Abstract
The aim of this research was to examine the influence of β-hydroxy-β-methylbutyrate (HMB) on changes in the profile of muscle fibers, whether these alterations were similar between the elevator and depressor muscles of the jaw, and whether the effects would be similar in male and female animals. Fifty-eight rats aged 60 days (29 animals of each gender) were divided into four groups: the initial control group (ICG) was sacrificed at the beginning of the experiment; the placebo control group (PCG) received saline and was fed ad libitum; the experimental group (EG) received 0.3 g kg−1 of HMB daily for 4 weeks by gavage as well as the same amount of food consumed by the PCG in the previous day; and the experimental ad libitum group (EAG) received the same dose of the supplement along with food ad libitum. Samples included the digastric and masseter muscles for the histoenzymological analysis. Data were subjected to statistical analysis with a significance level of P < 0.05. Use of HMB caused a decrease in the percentage of fast twitch glycolytic (FG) fibers and an increase in fast twitch oxidative glycolytic (FOG) fibers in males in both experimental groups (EG and EAG). However, it produced no increase in the muscle fiber area, in either gender, in the masseter muscle. In the digastric muscle, the HMB did not change the frequency or the area of any muscle fiber types in either gender. Our data suggest that the use of HMB caused small changes in the enzymological profile of fibers of the mastication muscles; the changes were different in the elevator and depressor muscles of the jaw and the results were different depending on gender.
Keywords: digastric muscle, masseter muscle, muscle fiber type, β-hydroxy-β-methylbutyrate
Introduction
The use of dietary supplements can bring major contributions to a person's health, but only one type of supplement is recommended for each purpose. Thus, knowing the effect of the supplement used and the possible changes and improvements is essential to achieve the desired goal.
The popularity of the use of nutritional supplements is increasing due to the rising demand for better results in sports activities, such as muscle mass gains and improved performance in physical activities (Castillo & Comstock, 2007; Zadik et al. 2009). However, only 14% of users consult a health professional before using these supplements (Chlopicka et al. 2007).
Recently, β-hydroxy-β-methylbutyrate (HMB) has become one of the most popular nutritional supplements (Chlopicka et al. 2007). There are more than 200 supplements that promise such effects, but only supplemental creatine and HMB produce them (Nissen & Sharp, 2003). The International Olympic Committee has accepted the use of HMB as legal (Alvares & Meirelles, 2008).
HMB has an anabolic protein that allows it to be used in the treatment of diseases that lead to weight loss, such as cancer (May et al. 2002; Smith et al. 2005; Aversa et al. 2011), acquired immunodeficiency syndrome (AIDS) (Clark et al. 2000), surgery, and chronic diseases in the elderly (Fitschen et al. 2013). It is used to increase both lean muscle mass and the mass and/or muscle strength in resistance exercise (Panton et al. 2000; Jówko et al. 2001; Nissen & Sharp, 2003; Rowlands & Thomson, 2009).
However, the use of supplements and anabolic steroids can have adverse consequences. When it comes to the muscles of mastication, the changes in muscle fibers can cause damage and myofascial dysfunction. Excessive contraction of the masseter can cause teeth clenching and bruxism, and excessive force can damage the facial structures. Also, an imbalance of these structures may cause TMJ arthralgia (Hansdottir & Bakke, 2004; Hugger et al. 2007, 2013).
None of these approaches addresses the issue of whether HMB influences muscles not subjected to physical exercise. Very little is known about the effect of HMB on the morphophysiology of mastigatory muscle fibers, for example, about the profile and the area of the different muscle fiber types.
We aimed to analyze the influence of HMB on changes in the profile of the muscle fibers; whether these alterations were similar between the elevator and depressor muscles of the jaw and whether the effects would be similar in male and female animals.
Materials and methods
The experimental procedures used in this study were approved by the local ethics committee on animal experimentation of the Ethics Committee on Animal Research of the University of São Paulo, Bauru School of Dentistry, Brazil (CEEPA-Proc No. 009/2011).
Animals and groups
Fifty-eight Wistar rats (29 females and 29 males), aged 60 days, were used. The animals were kept in individual boxes with individual feeders and water as well as with a controlled temperature and 12-h light-dark periods.
Initially, the animals were weighed and distributed in such a way that animals of similar weights were grouped together. Then, the four groups were randomly identified, and each one was divided into a male and a female group.
In the initial control group (ICG) the animals were sacrificed at the beginning of the experiment; in the placebo control group (PCG), the animals received saline under the same conditions the experimental groups did; in the experimental group (EG), the animals received HMB supplements and controlled feeding; and in the experimental ad libitum group (EAG), the animals received HMB supplements and had free access to a commercial diet.
The PCG and EAG groups received water and food ad libitum. The feed given to the PCG was accurately weighed (30 g) for subsequent calculation of daily consumption. The EG animals received the same average amount of food as the animals of the PCG consumed the previous day.
HMB supplementation
Supplementation of HMB was carried out in the experimental groups with a dose of 0.3 g kg−1 of bodyweight per day of HMB supplementation diluted in saline in a volume of 1 mL by gavage for 4 weeks. PCG animals received the same volume of saline in the same experimental conditions (Holecek et al. 2009; Gerlinger-Romero et al. 2011).
Histological analysis
The IG animals were sacrificed at the beginning of the experiment and the other groups were sacrificed after 4 weeks of treatment with the supplement/placebo.
The animals were sacrificed by overdose of anaesthetic via intramuscular injection of xylazine hydrochloride with ketamine hydrochloride.
Samples included masseter muscle (superficial part) and digastric muscle (anterior belly), which were wrapped in neutral talc and then soaked in a freezing medium for Tissue-Tek (OCT, Sakura Finetek, Torrance, CA, USA) and immediately immersed in liquid nitrogen until effervescence ceased (Werneck, 1981). After freezing, the samples were stored in a freezer (Indrel, IULT 2430, Londrina, Paraná, Brazil) at −80 °C for subsequent histological processing.
Histological sections obtained from the muscle samples were stained with hematoxylin-eosin (HE) used for checking the quality of freezing) and m-ATPase (adenosine triphosphate myofibrillar) reactions at pH 4.35 and 10.3, as well as NADH-TR (nicotinamide adenine tetrazolium reductase).
With the aid of the light microscope Olympus BX 50, 220 identical fibers from each animal were classified as FG (fast twitch glycolytic), FOG (fast twitch oxidative glycolytic) and SO (slow twitch oxidative), according to Peter et al. (1972). The same areas were then calculated with the aid of the image analysis program image-pro plus version 6.2.
Statistical analysis
For a comparison of area and frequency of fibers types between groups and genders, data were submitted to two-way analysis of variance (anova) with post-hoc test (Tukey) when the first showed a statistically significant difference between groups. For all analyses, values were considered statistically significant for P < 0.05.
Results
For a data analysis of the area and for an analysis of the frequency of muscle fiber types, the following observations were made: (i) normal muscle development in the animals during the experiment determined by comparing the ICG and PCG control groups; (ii) effect of HMB when comparing groups PCG and EG; and (iii) comparison between genders.
Although it was not the objective of this study, two factors were found to control the experiment: (i) the comparison of the chewing cycle EG vs. EAG and (ii) the combination of the chewing cycle over the use of HMB compared with PCG vs. EAG.
Masseter muscle
The results of the reactions do not show the presence of type SO fibers in the superficial portion of the masseter muscle in rats.
In relation to the frequency of different fiber types, use of HMB caused a decrease in the percentage of FG fibers and an increase in FOG type fibers when compared with the PCG between EG and EAG in males (Table 1). In females, only the GEA had decreased fiber FG and increased FOG fibers in relation to GCP.
Table 1.
Comparison of the percentage of muscle fiber types between the groups in the masseter muscle of rats
| Male |
Female |
|||
|---|---|---|---|---|
| Groups | %FG | %FOG | %FG | %FOG |
| ICG | 30.72 ± 5.54a | 69.28 ± 5.54a | 27.3 ± 4.18a | 72.69 ± 4.18a |
| PCG | 34.83 ± 5.51ab | 65.17 ± 5.51ab | 31.07 ± 4.56ab | 68.93 ± 4.56ab |
| EG | 24.55 ± 4.64a | 75.45 ± 4.64a | 26.36 ± 2.66abc | 73.64 ± 2.66abc |
| EAG | 23.52 ± 2.75a | 76.48 ± 2.75a | 23.64 ± 2.37ac | 76.36 ± 2.37ac |
EAG, experimental ad libitum group; EG, experimental group; ICG, initial control group; PCG, placebo control group.
Values are means ± SD. Mean values in a column with different superscript letters differ significantly [P < 0.05, by analysis of variance (anova), followed by Tukey test].
HMB produced no increase in the area of the fibers in either gender (Table 2).
Table 2.
Comparison between the areas (μm2) of muscle fibers between the groups in the masseter muscle of rats
| Male |
Female |
|||
|---|---|---|---|---|
| Groups | FG | FOG | FG | FOG |
| ICG | 1121.44 ± 215.17a | 908.11 ± 118.78a | 1053.05 ± 124.04a | 825.52 ± 118.66a |
| PCG | 1643.17 ± 185.76b | 1186.23 ± 130.63b | 1487.68 ± 332.77b | 1153.77 ± 227.38b |
| EG | 1625.06 ± 346.59b | 1212.52 ± 206.70b | 1606.35 ± 191.80b | 1225.46 ± 162.15b |
| EAG | 1898.55 ± 305.99b | 1346.47 ± 177.10b | 1528.66 ± 254.77b | 1148.23 ± 193.59b |
EAG, experimental ad libitum group; EG, experimental group; ICG, initial control group; PCG, placebo control group.
Values are means ± SD. Mean values in a column with different superscript letters differ significantly [P < 0.05, by analysis of variance (anova), followed by Tukey test].
Combination of the chewing cycle with HMB did not cause a change in the data of this study.
Digastric muscle
During the development of the muscle, a change occurred in the frequency of the FOG fiber type (Table 3). The HMB did not alter the frequency of the fibers and the results were similar for males and females. Figure 1 shows the presence of the three different types of fibers found in the digastric muscle.
Table 3.
Comparison of the percentage of muscle fiber types between the groups in the digastric muscle of rats
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Groups | %FG | %FOG | %SO | %FG | %FOG | %SO |
| ICG | 21.27 ± 3.44a | 70.91 ± 2.87a | 7.82 ± 4.58a | 21.36 ± 9.42a | 72.16 ± 4.40a | 6.48 ± 5.15a |
| PCG | 19.37 ± 2.17a | 76.65 ± 3.98b | 3.98 ± 2.77ab | 17.12 ± 3.08a | 80.68 ± 1.99b | 2.20 ± 1.87ab |
| EG | 23.25 ± 3.47a | 76.30 ± 3.27ab | 0.45 ± 0.79b | 21.34 ± 4.88a | 76.16 ± 3.02ab | 2.50 ± 2.95ab |
| EAG | 20.71 ± 4.28a | 77.47 ± 3.74b | 1.82 ± 2.65b | 20.74 ± 3.40a | 77.78 ± 4.29ab | 1.48 ± 1.73b |
EAG, experimental ad libitum group; EG, experimental group; ICG, initial control group; PCG, placebo control group.
Values are means ± SD. Mean values in a column with different superscript letters differ significantly [P < 0.05, by analysis of variance (anova), followed by Tukey test].
Fig. 1.
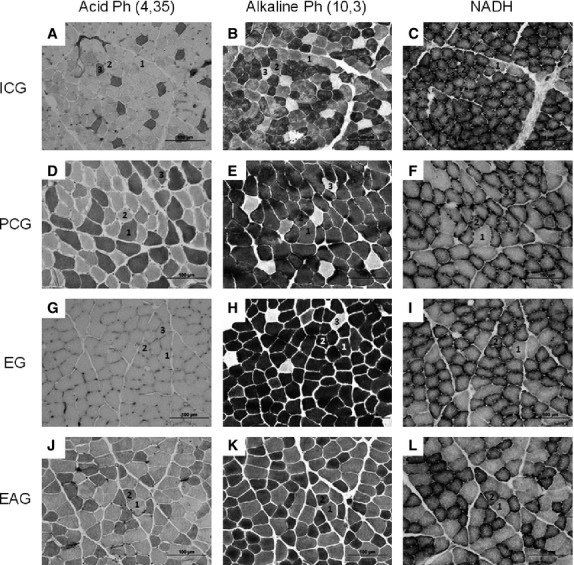
Photomicrograph of digastric muscle in male rats subjected to reactions to mATPase (acid and alkaline) and NADH. (A–C) ICG. (D–F) PCG. (G–I) EG. (J–L) EAG. In, 1 = FG fiber type, 2 = FOG fiber type, and 3 = SO fiber type.
Also during development of the digastric muscle, there was a significant increase in the area of fast-twitch fibers (FG and FOG) in males; in females only FOG fibers showed a significantly increased area (Table 4). Use of HMB did not alter the area of any fiber type. Comparing the experimental groups (EG and EAG), the use of HMB decreased the area of fast fibers of the EAG in males.
Table 4.
Comparison between the areas (μm2) of muscle fibers between the groups in the digastric muscle of rats
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Groups | FG | FOG | SO | FG | FOG | SO |
| ICG | 1123.92 ± 191.82a | 803.30 ± 108.59a | 660.47 ± 382.67a | 1046.26 ± 208.50ª | 791.93 ± 203.43ª | 515.87 ± 357.29ª |
| PCG | 1656.12 ± 229.27b | 1076.93 ± 110.03b | 992.03 ± 121.76a | 1613.00 ± 259.55a | 1093.13 ± 140.01b | 761.77 ± 387.38ª |
| EG | 1749.52 ± 201.98b | 1148.41 ± 129.16b | 400.85 ± 631.14a | 1567.66 ± 473.59a | 1059.93 ± 189.19b | 900.02 ± 179.59ª |
| EAG | 1402.08 ± 130.01bc | 977.44 ± 92.50bc | 961.11 ± 172.25a | 1346.02 ± 194.80a | 915.54 ± 90.22ab | 730.99 ± 151.85ª |
EAG, experimental ad libitum group; EG, experimental group; ICG, initial control group; PCG, placebo control group.
Values are means ± SD. Mean values in a column with different superscript letters differ significantly [P < 0.05, by analysis of variance (anova), followed by Tukey test].
Data on this muscle noted that if the combination of chewing cycle (amount of ingested food) with use of HMB is not controlled it may interfere on fiber area.
Discussion
The initial weighing of the animals for the subsequent group draw was used because it is known that there may be a direct relationship between the weight of the animals and the area of different types of muscle fibers (Maxwell et al. 1979). Initially weighing the animals and distributing them so that the groups stayed equivalent, avoided a situation in which a group could have been formed by animals that would supposedly show larger muscle fibers at the beginning of the experiment, and could cast doubt on the results obtained at the end of research.
In the muscles of mastication that were studied, it was necessary to carefully control the amount of feed intake, because animals that eat solid feed chew more and thereby exercise the muscles that perform this task more, especially the mandibular elevators. Therefore the experimental group (EG) received the same amount of feed that the placebo control group (PCG) had the previous day.
It has been shown that a combination of HMB treatment and exercise alters the biology of striated muscles (Panton et al. 2000; Jówko et al. 2001; Zanchi et al. 2011; Fitschen et al. 2013); in this study, therefore, a group received HMB and food ‘ad libitum’ in order to verify whether such an effect could also occur in the muscles of mastication.
Due to hormonal interference, females are often not used in studies (Silva & Cecanho, 2009), but this did not prevent researchers from analysing the effect of HMB on sarcopenia, especially in older rats. There is a higher disease incidence according to both gender and age in humans (Kim et al. 2012). Flakoll et al. (2004) also studied the effect of HMB associated with arginine and lysine on muscle mass, function, and protein metabolism in elderly women. Another aspect that led to the inclusion of females in this study was that the number of women attending gyms and using dietary supplements is increasing.
Studies have been done on the effect of HMB muscle growth in animals (Moore et al. 2005), muscles of humans affected by various diseases (Werneck, 1981; Aversa et al. 2011), in the young (Rowlands & Thomson, 2009; Portal et al. 2011) and elderly humans (Hsieh et al. 2010; Fitschen et al. 2013) who had not previously done physical activities but started doing so them together with the use of this substance. Nevertheless, the literature contains few data on the effect of HMB in muscles of young animals that have a normal life without exercising, as did this study. It must be remembered that Kornasio et al. (2009), working with cell cultures, observed a positive effect of the use of HMB on muscle tissues, where practising physical activity could not have interfered.
Skeletal muscles have a heterogeneous composition of fiber types with different physiological properties (Kawai et al. 2010), which are not immutable. Muscle fibers have the ability to adapt according to functional demands, and can switch from one fiber type to another (Roy et al. 1991; Pette, 2002). Furthermore, the composition of the particular type of muscle fiber is related to its daily routine use (Langenbach et al. 2008).
Muscles of mastication are often studied, as an imbalance between these muscles and temporomandibular disorders leads to myofascial dysfunction. This study shows that the HMB supplementation did not cause fiber hypertrophy of the masseter or the digastric muscles.
Both the masseter muscle and the anterior belly of the digastric muscle used in this research show heterogeneity in the fiber type. This heterogeneity reflects the function of muscles and demonstrates the complex role of both during mastication (Sano et al. 2007).
On the masticatory muscles, large changes in the fiber type composition can be observed mainly during the postnatal development (Bredman et al. 1992; Anapol & Herring, 2000; Korfage et al. 2006), as observed in this study.
Considering the different types of fibers present on the surface region of the masseter muscle, only the presence of fast-twitch FG and FOG fibers was reported in this study in both genders, coinciding with the findings of other researchers (Suzuki, 1977; Rokx et al. 1984; Roy et al. 1991; Andreo et al. 2005; Kawai et al. 2007).
The area of the FG fiber types was larger than that of the FOG types on the masseter muscle of all groups in both genders in this study, corroborating previous research (Kowalewski & Miltzow, 1990; Andreo et al. 2005).
Data from this study showed a trend for the area of fiber types FG and FOG to be higher in masseter muscle, in the experimental group that received food ad libitum than in other groups. Some questions remain:
Does 2the experiment need be performed for a longer period? Would this tendency then become statistically significantly different? Many researchers have noted the need to study the effect of HMB for longer periods of use (Portal et al. 2010; Pimentel et al. 2011).
This tendency can be explained by a greater feed intake and a larger amount of amino acid. Why? Scientific studies show that the increasing availability of amino acids resulted in increased rates of muscle protein synthesis in both elderly and young (Welle et al. 1994; Volpi et al. 1998, 1999).
Concerning the frequency of different types of masseter muscle fibers found in this study, the amount of FOG fibers was always higher than the amount of FG fibers in both genders; however, these data do not agree with results in previous studies (Kowalewski & Miltzow, 1990; Andreo et al. 2005).
In this study, when comparing the masseter muscle between the initial group and the placebo group, changes were not observed in the phenotypic profile of the fibers. However, in males, with HMB supplementation, there was a change from fiber type FG to FOG, suggesting that there HMB might influence modulation of these fiber types, decreasing fiber type FG and increasing type FOG. Similar results were found by Pinheiro et al. (2012) when studying the gastrocnemius muscle; they found that metabolic changes associated with increased strength and prevention of muscle fatigue are typical characteristics of the FOG fiber type. In females, only the group that ate ad libitum, i.e. who did more chewing cycles, presented these changes in fiber types.
There is little information in the literature concerning the enzymology of fibers of the anterior belly of the digastric muscle, making it difficult to discuss the results of this study.
It was observed in animals of both genders that the digastric muscle consists of fast-twitch and slow-twitch (FG, FOG and SO) fibers, corroborating data from previous work (Bennet et al. 1977; Kiliaridis & Shyu, 1988; Andreo et al. 2004).
In this study, it was observed that there were larger areas of the fast-twitch fibers than of the slow-twitch ones, and between fast-twitch fibers FG fibers were larger than FOG fibers in both genders.
Observing the area of fast-twitch fibers (FG and FOG) in the digastric muscle in both genders, it is noted that they behaved differently from fibers in the masseter muscle, showing a decrease in animals that ate ad libitum (EAG group). The reason for this may be that these types of fibers are best suited for force production. In other words, the anterior belly of the digastric muscle would be less in use because it is a depressor of the mandible, whereas the masseter muscle would be used because it raises the jaw.
Regarding the percentage of the different types of fibers found in the digastric muscle of animals in this research, it was noted that the fast-twitch fibers were predominant among them, and the type FOG was found most frequently.
During normal development of the animals, in this study the digastric muscle showed a change in the composition of the fibers, with an increase of FOG fiber, and a tendency of a decrease in slow fiber types. Note that the change in the types of fibers occurred between SO and FOG fibers but that the fiber content of the FG remained the same. This was observed in both genders.
In the other groups, there were no differences regarding the frequencies of the different types of fibers. These observations suggest that during the supplementation period, HMB did not affect the modulation of the types of fibers of the digastric muscle. This was observed in animals of both genders.
It should be noted that in the groups treated with HMB and that had controlled alimentation, there was a tendency of increasing fiber area in males. In future research, increasing the duration of the experiment and increasing the daily dose could confirm this increase in the fiber area.
Conclusion
Based on the data of this study, it can be concluded that the daily use of 0.3 g kg−1 of HMB for a period of 4 weeks, caused small changes in the enzymological profile of fibers of the muscles of mastication, with differences between the elevator and depressor muscles of the jaw, and between genders.
Acknowledgments
Acknowledgments to Capes for the material support.
References
- Alvares TS, Meirelles CM. Effects of beta-hydroxy-beta-methylbutyrate supplementation on strength and hypertrophy: [review] Rev Nutr. 2008;21:49–61. [Google Scholar]
- Anapol F, Herring SW. Ontogeny of histochemical fiber types and muscle function in the masseter muscle of miniature swine. Am J Phys Anthropol. 2000;112:595–613. doi: 10.1002/1096-8644(200008)112:4<595::AID-AJPA11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Andreo JC, dos Santos NB, Moraes LHR, et al. Effect of ethanol in the Digastric muscle in rats (Rattus Norvegicus): an histoenzymologic evaluation. Salusvita. 2004;23:371–386. [Google Scholar]
- Andreo JC, Santos NB, Moraes LHR, et al. Is there morphological difference between branchiomeric and somitic muscles submitted to the alccohol consumption? An experimental study in rats (Rattus norvegicus. J Appl Oral Sci. 2005;13:296–304. doi: 10.1590/s1678-77572005000300018. [DOI] [PubMed] [Google Scholar]
- Aversa Z, Bonetto A, Costelli P, et al. β-Hydroxy-β-methylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int J Oncol. 2011;38:713. doi: 10.3892/ijo.2010.885. [DOI] [PubMed] [Google Scholar]
- Bennet JH, Jeffery PK, Weber WV. The histochemistry of the jaw muscles of the mature and maturing rat (proceedings) J Physiol. 1977;270:26–27. [PubMed] [Google Scholar]
- Bredman J, Weijs W, Korfage H, et al. Myosin heavy chain expression in rabbit masseter muscle during postnatal development. J Anat. 1992;180:263. [PMC free article] [PubMed] [Google Scholar]
- Castillo EM, Comstock RD. Prevalence of use of performance-enhancing substances among United States adolescents. Pediatr Clin North Am. 2007;54:663–675. doi: 10.1016/j.pcl.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Chlopicka J, Wandas P, Zachwieja Z. Dietary supplements selected by young people exercising in fitness rooms in Kraków and environs. Rocz Panstw Zakl Hig. 2007;58:185–189. [PubMed] [Google Scholar]
- Clark RH, Feleke G, Din M, et al. Nutritional treatment for acquired immunodeficiency virus-associated wasting using β-hydroxy β-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. J Parenter Enteral Nutr. 2000;24:133–139. doi: 10.1177/0148607100024003133. [DOI] [PubMed] [Google Scholar]
- Fitschen PJ, Wilson GJ, Wilson JM, et al. Efficacy of β-hydroxy-β-methylbutyrate supplementation in elderly and clinical populations. Nutrition. 2013;29:29–36. doi: 10.1016/j.nut.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Flakoll P, Sharp R, Baier S, et al. Effect of β-hydroxy-β-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition. 2004;20:445–451. doi: 10.1016/j.nut.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Gerlinger-Romero F, Guimarães-Ferreira L, Giannocco G, et al. Chronic supplementation of beta-hydroxy-beta methylbutyrate (HMb) increases the activity of the GH/IGF-I axis and induces hyperinsulinemia in rats. Growth Horm IGF Res. 2011;21:57–62. doi: 10.1016/j.ghir.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Hansdottir R, Bakke M. Joint tenderness, jaw opening, chewing velocity, and bite force in patients with temporomandibular joint pain and matched healthy control subjects. J Orofac Pain. 2004;18:108–113. [PubMed] [Google Scholar]
- Holecek M, Muthny T, Kovarik M, et al. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem Toxicol. 2009;47:255–259. doi: 10.1016/j.fct.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Hsieh LC, Chow CJ, Chang WC, et al. Effect of beta-hydroxy-beta-methylbutyrate on protein metabolism in bed-ridden elderly receiving tube feeding. Asia Pac J Clin Nutr. 2010;19:200–208. [PubMed] [Google Scholar]
- Hugger A, Schindler HJ, Böhner W, et al. Therapy of temporomandibular joint pain: recommendations for clinical management. Schmerz. 2007;21:116–130. doi: 10.1007/s00482-007-0532-9. [DOI] [PubMed] [Google Scholar]
- Hugger S, Schindler HJ, Kordass B, et al. Surface EMG of the masticatory muscles (Part 3): impact of changes to the dynamic occlusion. Int J Comput Dent. 2013;16:119–123. [PubMed] [Google Scholar]
- Jówko E, Ostaszewski P, Jank M, et al. Creatine and β-hydroxy-β-methylbutyrate (HMB) additively increase lean body mass and muscle strength during a weight-training program. Nutrition. 2001;17:558–566. doi: 10.1016/s0899-9007(01)00540-8. [DOI] [PubMed] [Google Scholar]
- Kawai N, Tanaka E, Langenbach GEJ, et al. Daily jaw muscle activity in freely moving rats measured with radio-telemetry. Eur J Oral Sci. 2007;115:15–20. doi: 10.1111/j.1600-0722.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- Kawai N, Sano R, Korfage JAM, et al. Adaptation of rat jaw muscle fibers in postnatal development with a different food consistency: an immunohistochemical and electromyographic study. J Anat. 2010;216:717–723. doi: 10.1111/j.1469-7580.2010.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiliaridis S, Shyu B. Isometric muscle tension generated by masseter stimulation after prolonged alteration of the consistency of the diet fed to growing rats. Arch Oral Biol. 1988;33:467–472. doi: 10.1016/0003-9969(88)90026-x. [DOI] [PubMed] [Google Scholar]
- Kim JS, Park YM, Lee SR, et al. β-Hydroxy-β-methylbutyrate did not enhance high intensity resistance training-induced improvements in myofiber dimensions and myogenic capacity in aged female rats. Mol Cells. 2012;34:439–448. doi: 10.1007/s10059-012-0196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfage J, Van Wessel T, Langenbach G, et al. Postnatal transitions in myosin heavy chain isoforms of the rabbit superficial masseter and digastric muscle. J Anat. 2006;208:743–751. doi: 10.1111/j.1469-7580.2006.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornasio R, Riederer I, Butler-Browne G, et al. β-Hydroxy-β-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta. 2009;1793:755–763. doi: 10.1016/j.bbamcr.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Kowalewski R, Miltzow M. Postnatal development of the masticatory muscles of the Wistar rat (Rattus norvegicus Berkenhout). A histochemical study. Anat Anz. 1990;170:205–211. [PubMed] [Google Scholar]
- Langenbach G, Van Wessel T, Brugman P, et al. Is fiber-type composition related to daily jaw muscle activity during postnatal development? Cells Tissues Organs. 2008;187:307–315. doi: 10.1159/000112791. [DOI] [PubMed] [Google Scholar]
- Maxwell LC, Carlson DS, McNamara JA, Jr, et al. Histochemical characteristics of the masseter and temporalis muscles of the Rhesus monkey. Anat Rec. 1979;193:389–402. doi: 10.1002/ar.1091930306. [DOI] [PubMed] [Google Scholar]
- May PE, Barber A, D'Olimpio JT, et al. Reversal of cancer-related wasting using oral supplementation with a combination of β-hydroxy-β-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183:471–479. doi: 10.1016/s0002-9610(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Moore D, Ferket P, Mozdziak P. The effect of early nutrition on satellite cell dynamics in the young turkey. Poult Sci. 2005;84:748–756. doi: 10.1093/ps/84.5.748. [DOI] [PubMed] [Google Scholar]
- Nissen SL, Sharp RL. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol. 2003;94:651–659. doi: 10.1152/japplphysiol.00755.2002. [DOI] [PubMed] [Google Scholar]
- Panton LB, Rathmacher JA, Baier S, et al. Nutritional supplementation of the leucine metabolite β-hydroxy- β-methylbutyrate (HMB) during resistance training. Nutrition. 2000;16:734–739. doi: 10.1016/s0899-9007(00)00376-2. [DOI] [PubMed] [Google Scholar]
- Peter JB, Barnard RJ, Edgerton VR, et al. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochem. 1972;11:2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pette D. The adaptive potential of skeletal muscle fibers. Can J Appl Physiol. 2002;27:423–448. doi: 10.1139/h02-023. [DOI] [PubMed] [Google Scholar]
- Pimentel G, Rosa J, Lira F, et al. β-Hydroxy-β-methylbutyrate (HMβ) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr Metab. 2011;8:11. doi: 10.1186/1743-7075-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro CH, Gerlinger-Romero F, Guimarães-Ferreira L, et al. Metabolic and functional effects of beta-hydroxy-beta-methylbutyrate (HMB) supplementation in skeletal muscle. Eur J Appl Physiol. 2012;112:2531–2537. doi: 10.1007/s00421-011-2224-5. [DOI] [PubMed] [Google Scholar]
- Portal S, Eliakim A, Nemet D, et al. Effect of HMB supplementation on body composition, fitness, hormonal profile and muscle damage indices. J Pediatr Endocrinol Metab. 2010;23:641–650. doi: 10.1515/jpem.2010.23.7.641. [DOI] [PubMed] [Google Scholar]
- Portal S, Zadik Z, Rabinowitz J, et al. The effect of HMB supplementation on body composition, fitness, hormonal and inflammatory mediators in elite adolescent volleyball players: a prospective randomized, double-blind, placebo-controlled study. Eur J Appl Physiol. 2011;111:2261–2269. doi: 10.1007/s00421-011-1855-x. [DOI] [PubMed] [Google Scholar]
- Rokx J, Van Willigen J, Jansen H. Muscle fibre types and muscle spindles in the jaw musculature of the rat. Arch Oral Biol. 1984;29:25–31. doi: 10.1016/0003-9969(84)90038-4. [DOI] [PubMed] [Google Scholar]
- Rowlands DS, Thomson JS. Effects of beta-hydroxy-beta-methylbutyrate supplementation during resistance training on strength, body composition, and muscle damage in trained and untrained young men: a meta-analysis. J Strength Cond Resh. 2009;23:836–846. doi: 10.1519/JSC.0b013e3181a00c80. [DOI] [PubMed] [Google Scholar]
- Roy RR, Baldwin KM, Edgerton VR. The plasticity of skeletal muscle: effects of neuromuscular activity. Exerc Sport Sci Rev. 1991;19:269–312. [PubMed] [Google Scholar]
- Sano R, Tanaka E, Korfage J, et al. Heterogeneity of fiber characteristics in the rat masseter and digastric muscles. J Anat. 2007;211:464–470. doi: 10.1111/j.1469-7580.2007.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva HCFP, Cecanho R. Cephalometric changes produced by locally applied anabolic steroid in Wistar rats. Arch Oral Biol. 2009;54:389–395. doi: 10.1016/j.archoralbio.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Smith HJ, Mukerji P, Tisdale MJ. Attenuation of proteasome-induced proteolysis in skeletal muscle by β-hydroxy-β-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005;65:277–283. [PubMed] [Google Scholar]
- Suzuki A. A comparative histochemical study of the masseter muscle of the cattle, sheep, swine, dog, guinea pig and rat. Histochemistry. 1977;51:121–131. doi: 10.1007/BF00567218. [DOI] [PubMed] [Google Scholar]
- Volpi E, Ferrando AA, Yeckel CW, et al. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Wolf SE, et al. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab. 1999;277:513–520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M, et al. Postprandial myofibrillar and whole body protein synthesis in young and old human subjects. Am J Physiol Endocrinol Metab. 1994;267:599–604. doi: 10.1152/ajpendo.1994.267.4.E599. [DOI] [PubMed] [Google Scholar]
- Werneck L. O valor da biópsia muscular em neurologia: análise de 290 exames a fresco e pela histoquímica. Rev Bras Clin Terap. 1981;10:2–22. [Google Scholar]
- Zadik Z, Nemet D, Eliakim A. Hormonal and metabolic effects of nutrition in athletes. J Pediatr Endocrinol Metab. 2009;22:769–778. doi: 10.1515/jpem.2009.22.9.769. [DOI] [PubMed] [Google Scholar]
- Zanchi NE, Gerlinger-Romero F, Guimarães-Ferreira L, et al. HMB supplementation: clinical and athletic performance-related effects and mechanisms of action. Amino Acids. 2011;40:1015–1025. doi: 10.1007/s00726-010-0678-0. [DOI] [PubMed] [Google Scholar]


