius robustus (neonate) | M | 2.20 | 5.00 | 0.50 | 0.08 | 11.00 | This study |
| Eschrichtius robustus (adult) | M | 2.10 | 4.50 | 0.50 | 0.07 | 9.50 | This study |
| Eschrichtiidae† | M | 2.40 | 4.40 | 0.60 | 0.08 | 10.60 | This study |
| Eubalaena glacialis | M | 2.40 | 3.80 | 0.60 | 0.07 | 9.10 | This study |
| ‘Megaptera’ miocaena† | M | 2.70 | 3.60 | 0.70 | 0.06 | 9.60 | This study |
| Megaptera novaeangliae | M | 2.20 | 4.20 | 0.50 | 0.07 | 9.20 | This study |
Daggers (†) indicate extinct taxa.
The basal ratio of the cochlear spiral (spiral height over width of the basal turn) is the greatest in extant Balaenoptera (0.66), although the ratio is similar for ‘Megaptera’ miocaena (Table 1). Except for Eubalaena (ratio equals 0.59), all of the remaining mysticetes and Zygorhiza have ratios around 0.50, indicating that the diameter of the basal turn is approximately twice as wide as the spiral is high. The axial pitch (spiral height over number of turns) is greatest for Eschrichtius, although there is variation between the two specimens (Table 1). The cochlea of Zygorhiza has a smaller axial pitch ratio than that of any mysticete examined here. The slope of the cochlea (spiral height over spiral length over number of turns) does not vary greatly among mysticetes, and the slope of Zygorhiza falls within the small range calculated for mysticetes.
Yamada & Yoshizaki (1959) described the apical coiling as loose in mysticetes in which the ‘vortex is open on the apex’ (p. 302). When the endocasts of the mysticete cochleae examined here are oriented in vestibular view, the space enclosed by the apical turn is open (Fig. 6B–K). The modiolus is extremely narrow at the apex of Zygorhiza, resulting in a closed space (Fig. 2D). Among mysticetes, relatively tight apical coiling is observed in Eubalaena, the extinct eschrichtiid, ‘Megaptera’ miocaena, and Balaenoptera (Fig. 6C,D,I, and K). In addition, Yamada & Yoshizaki (1959) noted that the proximal (basal) quarter of the cochlea diverges radially from the modiolus in odontocetes, and primarily delphinids. The divergence is a result, in part, of a wide bony separation between the first and second turns in odontocetes (e.g. Fig. 4B). An apparent divergence is observed in Zygorhiza in vestibular aspect (Fig. 2D), and the thickness of the wall between the turns at the first quarter is wider relative to the diameter of the basal turn (Tables S2 and S3). Among mysticetes, the relative thickness of the walls at the first quarter turn is thinnest in Balaenoptera and thickest in ‘Megaptera’ miocaena (Table S3; Fig. 5).
The tightness of apical coiling in some taxa may be described by the graded curvature of the cochlear spiral (following Manoussaki et al. 2008). Among mysticetes, the steepest grades are observed in Eubalaena (9.6), indicating a large difference between the basal and apical radii (Table S2). The lowest grade is observed for Balaena (5.6), which might be the result of the extreme loose coiling in that taxon (see Fig. 6B). Excluding Balaena, the range for extinct and extant mysticetes (6.6–9.6) falls within the range of mammals that have lower frequency limits relative to other taxa reported by Manoussaki et al. (2008). Interestingly, the grade calculated for Zygorhiza (10.0) is steeper than any of the mysticetes in this study (Table S2), as well as any of the taxa considered by Manoussaki et al. (2008).
The primary and secondary bony laminae are present in all of the mysticetes examined (Figs 4C–L and 6B–K). The secondary bony lamina nearly extends throughout the entire basal turn of the cochlea in Balaenoptera (Table S2; Figs 5L and 6K), but the lamina is not present beyond the first half of the basal turn in Balaena, Eubalaena, ‘Megaptera’ miocaena, and Megaptera novaeangliae (Table S2; Figs 5C,D,J, and K, and 6B,C,I, and J). The secondary bony lamina is restricted to the first two thirds of the basal turn in Zygorhiza (Table S2; sl in Fig. 2). The ratio of the curvilinear length of the secondary lamina to that of the cochlear canal is the lowest in Eubalaena (only present for 14% of the cochlear canal), although the secondary bony lamina is present for only around 30% in Balaena and ‘M.’ miocaena (Table S2). The secondary bony lamina of Zygorhiza is present for half the length of the cochlear canal, which falls within the range of mysticetes but below the range that has been reported for phocoenid odontocetes (e.g. Ketten & Wartzok, 1990; Ketten, 2000).
Discussion
One extreme difficulty in reconstructing the auditory physiology of extinct cetaceans is the lack of published audiograms for any extant mysticete. Thus, any comparisons using the morphology of baleen whales can only be made in a relative sense. Among extant cetaceans, odontocetes have been divided into two categories based on the ultrasonic frequencies of their vocalizations, which appear to correspond to auditory sensitivities, and morphological differences in cochlear shape (‘type I’ and ‘type II’ of Ketten, 1984; Ketten & Wartzok, 1990; Table 1). In general, each of those measurements is greater for the ‘type II’ cochlea of odontocetes than ‘type I’ (Ketten & Wartzok, 1990). The peak vocal frequencies of mysticetes are much lower than those of odontocetes (Edds, 1982; Ljungblad et al. 1982; Cummings & Holliday, 1987; Edds et al. 1993; Wartzok & Ketten, 1999; Erbe, 2002; Stimpert et al. 2007; Buchan et al. 2010), and all mysticete ears were binned within a single category (‘type M’; Ketten, 2000).
There have been a few attempts to compare cochlear anatomy among archaeocetes, odontocetes, and mysticetes in order to reconstruct the auditory physiology of Zygorhiza and allied taxa (e.g. Fleischer, 1976; Ketten, 1992b; Uhen, 2004). The bony laminae were poorly preserved in a specimen of Zygorhiza kochii examined by Fleischer (1976), although he argued that the overall structure of the Zygorhiza cochlea was significantly different from either extant Odontoceti or Mysticeti in terms of basal ratio. However, there is little difference among most cetaceans examined here and elsewhere (Table 1). Ketten (1992b) reexamined Fleischer's data for Zygorhiza and supplemented those analyses with additional archaeocete data compiled from Kellogg (1936). Ketten concurred with Fleischer that the basilosaurid cochlea is unlike either odontocetes or mysticetes and she classified Zygorhiza as ‘types I’, ‘II’, and ‘M’. Ultimately, she hypothesized that Eocene archaeocetes likely were sensitive to high frequencies with subsequent evolution of low frequency sensitivity in mysticetes, based on the incomplete cochlea initially described by Fleischer (1976). Uhen (2004) studied the cochlea of the closely related basilosaurid Dorudon atrox, which was better preserved than the Zygorhiza cochlea examined by Fleischer (1976). Uhen concluded that Dorudon was much more comparable to extant Mysticeti than extant and extinct Odontoceti, particularly in terms of the laminar gap, and he hypothesized that basilosaurids likely were sensitive to similar lower frequency sound waves as has been hypothesized for extant Mysticeti (Uhen, 2004).
Although some previous studies were unable to unite the cochlea of Zygorhiza with odontocetes or mysticetes (Fleischer, 1976; Ketten, 1992b), the dimensions of the cochlea can be compared. There are published data available for several odontocete species Ketten and Wartzok, 1990) that can be combined with the novel measurements of mysticetes produced in the present study. In terms of number of turns, the range of both types ‘II’ and ‘M’ overlap (1.7–2.5 and 2.0–2.7, respectively) and Zygorhiza (2.5) falls within that range (Table 1). Note that the number of turns calculated for the Tursiops truncatus specimen examined by Ekdale (2013) is nearly one half turn less than the value reported by previous authors (e.g. Ketten & Wartzok, 1990; Ketten, 1992b, 2000). In terms of axial pitch and absolute axial height, Zygorhiza falls between the ranges of odontocetes and mysticetes (Table 1). The basal and slope ratios of Zygorhiza fall within the ranges of mysticetes examined here and are separate from values calculated for ‘type I’ odontocetes (Table 1). Taking those observations together, it is unlikely that the cochlea of Zygorhiza is of ‘type I’, and although it may be classified as ‘type II’, there is more evidence for a ‘type M’ or mysticete-like classification. Because the anatomy of the cetacean cochlea is related to auditory function and auditory thresholds initially defined the ‘types’, Zygorhiza likely would have been sensitive to similar low frequencies as are extant mysticetes.
Most previous attempts to reconstruct the hearing physiology of extinct mammals have focused on the secondary bony lamina, and the laminar gap in particular (e.g. Fleischer, 1976; Geisler & Luo, 1996; Ekdale & Rowe, 2011). In life, the laminar gap and the robusticity of each bony spiral lamina affect the rigidity of the basilar membrane in such a way that a narrow gap and robust laminae indicate a stiffer membrane (Wever et al. 1971b; Pye, 1979). Stiffness and thickness of the membrane in turn are related to frequency sensitivity, in that a mammal with a stiffer basilar membrane will be sensitive to higher frequency vibrations than a mammal with a more flexible membrane (Békésy, 1970; Echteler et al. 1994; Ketten, 1994, 1997; Wartzok & Ketten, 1999). Following a transitive property, one mammal with a narrower laminar gap than another mammal will have a stiffer basilar membrane, and in turn will be more sensitive to higher frequencies. Even in the absence of neurophysiological data, the laminar gap can be used to interpret the relative hearing differences among mammals. However, the primary and secondary bony laminae are extremely thin and delicate, and their distal edges towards the center of the cochlear lumen often are missing or are not resolved in CT images of even the best preserved recent specimens (Fig. 4).
The delicate nature of the bony laminae decreases the accuracy of basilar membrane width such that laminar gap estimates may be off by more than 100% when estimating actual membrane width at the basal end of the mysticete cochlea (Ketten, 2000). For odontocetes, estimates near the apex can be off by approximately 25%. Although the distal portions of the laminae are delicate, the bases are more robust and are more readily preserved, even in fossils. The proportion of the cochlear canal in which the secondary bony lamina is present is an indicator of relative high vs. low frequency sensitivity. Indeed, the secondary bony lamina is present for nearly 90% of the cochlea in Tursiops, but is restricted to the first three quarters in all mysticetes (Table S2). Among mysticetes, the secondary bony lamina is reduced to the greatest degree in extant balaenids, where it is restricted to the first third of the basal turn (Table S2). The secondary bony lamina extends for only half the cochlear canal in Zygorhiza, which suggests comparable development of the secondary bony laminae between the archaeocete and most mysticetes.
Although gracile and short (less extensive) with respect to odontocetes, the secondary bony lamina is present in mysticetes. However, it has been hypothesized that the secondary bony laminae in mysticetes ‘are not functional equivalents’ of the secondary bony lamina in odontocetes and that ‘presence of the [secondary bony] lamina in mysticetes is a residual ancestral condition rather than a derived structure related to mysticete frequency ranges’ (Ketten, 1992a: p. 736). A secondary bony lamina is not visible in the endocasts of the early cetaceans Ichthyolestes and Indocetus that are figured by Spoor et al. (2002), although it is unclear whether the structure is in fact absent in those taxa. Both Ichthyolestes and Indocetus are archaeocetes, but they represent very different stages in the evolution of whales and degree of secondarily aquatic adaptation than Zygorhiza, which might account for morphological differences. The secondary bony lamina is described as absent for the terrestrial artiodactyl Sus (Ekdale, 2013), although the structure is described and figured for the early artiodactyl Diacodexis (Orliac et al. 2012), extinct notoungulates (Macrini et al. 2010, 2013), and the extant mouse deer Moschiola (Orliac et al. 2012). We agree that the secondary bony lamina is an ancestral retention in mysticetes, especially given the similarities in structure between mysticetes and Zygorhiza, but given that the bony lamina is attached to the basilar membrane, its very presence would affect the function of the membrane.
A combination of basilar membrane length and number of cochlear turns is hypothesized to be useful in predicting high and low frequency thresholds in terrestrial mammals (West, 1985). In particular, West argued that the low frequency limit of hearing in terrestrial mammals could be predicted by the product of basilar membrane length multiplied by number of turns. Manoussaki et al. (2008) recovered a similar correlation, thereby corroborating West's hypothesis. The product of length times turns calculated for Zygorhiza falls between extant odontocetes and mysticetes (Table S2), but the metric is not entirely independent of body mass (length of the cochlear canal scales to body mass; Ekdale, 2013). Furthermore, West's calculations were based on audiograms measured in air. Pinnipeds have different auditory thresholds in water and air (e.g. Kastak & Schusterman, 1998, 1999), and subsequent investigations of West's correlations indicate that the dimensions may not hold for marine mammals, at least where the low frequency limit is concerned (Manoussaki et al. 2008). Therefore, this product may not be appropriate for the interpretation of auditory physiologies of extinct marine mammals, although future research is needed in this regard.
The graded curvature of the cochlea appears to be more important for reconstructing auditory capabilities of extinct mammals (Manoussaki et al. 2006, 2008). Manoussaki et al. (2008) examined a broad range of placental mammals and concluded that taxa with wider basal turns relative to apical turns had a downward shift in low frequency thresholds. As might be expected, the grade calculated for most mysticetes is greater than that calculated for Tursiops (Table S2). In fact, the taxa examined by Manoussaki et al. (2008) that have grades approaching mysticetes are the cow and the elephant, both of which are known to be sensitive to very low frequency noises (Heffner & Heffner, 1982, 1983; Payne et al. 1986; Poole et al. 1988). Using the equation provided by Manoussaki et al. (2008), the low frequency threshold for Tursiops was estimated to be 187 Hz (in water at 120 dB re 1 μPa; range in the literature from 150 to 200 Hz; Ketten, 2000; Manoussaki et al. 2008) and extinct and extant mysticetes ranged from 10 Hz (Eubalaena) to 107 Hz (Balaena) (Table S2). Balaena is an outlier – the next highest threshold was estimated for the extinct eschrichtiid (60 Hz). A low frequency threshold for Zygorhiza was estimated at 8 Hz, which not only is below the range of normal human hearing (20 Hz to 20 kHz) but is lower than that estimated for any mysticete considered here. Although these results support the hypothesis that mysticetes are sensitive to low frequency noises, the specific frequency values that are estimated here should be considered tentative until audiograms of mysticetes are obtained and tested against the equation of Manoussaki et al. (2008), which was based primarily on hearing of terrestrial mammals in air.
Related to the ‘tightness’ of coiling of the cochlea is the separation between basal and apical turns. Moving through the cochlea from the base to the apex, the walls separating the turns become thinner in all of the cetaceans examined here (Table S3; Fig. 5). The thickness of the wall at one-quarter turn of the basal whorl relative to the basal diameter is nearly twice as large in Tursiops than in any mysticete, but Zygorhiza falls in between. However, when the cochlea is observed in cross-section, it can be seen that the walls are really quite thin in apical regions of Zygorhiza (w in Fig. 3, slice 80, and Fig. 4A), and the walls of successive turns overlap as observed in mysticetes (Fig. 4), indicating more similar hearing lifestyles in those taxa.
When compared with extant cetaceans, the cochlea of Zygorhiza is longer and coils to a greater degree, the wall separating the basal and apical whorls is relatively thinner, subsequent turns overlap, the secondary bony lamina is less robust and extends to a lesser degree within the cochlear canal, and the apical turn is coiled tighter (has a smaller relative radius) than the basal turn. All of these features point to physiology in line with extant mysticetes, which is a presumed sensitivity to low frequencies. If archaeocetes such as Zygorhiza were sensitive to low frequencies, then low frequency sensitivity would be the ancestral condition for crown Cetacea that is retained by extant mysticetes (Fig. 7). A subsequent specialization for high frequency sensitivity was developed in odontocetes.
Fig. 7.
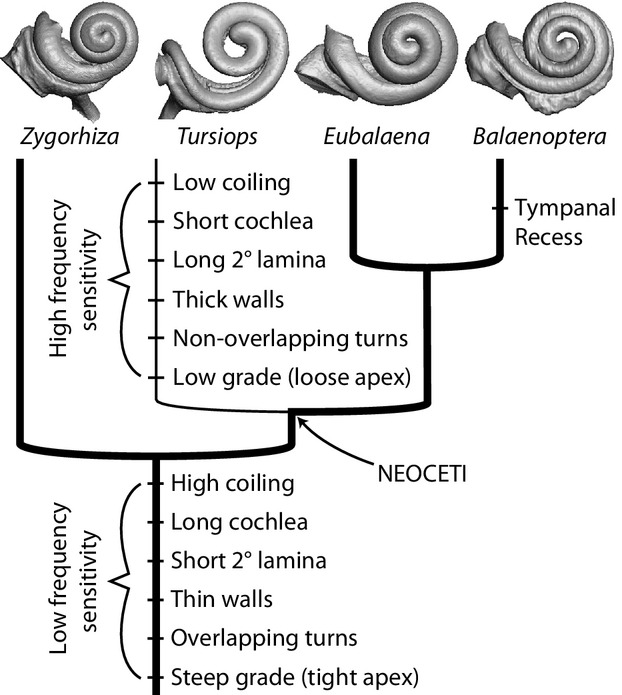
Generalized cladogram of Cetacea with mapped characters associated with auditory physiology. Thick line indicates low frequency sensitivity and thin line indicates high frequency sensitivity. Physiological significance of the characters is provided in the text.
Although structure of the cochlea supports low frequency sensitivity in Zygorhiza, high frequency sensitivity in archaeocetes was hypothesized based on cranial asymmetry and mandibular architecture (Fahlke et al. 2011). The mandibular features that have been used to argue high frequency hearing sensitivity in archaeocetes include a possible pan bone and enlarged mandibular canal for a fat pad (Bianucci & Gingerich, 2011; Fahlke et al. 2011). However, recent evidence has shown that the acoustic fats and pan bone may not serve as the primary ‘acoustic window’ for reception of high frequency sounds as originally thought (e.g. Norris, 1968), but rather the primary sound transmission pathway of sound to the ear is through the gular region and into the fat pads leading to the ear complex (Cranford et al. 2008). In this sense, the enlarged mandibular foramen would serve as an ‘open door’ (Cranford et al. 2010) that likely facilitates hearing underwater in general for early whales, rather than hearing at specific frequencies. Furthermore, an enlarged mandibular foramen would allow access for sound waves with larger wavelengths (indicating lower frequencies) and higher amplitudes (Barroso et al. 2012). This should not imply that the animals were detecting low frequency vibrations, but that those vibrations could be transported to the ear. Likewise, cranial asymmetry in archaeocetes, as argued by Fahlke et al. (2011), likely is an adaptation for directional hearing underwater rather than evidence of biosonar or a particular bandwidth of hearing sensitivity, but more research is needed in this area.
When considering the vestibular system and its relationship to agility and locomotion, cetaceans have been excluded from most correlations on account of the reduction of the vestibular system (e.g. Spoor et al. 2002) and cetacean estimates may not be comparable to terrestrial mammals (Spoor et al. 2007). However, the deviation of ipsilateral (same side) semicircular canal pairs from orthogonality (Malinzak et al. 2012; Berlin et al. 2013) is independent of body mass (unlike correlations based on semicircular canal arc radius; Spoor et al. 2007; Silcox et al. 2009) and it is perhaps more appropriate for extinct and extant cetaceans. In general, the closer two semicircular canal planes are to orthogonal (90°), the more sensitive the canals are to head rotations (Malinzak et al. 2012; Berlin et al. 2013). The average deviation from orthogonality for the three semicircular canal pairs is less for Zygorhiza than for any other cetacean examined, including Tursiops (Table S4), indicating high rotational sensitivity. Interestingly, the average deviation is low for extant Balaena, too. These results support a hypothesis that the deviation from orthogonality of the semicircular canals in mysticetes is likely a result of their shift from raptorial, single-prey predation as hypothesized for basilosaurids (Uhen, 2004; Fitzgerald, 2010) to bulk filter feeding in later diverging mysticetes. One might argue that bulk filter feeding would not require semicircular canals as sensitive as those needed for active pursuit predators. However, the agility of mysticetes has not been investigated across the clade and several mysticete species exhibit elaborate movements during breaching and feeding (e.g. lunging in balaenopterids). Future investigations into the sensitivity of the semicircular canals to rotations of the head should assess intraspecific variation in canal shape and orientation. For example, a greater degree of variation in the orientations and morphology of the semicircular canals was observed in slow moving xenarthrans compared with faster moving species (Billet et al. 2012). We predict that if there is a reduction in the sensitivity of the semicircular canals that is associated with bulk filter feeding, and in turn agility, then a greater degree of variation in the deviation of the canal planes from orthogonality would be observed in mysticetes than in earlier diverging raptorial cetaceans.
In summary, the morphology of the inner ear of Zygorhiza supports the hypothesis that low frequency sensitivity was ancestral for cetaceans and was retained in mysticetes with subsequent high frequency sensitivity in odontocetes. Given the potentially harmful effects of anthropogenic noise on cetaceans and other marine mammals, an accurate interpretation of auditory physiologies of living whales is of paramount importance. Thus, additional information from extinct mysticetes and odontocetes will uncover further patterns of the sensory evolution in cetaceans, which will lead to a better understanding of the physiologies of the living biota.
Acknowledgments
This project was funded by the National Science Foundation, DEB-0743869, DEB-0743861, and DEB-1146371. We thank M. Colbert and J. Maisano at UTCT for generating the CT data used in this study. We also thank A. Berta and T. Deméré for helpful discussions during the development of this project. Three anonymous reviewers provided helpful comments during the review process of the manuscript.
Author contributions
Both authors contributed to the design of the project, acquisition of data and data analysis. E.G.E. drafted the original manuscript and both E.G.E. and R.A.R. critically revised and approved the completed manuscript prior to submission.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Parameters used during novel CT scanning of cetaceans considered here. Daggers (†) indicate extinct taxa. Parameter abbreviations: Interslice – interslice spacing in μm; Interpixel – interpixel spacing in μm, calculated as field of reconstruction/image resolution; Reconstruction – field of reconstruction in mm; Resolution – image resolution of individual CT slices in pixels; Spec No – specimen numbers. Institutional abbreviations: HSU VM – Humboldt State University, Arcata, CA; LACM – Natural History Museum Los Angeles County, Los Angeles, CA; SDNHM/SDSNH – San Diego Natural History Museum, San Diego, CA; USNM – United States National Museum of Natural History, Washington, DC.
Table S2. Dimensions and shape ratios of the bony labyrinths of cetaceans. Data from the bony labyrinths of Balaenopteridae (NC) (from Pliocene of North Carolina) and Tursiops truncatus were taken from Ekdale (2013). Daggers (†) indicate extinct taxa. #T – number of turns completed by cochlea; %Cv – volumetric contribution (%) of cochlea to entire bony labyrinth, calculated as Cv/(Cv + Vv); 2L/C – extension (%) of secondary bony lamina within cochlear canal, calculated as 2Ll/Cl; 2Ll – spiral length of secondary bony lamina (mm); Ap – axial pitch of cochlea, calculated as Ch/#T (mm); BLl – total length of bony labyrinth (mm); Br – basal (aspect) ratio of cochlear spiral, calculated as Ch/Cw; Ch – height of cochlear spiral (mm); Cl – spiral length of cochlear canal (mm); Cra – radius of apical turn of cochlea (mm); Crb – radius of basal turn of cochlea (mm); Cs – cochlear slope, calculated as Ch/Cl/#T; CT – product of length of cochlear canal and number of turns, calculated as Cl x #T; Cv – volume of cochlear canal (mm3); Cw – width of basal turn of cochlea (mm); LF – estimated low frequency limit (Hz) based on graded curvature (following Manoussaki et al. 2008); Vv – volume of vestibular system including ampullae and semicircular canals (mm3); ρ – graded curvature of cochlea, calculated as Crb/Cra).
Table S3. Dimensions of the internal structures of the cochlear canal at each quarter of every turn. First measurement location (0/4) is immediately behind the center of the fenestra cochleae. Values expressed in mm. Dashes (–) indicate that the structure is not present, and ‘NP’ indicates that the structure is not preserved. Bony labyrinths of Balaenopteridae (NC) (from Pliocene of North Carolina) and Tursiops truncatus are described further by Ekdale (2013). Daggers (†) indicate extinct taxa.
Table S4. Dimensions and orientations of anterior (A), lateral (L), and posterior semicircular canals (P). Canal arc radii and lengths expressed in mm, and 90var refers to average deviation of canal pair angles (90A-L, 90A-P, 90L-P) and expressed in degrees. Data from the bony labyrinths of Balaenopteridae (NC) (from Pliocene of North Carolina) and Tursiops truncatus were taken from Ekdale (2013). Daggers (†) indicate extinct taxa.
References
- Au WWL, Thomas JA, Ramirez KT. Characteristics of the auditory brainstem evoked potential of a Pacific white-sided dolphin (Lagenorhynchus obliquidens. Aquat Mamm. 2007;33:76–84. [Google Scholar]
- Barroso C, Cranford TW, Berta A. Shape analysis of odontocete mandibles: functional and evolutionary implications. J Morphol. 2012;273:1021–1030. doi: 10.1002/jmor.20040. [DOI] [PubMed] [Google Scholar]
- Békésy G. Travelling wave as frequency analysers in the cochlea. Nature. 1970;225:1207–1209. doi: 10.1038/2251207a0. [DOI] [PubMed] [Google Scholar]
- Benoit J, Orliac M, Tabuce R. The petrosal of the earliest elephant-shrew Chambius (Macroscelidea: Afrotheria) from the Eocene of Djebel Chambi (Tunisia) and the evolution of middle and inner ear of elephant shrews. J Syst Palaeontol. 2013;11:907–923. [Google Scholar]
- Berlin JC, Kirk EC, Rowe TB. Functional implications of ubiquitous semicircular canal non-orthogonality in mammals. PLoS ONE. 2013;8:e79585. doi: 10.1371/journal.pone.0079585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianucci G, Gingerich PD. Aegyptocetus tarfa, n. gen. et sp. (Mammalia, Cetacea), from the middle Eocene of Egypt: clinorhynchy, olfaction, and hearing in a protocetid whale. J Vertebr Paleontol. 2011;31:1173–1188. [Google Scholar]
- Billet G, Hautier L, Asher RJ, et al. High morphological variation of vestibular system accompanies slow and infrequent locomotion in three-toed sloths. Proc R Soc B. 2012;279:3932–3939. doi: 10.1098/rspb.2012.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billet G, Germain D, Ruf I, et al. The inner ear of Megatherium and the evolution of the vestibular system in sloths. J Anat. 2013;223:557–567. doi: 10.1111/joa.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisconti M. New description of ‘Megaptera’ hubachi Dathe, 1983 based on the holotype skeleton held in the Museum für Naturkunde, Berlin. Quad Mus St Nat Livorno. 2010;23:37–68. [Google Scholar]
- Bisconti M. Comparative osteology and phylogenetic relationships of Miocaperea pulchra, the first fossil pygmy right whale genus and species (Cetacea, Mysticeti, Neobalaenidae) Zool J Linn Soc. 2012;166:876–911. [Google Scholar]
- Brill RL, Moore PWB, Dankiewicz LA. Assessment of dolphin (Tursiops truncatus) auditory sensitivity and hearing loss using jawphones. J Acoust Soc Am. 2001;109:1717–1722. doi: 10.1121/1.1356704. [DOI] [PubMed] [Google Scholar]
- Buchan SJ, Rendell LE, Hucke-Gaete R. Preliminary recordings of blue whale (Balaenoptera musculus) vocalizations in the Gulf of Corcovado, northern Patagonia, Chile. Mar Mamm Sci. 2010;26:451–459. [Google Scholar]
- Court N. Cochlea anatomy Numidotherium koholense: auditory acuity in the oldest known proboscidean. Lethaia. 1992;25:211–215. [Google Scholar]
- Cranford TW, Krysl P, Hildebrand JA. Acoustic pathways revealed: simulated sound transmission and reception in Cuvier's beaked whale (Ziphius cavirostris. Bioinspir Biomim. 2008;3:1–10. doi: 10.1088/1748-3182/3/1/016001. [DOI] [PubMed] [Google Scholar]
- Cranford TW, Krysl P, Amundin M. Acoustic portal into the odontocete ear and vibrational analysis of the tympanoperiotic complex. PLoS ONE. 2010;5:e11927. doi: 10.1371/journal.pone.0011927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings WC, Holliday DV. Sounds and source levels from bowhead whales off Pt. Barrow, Alaska. J Acoust Soc Am. 1987;82:814–821. doi: 10.1121/1.395279. [DOI] [PubMed] [Google Scholar]
- Deméré TA, Berta A, McGowen M. The taxonomic and evolutionary history of fossil and modern balaenopteroid mysticetes. J Mamm Evol. 2005;12:99–143. [Google Scholar]
- Deméré TA, McGowen MR, Berta A, et al. Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Syst Biol. 2008;57:15–37. doi: 10.1080/10635150701884632. [DOI] [PubMed] [Google Scholar]
- Echteler SM, Fay RR, Popper AN. Structure of the mammalian cochlea. In: Fay RR, Popper AN, editors. Comparative Hearing: Mammals. Springer Handbook of Auditory Research. Vol. 4. New York: Springer-Verlag; 1994. pp. 134–171. [Google Scholar]
- Edds PL. Vocalizations of the blue whale, Balaenoptera musculus, in the St. Lawrence River. J Mammal. 1982;63:345–347. [Google Scholar]
- Edds PL, Odell DK, Tershy BR. Vocalizations of a captive juvenile and free-ranging adult-calf pairs of Bryde's whales, Balaenoptera edeni. Mar Mamm Sci. 1993;9:269–284. [Google Scholar]
- Edinger T. Hearing and smell in cetacean history. Monatsschr Psychiatr Neurol. 1955;129:37–58. doi: 10.1159/000139733. [DOI] [PubMed] [Google Scholar]
- Ekdale EG. Ontogenetic variation in the bony labyrinth of Monodelphis domestica (Mammalia: Marsupialia) following ossification of the inner ear cavities. Anat Rec. 2010;293:1896–1912. doi: 10.1002/ar.21234. [DOI] [PubMed] [Google Scholar]
- Ekdale EG. Physiological and evolutionary implications of the cochlear morphology of Miocene Mysticeti (Cetacea) J Vertebr Paleontol. 2012;2012:90. Prog Abstr. [Google Scholar]
- Ekdale EG. Comparative anatomy of the bony labyrinth (inner ear) of placental mammals. PLoS ONE. 2013;8:e66624. doi: 10.1371/journal.pone.0066624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdale EG, Rowe T. Morphology and variation within the bony labyrinth of zhelestids (Mammalia, Eutheria) and other therian mammals. J Vertebr Paleontol. 2011;31:658–675. [Google Scholar]
- Ekdale EG, Berta A, Deméré TA. The comparative osteology of the petrotympanic complex (ear region) of extant baleen whales (Cetacea: Mysticeti) PLoS ONE. 2011;6:e21311. doi: 10.1371/journal.pone.0021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbe C. 2002. pp. 1–28. Hearing abilities of baleen whales. Defense R&D Canada Atlantic Contractor Report 2002–65.
- Fahlke J, Gingerich PD, Welsh RC, et al. Cranial asymmetry in Eocene archaeocete whales and the evolution of directional hearing in water. Proc Natl Acad Sci U S A. 2011;108:14545–14548. doi: 10.1073/pnas.1108927108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EMG. The morphology and systematics of Mammalodon colliveri (Cetacea: Mysticeti), a toothed mysticete from the Oligocene of Australia. Zool J Linn Soc. 2010;158:367–476. [Google Scholar]
- Fleischer G. Hearing in extinct cetaceans as determined by cochlear structure. J Paleontol. 1976;50:133–152. [Google Scholar]
- Fordyce RE, Marx FG. The pygmy right whale Caperea marginata: the last of the cetotheres. Proc R Soc B. 2013;280:20122645. doi: 10.1098/rspb.2012.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler JH, Luo Z-X. The petrosal and inner ear of Herpetocetus sp. (Mammalia: Cetacea) and their implications for the phylogeny and hearing of archaic mysticetes. J Paleontol. 1996;70:1045–1066. [Google Scholar]
- Geisler JH, Sanders AE. Morphological evidence for the phylogeny of Cetacea. J Mamm Evol. 2003;10:23–129. [Google Scholar]
- Geisler JH, McGowen MR, Yang G, et al. A supermatrix analysis of genomic, morphological, and paleontological data from crown Cetacea. BMC Evol Biol. 2011;11:1–33. doi: 10.1186/1471-2148-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild SR. A graphic reconstruction method for the study of the organ of Corti. Anat Rec. 1921;22:141–157. [Google Scholar]
- Hall JD, Johnson CS. Auditory thresholds of a killer whale Orcinus orca Linnaeus. J Acoust Soc Am. 1972;51:515–517. [Google Scholar]
- Heffner RS, Heffner HE. Hearing in the elephant (Elephas maximus): absolute sensitivity, frequency discrimination, and sound localization. J Comp Physiol Psychol. 1982;96:926–944. [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Hearing in large mammals: horses (Equus caballus) and cattle (Bos taurus. Behav Neurosci. 1983;97:299–309. [Google Scholar]
- Hemilä S, Nummela S, Reuter T. Modeling whale audiograms: effects of bone mass on high-frequency hearing. Hear Res. 2001;151:221–226. doi: 10.1016/s0378-5955(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Houser DS, Helweg DA, Moore PWB. A bandpass filter-bank model of auditory sensitivity in the humpback whale. Aquat Mamm. 2001;27:82–91. [Google Scholar]
- Kastak D, Schusterman RJ. Low-frequency amphibious hearing in pinnipeds: methods, measurements, noise, and ecology. J Acoust Soc Am. 1998;103:2216–2228. doi: 10.1121/1.421367. [DOI] [PubMed] [Google Scholar]
- Kastak D, Schusterman RJ. In-air and underwater hearing sensitivity of a northern elephant seal (Mirounga angustirostris. Can J Zool. 1999;77:1751–1758. [Google Scholar]
- Kellogg R. A review of the Archaeoceti. Carnegie Inst Wash Publ. 1936;482:1–366. [Google Scholar]
- Ketten DR. 1984. Correlations of morphology with frequency for odontocete cochlea: systematics and topology. Dissertation, Johns Hopkins University.
- Ketten DR. The marine mammal ear: specializations for aquatic audition and echolocation. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. New York: Springer-Verlag; 1992a. pp. 717–750. [Google Scholar]
- Ketten DR. The cetacean ear: form, frequency, and evolution. In: Thomas JA, Kastelein RA, Supin AY, editors. Marine Mammal Sensory Systems. New York: Plenum Press; 1992b. pp. 53–75. [Google Scholar]
- Ketten DR. 1994. pp. 264–270. Functional analyses of whale ears: adaptations for underwater hearing. IEEE Proc Underwater Acoust 1.
- Ketten DR. Structure and function in whale ears. Bioacoustics. 1997;8:103–135. [Google Scholar]
- Ketten DR. Cetacean ears. In: Au WWL, Popper AN, Fay RR, editors. Hearing by Whales and Dolphins. New York: Springer; 2000. pp. 43–108. [Google Scholar]
- Ketten DR. Marine mammal auditory systems: a summary of audiometric and anatomical data and implications for underwater acoustic impacts. Polarforschung. 2004;72:79–92. [Google Scholar]
- Ketten DR, Wartzok D. Three-dimensional reconstructions of the dolphin ear. In: Thomas J, Kastelein R, editors. Sensory Abilities of Cetaceans. New York: Plenum; 1990. pp. 81–105. [Google Scholar]
- Lancaster WC. The middle ear of the Archaeoceti. J Vertebr Paleontol. 1990;10:117–127. [Google Scholar]
- Ljungblad DK, Thompson PO, Moore SE. Underwater sounds recorded from migrating bowhead whales, Balaena mysticetus, in 1979. J Acoust Soc Am. 1982;71:477–482. [Google Scholar]
- Luo Z-X, Eastman ER. Petrosal and inner ear of a squalodontoid whale: implications for evolution of hearing in odontocetes. J Vertebr Paleontol. 1995;15:431–442. [Google Scholar]
- Luo Z-X, Gingerich PD. Terrestrial Mesonychia to aquatic Cetacea: transformation of the basicranium and evolution of hearing in whales. Univ Mich Pap Paleontol. 1999;31:1–98. [Google Scholar]
- Luo Z-X, Marsh K. Petrosal (periotic) and inner ear of a Pliocene kogiine whale (Kogiinae, Odontoceti): implications on relationships and hearing evolution of toothed whales. J Vertebr Paleontol. 1996;16:328–348. [Google Scholar]
- Luo Z-X, Ruf I, Schultz JA, et al. Fossil evidence on evolution of inner ear cochlea in Jurassic mammals. Proc R Soc B. 2011;278:28–34. doi: 10.1098/rspb.2010.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrini TE, Flynn JJ, Croft DA, et al. Inner ear of a notoungulate placental mammal: anatomical description and examination of potentially phylogenetically informative character. J Anat. 2010;216:600–610. doi: 10.1111/j.1469-7580.2010.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrini TE, Flynn JJ, Ni X, et al. Comparative study of notoungulate (Placentalia, Mammalia) bony labyrinths and new phylogenetically informative inner ear characters. J Anat. 2013;223:442–461. doi: 10.1111/joa.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinzak MD, Kay RF, Hullar TE. Locomotor head movements and semicircular canal morphology in primates. Proc Natl Acad Sci U S A. 2012;109:17914–17919. doi: 10.1073/pnas.1206139109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoussaki D, Dimitriadis EK, Chadwick RS. Cochlea's graded curvature effect on low frequency waves. Phys Rev Lett. 2006;96:1–4. doi: 10.1103/PhysRevLett.96.088701. [DOI] [PubMed] [Google Scholar]
- Manoussaki D, Chadwick RS, Ketten DR, et al. The influence of cochlear shape on low-frequency hearing. Proc Natl Acad Sci U S A. 2008;105:6162–6166. doi: 10.1073/pnas.0710037105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino L, Uhen MD, Frohlich B, et al. Endocranial volume of mid-late Eocene archaeocetes (Order: Cetacea) revealed by computed tomography: implications for cetacean brain evolution. J Mamm Evol. 2000;7:81–94. [Google Scholar]
- Marx FG. The more the merrier? A large cladistic analysis of mysticetes, and comments on the transition from teeth to baleen. J Mamm Evol. 2011;18:77–100. [Google Scholar]
- Meng J, Fox RC. Osseous inner ear structures and hearing in early marsupials and placentals. Zool J Linn Soc. 1995;115:47–71. [Google Scholar]
- Nachtigall PE, Mooney TA, Taylor KA, et al. Hearing and auditory evoked potential methods applied to odontocete cetaceans. Aquat Mamm. 2007;33:6–13. [Google Scholar]
- Norris KS. The evolution of acoustic mechanisms in odontocete cetaceans. In: Drake ET, editor. Evolution and Environment. New Haven: Yale University Press; 1968. pp. 297–324. [Google Scholar]
- Nummela S, Wägar T, Hemilä S, et al. Scaling of the cetacean middle ear. Hear Res. 1999;133:71–81. doi: 10.1016/s0378-5955(99)00054-4. [DOI] [PubMed] [Google Scholar]
- Nummela S, Thewissen JGM, Bajpai S, et al. Eocene evolution of whale hearing. Nature. 2004;430:776–778. doi: 10.1038/nature02720. [DOI] [PubMed] [Google Scholar]
- Nummela S, Thewissen JGM, Bajpai S, et al. Sound transmission in archaic and modern whales: anatomical adaptations for underwater hearing. Anat Rec. 2007;290:716–733. doi: 10.1002/ar.20528. [DOI] [PubMed] [Google Scholar]
- Orliac MJ, Benoit J, O'Leary MA. The inner ear of Diacodexis, the oldest artiodactyl mammal. J Anat. 2012;221:417–426. doi: 10.1111/j.1469-7580.2012.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks SE, Ketten DR, O'Malley JT, et al. Anatomical predictions of hearing in the North Atlantic right whale. Anat Rec. 2007;290:734–744. doi: 10.1002/ar.20527. [DOI] [PubMed] [Google Scholar]
- Payne KB, Langbauer WR, Jr, Thomas EM. Infrasonic calls of the Asian elephant (Elephas maximus. Behav Ecol Sociobiol. 1986;18:297–301. [Google Scholar]
- Poole JH, Payne K, Langbauer WR, Jr, et al. The social contexts of some very low frequency calls of African elephants. Behav Ecol Sociobiol. 1988;22:385–392. [Google Scholar]
- Popov VV, Supin AY, Pletenko MG, et al. Audiogram variability in normal bottlenose dolphins (Tursiops truncatus. Aquat Mamm. 2007;33:24–33. [Google Scholar]
- Pye A. The structure of the cochlea in some mammals. J Zool. 1979;187:39–53. [Google Scholar]
- Ramprashad F, Landolt JP, Money KE, et al. A morphometric study of the cochlea of the little brown bat (Myotis lucifugus. J Morphol. 1979;160:345–358. doi: 10.1002/jmor.1051600306. [DOI] [PubMed] [Google Scholar]
- Rice DW, Wolman AA. The life history and ecology of the gray whale (Eschrichtius robustus. Am Soc Mammal, Spec Publ. 1971;3:1–142. [Google Scholar]
- Ridgway SH, Bullock TH, Carder DA, et al. Auditory brainstem response in dolphins. Proc Natl Acad Sci U S A. 1981;78:1943–1947. doi: 10.1073/pnas.78.3.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth F. Mesocetus argillarius sp. n. (Cetacea, Mysticeti) from Upper Miocene of Denmark, with remarks on the lower jaw and the echolocation system in whale phylogeny. Zool Scr. 1978;7:63–79. [Google Scholar]
- Ruf I, Luo Z-X, Wible JR, et al. Petrosal anatomy and inner ear structures of the Late Jurassic Henkelotherium (Mammalia, Cladotheria, Dryolestoidea): insight into the early evolution of the ear region in cladotherian mammals. J Anat. 2009;214:679–693. doi: 10.1111/j.1469-7580.2009.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Schmelzle T. Anatomy and development of the bony inner ear in the woolly opossum, Caluromys philander (Didelphimorphia, Marsupialia) Mastozool Neotrop. 2007;15:53–60. [Google Scholar]
- Schuknecht HF. Techniques for study of cochlear function and pathology in experimental animals. Arch Otolaryngol. 1953;58:377–397. doi: 10.1001/archotol.1953.00710040399001. [DOI] [PubMed] [Google Scholar]
- Silcox MT, Bloch JI, Boyer DM, et al. Semicircular canal system in early primates. J Hum Evol. 2009;56:315–327. doi: 10.1016/j.jhevol.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Silva M, Downing JA. CRC Handbook of Mammalian Body Masses. Boca Raton: CRC Press; 1995. [Google Scholar]
- Spoor F, Bajpai S, Hussain ST, et al. Vestibular evidence for the evolution of aquatic behaviour in ear cetaceans. Nature. 2002;417:163–166. doi: 10.1038/417163a. [DOI] [PubMed] [Google Scholar]
- Spoor F, Garland T, Jr, Krovitz G, et al. The primate semicircular canal system and locomotion. Proc Natl Acad Sci U S A. 2007;104:10808–10812. doi: 10.1073/pnas.0704250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeman ME. A new baleen whale from the Late Miocene of Denmark and early mysticete hearing. Palaeontology. 2009;52:1169–1190. [Google Scholar]
- Stimpert AK, Wiley DN, Au WWL, et al. ‘Megapclicks’: acoustic click trains and buzzes produced during night-time foraging of humpback whales (Megaptera novaeangliae. Biol Letters. 2007;3:467–470. doi: 10.1098/rsbl.2007.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewissen JGM, Madar SI, Hussain ST. Ambulocetus natans, an Eocene cetacean (Mammalia) from Pakistan. Cour Forschungsinst Senckenb. 1996;191:1–86. [Google Scholar]
- Uhen MD. Form, function, and anatomy of Dorudon atrox (Mammalia, Cetacea): an archaeocete from the Middle to Late Eocene of Egypt. Univ Mich Pap Paleontol. 2004;34:1–222. [Google Scholar]
- Visualization Sciences Group – an FEI Company. Avizo: 3D Analysis Software for Scientific and Industrial Data, Standard Edition 8.0.0. Berlin: Konrad-Zuse-Zentrum für Informationstechnik; 2013. [Google Scholar]
- Wartzok D, Ketten DR. Marine mammal sensory systems. In: Reynolds J, Rommel S, editors. Biology of Marine Mammals. Washington, DC: Smithsonian Institution Press; 1999. pp. 117–175. [Google Scholar]
- West CD. The relationship of the spiral turns of the cochlea and length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J Acoust Soc Am. 1985;77:1091–1101. doi: 10.1121/1.392227. [DOI] [PubMed] [Google Scholar]
- Wever EG, McCormick JG, Palin J, et al. The cochlea of the dolphin, Tursiops truncatus: general morphology. Proc Natl Acad Sci U S A. 1971a;68:2381–2385. doi: 10.1073/pnas.68.10.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever EG, McCormick JG, Palin J, et al. Cochlea of the dolphin, Tursiops truncatus: the basilar membrane. Proc Natl Acad Sci U S A. 1971b;68:2708–2711. doi: 10.1073/pnas.68.11.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Yoshizaki F. Osseous labyrinth of Cetacea. Sci Rep Whales Res Inst. 1959;14:291–304. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Parameters used during novel CT scanning of cetaceans considered here. Daggers (†) indicate extinct taxa. Parameter abbreviations: Interslice – interslice spacing in μm; Interpixel – interpixel spacing in μm, calculated as field of reconstruction/image resolution; Reconstruction – field of reconstruction in mm; Resolution – image resolution of individual CT slices in pixels; Spec No – specimen numbers. Institutional abbreviations: HSU VM – Humboldt State University, Arcata, CA; LACM – Natural History Museum Los Angeles County, Los Angeles, CA; SDNHM/SDSNH – San Diego Natural History Museum, San Diego, CA; USNM – United States National Museum of Natural History, Washington, DC.
Table S2. Dimensions and shape ratios of the bony labyrinths of cetaceans. Data from the bony labyrinths of Balaenopteridae (NC) (from Pliocene of North Carolina) and Tursiops truncatus were taken from Ekdale (2013). Daggers (†) indicate extinct taxa. #T – number of turns completed by cochlea; %Cv – volumetric contribution (%) of cochlea to entire bony labyrinth, calculated as Cv/(Cv + Vv); 2L/C – extension (%) of secondary bony lamina within cochlear canal, calculated as 2Ll/Cl; 2Ll – spiral length of secondary bony lamina (mm); Ap – axial pitch of cochlea, calculated as Ch/#T (mm); BLl – total length of bony labyrinth (mm); Br – basal (aspect) ratio of cochlear spiral, calculated as Ch/Cw; Ch – height of cochlear spiral (mm); Cl – spiral length of cochlear canal (mm); Cra – radius of apical turn of cochlea (mm); Crb – radius of basal turn of cochlea (mm); Cs – cochlear slope, calculated as Ch/Cl/#T; CT – product of length of cochlear canal and number of turns, calculated as Cl x #T; Cv – volume of cochlear canal (mm3); Cw – width of basal turn of cochlea (mm); LF – estimated low frequency limit (Hz) based on graded curvature (following Manoussaki et al. 2008); Vv – volume of vestibular system including ampullae and semicircular canals (mm3); ρ – graded curvature of cochlea, calculated as Crb/Cra).
Table S3. Dimensions of the internal structures of the cochlear canal at each quarter of every turn. First measurement location (0/4) is immediately behind the center of the fenestra cochleae. Values expressed in mm. Dashes (–) indicate that the structure is not present, and ‘NP’ indicates that the structure is not preserved. Bony labyrinths of Balaenopteridae (NC) (from Pliocene of North Carolina) and Tursiops truncatus are described further by Ekdale (2013). Daggers (†) indicate extinct taxa.
Table S4. Dimensions and orientations of anterior (A), lateral (L), and posterior semicircular canals (P). Canal arc radii and lengths expressed in mm, and 90var refers to average deviation of canal pair angles (90A-L, 90A-P, 90L-P) and expressed in degrees. Data from the bony labyrinths of Balaenopteridae (NC) (from Pliocene of North Carolina) and Tursiops truncatus were taken from Ekdale (2013). Daggers (†) indicate extinct taxa.