Abstract
Even if different dissection, tractographic and connectivity studies provided pure anatomical evidences about the optic radiations (ORs), descriptions of both the anatomical structure and the anatomo-functional relationships of the ORs with the adjacent bundles were not reported. We propose a detailed anatomical and functional study with ‘post mortem’ dissections and ‘in vivo’ direct electrical stimulation (DES) of the OR, demonstrating also the relationships with the adjacent eloquent bundles in a neurosurgical ‘connectomic’ perspective. Six human hemispheres (three left, three right) were dissected after a modified Klingler's preparation. The anatomy of the white matter was analysed according to systematic and topographical surgical perspectives. The anatomical results were correlated to the functional responses collected during three resections of tumours guided by cortico-subcortical DES during awake procedures. We identified two groups of fibres forming the OR. The superior component runs along the lateral wall of the occipital horn, the trigone and the supero-medial wall of the temporal horn. The inferior component covers inferiorly the occipital horn and the trigone, the lateral wall of the temporal horn and arches antero-medially to form the Meyer's Loop. The inferior fronto-occipital fascicle (IFOF) covers completely the superior OR along its entire course, as confirmed by the subcortical DES. The inferior longitudinal fascicle runs in a postero-anterior and inferior direction, covering the superior OR posteriorly and the inferior OR anteriorly. The IFOF identification allows the preservation of the superior OR in the anterior temporal resection, avoiding post-operative complete hemianopia. The identification of the superior OR during the posterior temporal, inferior parietal and occipital resections leads to the preservation of the IFOF and of the eloquent functions it subserves. The accurate knowledge of the OR course and the relationships with the adjacent bundles is crucial to optimize quality of resection and functional outcome.
Keywords: anatomo-functional study, awake mapping, connectomic surgery, Klingler's technique, optic radiations, white matter
Introduction
The study of human white matter (WM) experienced a renewed interest over the last decade because of two main reasons. Firstly, diffusion tensor imaging (DTI) provided fascinating ‘in vivo’ reconstructions of brain fibres. Secondly, brain connectivity acquired a crucial role in the more recent model of organization of neurological functions, the so-called ‘hodotopical’ framework (Catani & Ffytche, 2005; Ffytche & Catani, 2005). According to this theory, the final functional output is the result of a complex integration of many functional sub-elaborations provided by largely distributed, multimodal and parallel processing networks subserved by long and short WM bundles, which connect different neural sub-populations. Many neuroimaging and direct electrical stimulation (DES) studies supported this hypothesis (Duffau, 2006; Vigneau et al. 2006). As a result of this modern concept of the anatomo-functional organization of the central nervous system, a mutation also in the surgical perspective has been proposed (De Benedictis & Duffau, 2011), especially concerning the approach to diffusive and infiltrating tumours. Extensive resections of high critical cortices, performed by respecting the eloquent subcortical WM without producing post-operative permanent deficits, demonstrated that the preservation of the networks’ connectivity is more important than the single cortical area sparing (De Benedictis et al. 2010; Sarubbo et al. 2011, 2012a; Duffau, 2012, 2013).
Recently, different methods have been proposed [i.e. direct electrical cortico-subcortical mapping; pre-operative and navigated DTI; intra-operative magnetic resonance (MR) and DTI sequences] to preserve the connectivity and to improve the functional outcome of neurosurgical patients with lesions harbouring eloquent subcortical regions. Moreover, considering the limitations of DTI, the interest for cadaveric dissections of human WM (particularly, for Klingler's technique) was renewed in order to confirm the DTI results, to provide new evidences in the brain anatomy and to improve the technical skills of neurosurgeons in performing safer approaches to diffusive and/or deep-sited lesions. The combination of evidences provided by DTI, anatomical dissections and intra-operative DES during functional tasks, provided encouraging results in the knowledge of the anatomical and functional brain connectivity (Sarubbo et al. 2012b).
In this context, the anatomical structure of the visual pathways was also better defined over the last decade (Sincoff et al. 2004; Rubino et al. 2005; Peltier et al. 2006; Yogarajah et al. 2009), even if the close relationships with the main associative bundles crossing and kissing these fibres were poorly reported. Moreover, the crucial functional role of this structure in daily living is not highly regarded in the approach to brain tumours, especially in case of gliomas. Visual field deficits (VFDs) after removal of low-grade (LGGs) or high-grade gliomas (HGGs) or after temporal resection for epilepsy were, in fact, frequently reported (Winston et al. 2012), even though the VFDs constitute invalidating symptoms, strongly impacting on the patients’ quality of life. Patients’ motility and daily living activities (including driving and work abilities) are significantly reduced, in fact, by homonymous hemianopia, especially considering young-age patients or longer survivals. Moreover, damage to the optic radiations (ORs) within the non-dominant hemisphere can be related also to a complex visuo-spatial perceptive syndrome (i.e. neglect).
Many anatomical, functional magnetic resonance imaging (MRI), DTI and connectivity studies demonstrated the structural substrate of visual pathways and showed also the role of modern imaging in improving the results of surgery involving the subcortical connectivity (Sincoff et al. 2004; Rubino et al. 2005; Nilsson et al. 2007; Duncan, 2010). Recently, the first series of visual monitoring with DES during awake surgery (Gras-Combe et al. 2012) has demonstrated the reliability of this technique and the benefits for the patients’ outcome. To our knowledge, there are no reports describing both the anatomical structure obtained with Klingler's dissection, the anatomo-functional relationships with the other eloquent bundles, and the results of the surgical mapping of the OR in the most critical and frequently encountered course of these fibres.
In this paper, we analysed the anatomical course of the visual pathways from the occipital lobe to the temporal stem (TS), with particular emphasis to the anatomical and functional relationships with the adjacent bundles. We also provided some evidences from DES during awake resections, in order to describe the main anatomo-functional surgical challenges for the resection of the lesions harbouring these fibres.
Materials and methods
All the parts of this study, including dissections and integration of the anatomical data with the evidences provided from the DES during awake surgery and the DTI reconstructions, were authorized from the Ethical Committee of the Azienda Sanitaria per i Servizi Sanitari (APSS) of Trento (Italy) and from the Ethical Committee of the ‘S. Anna’ University-Hospital of Ferrara (Italy).
WM dissection
Six fresh cerebral hemispheres (three left and three right) were fixed in a 10% formalin solution for 40 days and then frozen for 30 days at −20 °C. After gradual defrosting of the specimens, the arachnoids and vessels were gently removed. The dissection was performed by means of wooden spatulas, and we started from the removal of the grey matter of the sulci. The grey matter at the tip of the brain gyri was preserved according to a cortex-sparing technique (Martino et al. 2011), to obtain the correct definition of the bundles and their terminations territories. The dissection proceeded ‘layer-by-layer’ in a latero-medial direction. The specimens underwent progressive formalin re-injections and the frost–defrost process to improve also the anatomical definition of the deeper WM layers, according to the protocol specifically developed in our laboratory. The relationships among the bundles [i.e. indirect posterior and anterior indirect portions of the superior longitudinal fascicle (SLF), arcuate fasciculus (AF), inferior longitudinal fascicle (ILF), inferior fronto-occipital fascicle (IFOF), OR] were highlighted and the main stem of the more superficial bundles was preserved, over the different steps of dissection, providing a more integrated final vision of the entire fibres course.
Surgical population and procedures
In order to confirm and discuss the anatomical results of the dissections and the functional role of the eloquent bundles adjacent to the OR, we selected three cases of patients submitted to resections of lesions harbouring the visual pathways using intra-operative DES. All the patients were operated from the first author (S.S.) at the ‘S. Anna’ University-Hospital of Ferrara, and gave their informed consent to the surgery and to the use of the data for scientific purposes. Two of those (cases 1 and 2) were located in the postero-inferior portion of the OR course, in the right and left hemisphere, respectively. The third case concerns the resection of a diffusive LGG located along the anterior temporal course of the OR. This case was selected with the aim to confirm and to highlight the results of the dissections with the intra-operative stimulations of the OR at the level of the anterior temporal lobe, the anterior portion of the temporal horn and within the TS.
Case 1 was a 39-year-old right-handed man with a diffusive non-enhancing lesion, at the pre-operative MR (Fig. 1A), harbouring the middle and posterior thirds of the basal and medial surfaces of the right non-dominant temporal lobe (T5, T4 and partially T3). This lesion was strongly suggestive for a LGG. The patient suffered of progressively worsening visual seizures not controlled by combined medical treatment.
Fig. 1.
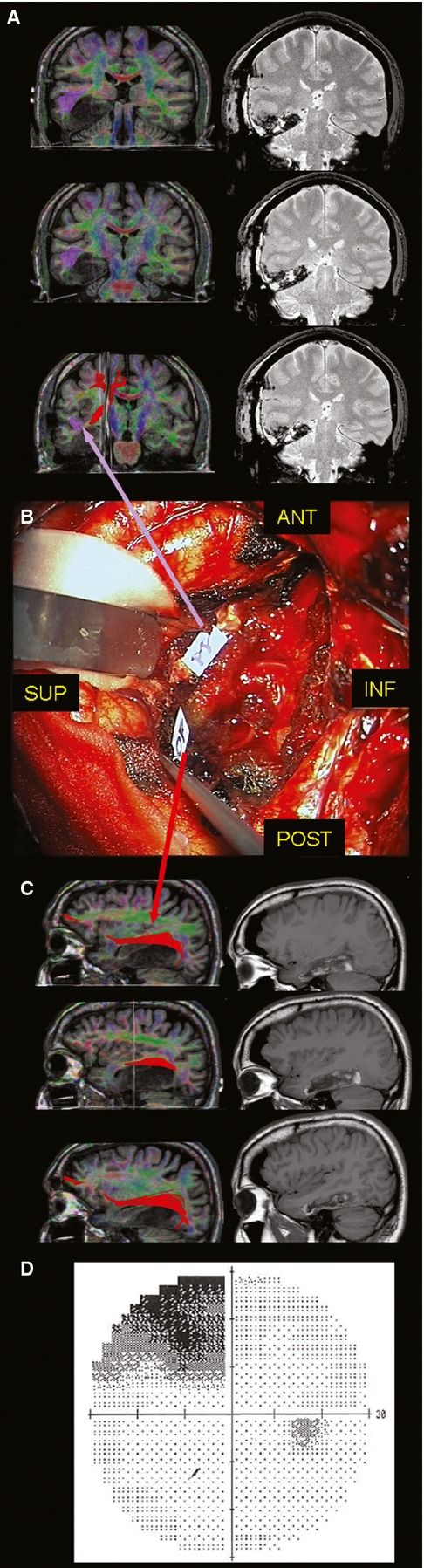
(A) On the left side, the pre-operative coronal T1-wighted MRI with integrated 60-directions DTI (superior OR in purple and IFOF in red) shows the LGG harbouring the posterior two-third of T5, T4 and partially T3. On the right side, the early post-operative coronal FLAIR sequence demonstrated the LGG resection with the opening of the floor of the trigone and temporal horn. (B) The tag 11 highlights the subcortical limit of resection at the inferior border of the trigone evoking flashes at the limit between the inferior and superior contralateral visual quadrants. Posteriorly, the tag 10 shows the point evoking also visual recognition troubles, suggesting the deactivation of the IFOF at its inferior margin, overlapping the superior OR, according to the course of this bundle in the pre-operative DTI (C). (D) The post-operative digital visual field examination showed the contralateral quadrantopia tailored with the DES-guided resection. DES, direct electrical stimulation; DTI, diffusion tensor imaging; IFOF, inferior fronto-occipital fascicle; LGG, low-grade glioma; MRI, magnetic resonance imaging; OR, optic radiation.
Case 2 was a 48-year-old right-handed man who experienced two episodes of transient expressive aphasia associated with positive visual symptoms (i.e. flashes). The pre-operative MRI showed a diffuse infiltrative lesion of the postero-inferior left dominant temporal lobe with multifocal contrast enhancement, suggesting a HGG (Fig. 2A).
Fig. 2.
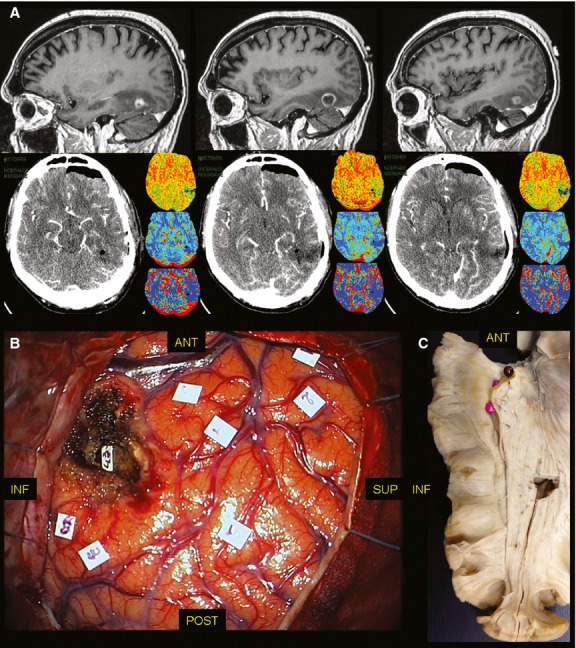
(A) On the upper line, the pre-operative sagittal T1 contrast-enhancing MRI shows a HGG with a nodular enhancing lesion surrounded by hypointense tissue. On the lower line, the post-operative perfusion CT-scan demonstrated the resection of the lesion. (B) The DES allowed to push the resection beyond the non-functional nodular enhancing tissue up to the identification of the inferior limit of the OR (tag 43), at the inferior margin of the lateral wall of the trigone, such as demonstrated in the anatomical cadaver dissection (C) with Klingler's technique. CT, computer tomography; DES, direct electrical stimulation; HGG, high-grade glioma; MRI, magnetic resonance imaging; OR, optic radiation.
Case 3 was a 38-year-old right-handed man with a diffusive lesion within the anterior and middle portions of the temporal lobe and the insula on the right non-dominant hemisphere, without contrast enhancing at the pre-operative MRI (Fig. 3A). He experienced generalized seizures partially controlled with the combined medical treatment.
Fig. 3.
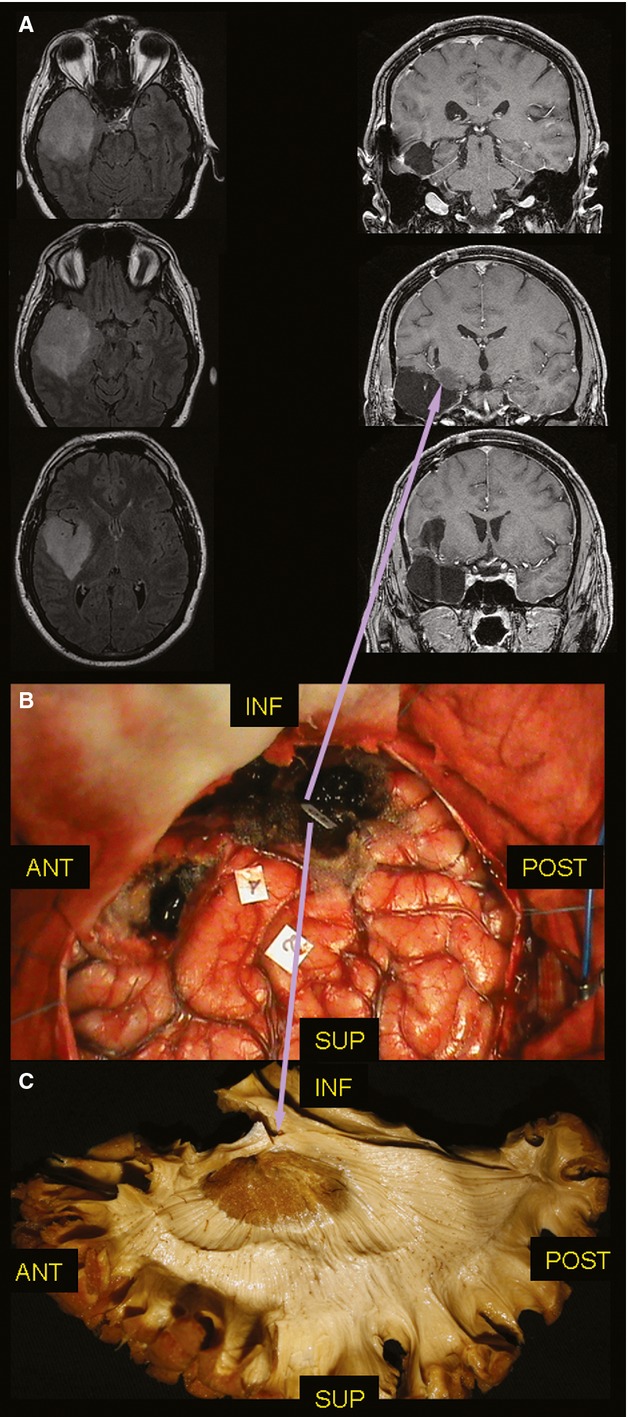
(A) On the left side, the pre-operative axial FLAIR MRI shows a diffuse lesion infiltrating the whole temporal lobe and the insula. On the right side, the post-operative coronal T1 MRI confirmed the limits of resection, at the level of the TS. (B) The DES allowed the resection of the temporal lobe and of the entire insula with a 6 cc residual tumour within the temporal stem. (C) The stimulation site of the superior OR (tag 40) matches (inferior purple arrow) with the course of these fibres at the level of the temporal stem identified with post-mortem dissection of the WM and the residual tissue at the early post-operative MRI (superior purple arrow). DES, direct electrical stimulation; MRI, magnetic resonance imaging; OR, optic radiation; TS, temporal stem; WM, white matter.
All the patients underwent to complete pre-operative neuropsychological assessment, according to the methodology previously described (Sarubbo et al. 2011, 2012a). In all the cases we performed fully awake surgery procedures in the park-bench position on the right side for patient no. 2, and on the left side for patients no. 1 and no. 3. The remifentanil and propofol injections were stopped at the dura opening, and the patients started the intra-operative task of combined monitoring of visual field, language and attentive functions. The visual task is composed of two images (from the Italian version of DO 80) in opposite quadrants separated by dotted lines (Fig. 4). The first image is sited in the quadrant we aimed to preserve (i.e. superior or inferior) of the contralateral visual field, in respect to the surgical side. The second one is sited in the opposite quadrant of the other side of the visual field (omolateral to the surgical side). The patients were asked to name both the items. This task has the double aim to preserve the quadrant not involved by the pathological MR signal, and to provide the monitoring of the denomination functions subserved by the language bundles on the dominant hemisphere and of the visual recognition functions subserved by the non-dominant hemisphere bundles. The dotted line bordering the four quadrants is used for monitoring the distribution of the positive effect of the DES of the OR (i.e. flashes, lightening), in order to define the real functional limit between the fibres of the superior and inferior quadrants on each hemisphere. The patients were asked to stare at the centre of the screen marked by the cross and the neuropsychologist monitored eye movements, in order to avoid false-negative responses secondary to ocular saccades.
Fig. 4.
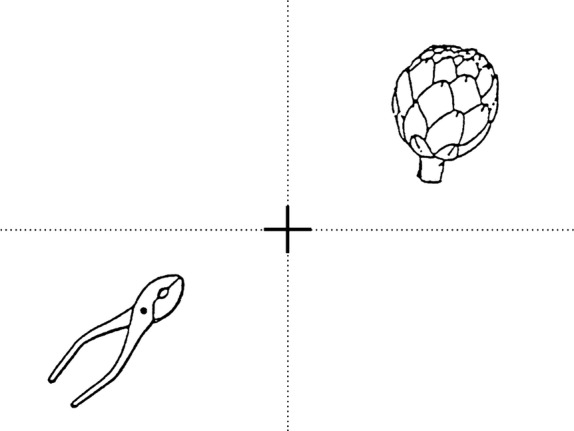
The task for visual and language monitoring is composed by the four quadrants of the visual fields divided by a dotted line. Two pictures are contemporarily shown. The former is located in the visual quadrant to be preserved and the latter in the opposite one.
A bipolar stimulator 0.7 mm spaced was used and the stimulation threshold was set after eliciting a complete speech arrest at the level of the ventral pre-motor cortex, according to previous reports (Sarubbo et al. 2011, 2012a). The continuous electrocorticography was achieved with 16-pole recording electrodes on the fronto-parietal convexity. The subpial resection with ultrasound aspirator (Dissectron, LifeSciences, Lyon, France) was alternated to subcortical DES during task execution, in order to tailor a customized resection according to the oncological and functional limits (i.e. preservation at least of one visual quadrant of the visual field).
Brain tractography
Patient no. 1 underwent a 60-direction diffusion-weighted imaging brain tractography with 1.5-T MRI scanner (GE Healthcare, UK) and an eight-channel head coil. The DTI was performed using a single-shot multislice spin echo–echo planar sequence with the following attributes: 40 slices; slice thickness: 2.6 mm; matrix 256 × 256; TR: 10 000; TE: 92.7; flip angle: 90. After bet extraction, noise reduction and eddy current correction obtained with the specific tools of the FMRIB Software Library (FSL; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/), the DTI calculation and tracking were performed with Diffusion Toolkit 0.6.1 and TrackVis 0.5.1 (http://trackvis.org), respectively. The FACT algorithm was selected for tacking, excluding all voxels with a fractional anisotropy value below 0.05 and setting the angle threshold to 35. The T1-weighted volume was co-registered to the fractional anisotropy map obtained after diffusion tensor calculation. We applied a knowledge-based multiple regions-of-interest inclusion and exclusion approach in which the tracking algorithm was initiated from user-defined seed regions, according also to direct visualization of the stem of the single bundle analysed.
Results
WM dissection
After identification and storage of the images of the occipital, temporal and inferior parietal sulci and U-fibres, we started with the removal of the mid-posterior portion of the middle temporal gyrus (MTG; Fig. 5A). Firstly, we encountered the indirect posterior portions of the SLF, terminating inferiorly at the level of the posterior thirds of the superior temporal gyrus (STG) and MTG and at the occipito-temporal junction, and superiorly at the level of the most ventral portion of the inferior parietal lobule (IPL; i.e. supramarginal and angular gyri). At the level of the IPL, we demonstrated also the terminations of the indirect anterior portion of the SLF, dorsally overlapped in respect to those of the posterior indirect component and connecting the inferior and middle frontal gyrus (respectively, IFG, MFG; Fig. 5B). After removal of the posterior indirect portion of the SLF, the fibres of the ventro-inferior portion of the ‘C’ course of the AF are demonstrated in a deeper layer (Fig. 5B,C). The inferiorly and anteriorly directed fibres of this portion of the AF completely cover the lateral wall of the ventricular trigone. After complete removal of the temporal course of the AF and cutting of the stem at the level of the fronto-parietal WM junction, the fibres of the IFOF and the ILF were demonstrated (Fig. 5C). The ILF runs inferiorly and mild-laterally with respect to the OR, connecting the extra-striate occipital cortices to the middle and anterior thirds of the temporal lobe (Fig. 5C). The course of this bundle is quite antero-inferiorly directed, with respect to the OR. In the posterior third of its course, just at the junction between the occipital, temporal and parietal lobes, it partially overlaps and crosses the OR (Fig. 5C). After this point, the two bundles have divergent courses. The OR runs parallel to the ventricle (following the lateral wall and the floor up to the temporal tip), and the ILF is antero-inferior directed to the temporo-basal and lateral cortices.
Fig. 5.
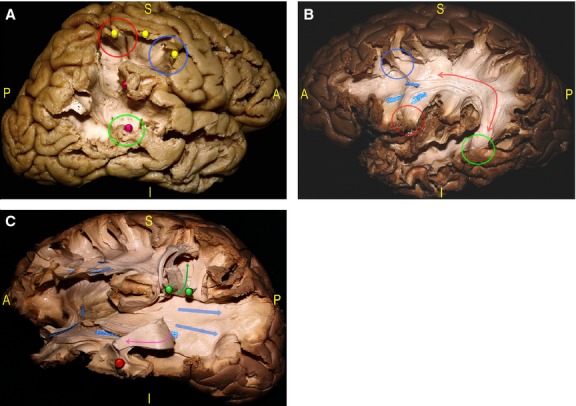
(A) The dissection started with the resection of the posterior MTG demonstrating the posterior indirect component of the SLF, connecting the Wernicke's territories (red pin, green circle) to the Geschwind territories within the inferior parietal lobule (IPL; red pin), and the anterior indirect component, connecting the Geshwind territories to the posterior IFG (yellow, pins, red and blue circles). (B) Deeper the AF (red arrow) is shown connecting the IFG (red circle) and MFG (blue circle) to the STG, MTG and ITG (green circle). (C) The AF and its termination are then cut and turned up (green arrow, green pins), and the ILF is detached and turned anteriorly (red pin, pink arrow) to show the deeper layer of the SS composed of the IFOF and superior and inferior components of the OR (blue arrows). AF, arcuate fasciculus; IFG, inferior frontal gyrus; IFOF, inferior fronto-occipital fascicle; ILF, inferior longitudinal fascicle; IPL, inferior parietal lobule; ITG, inferior temporal gyrus; MFG, middle frontal gyrus; MTG, middle temporal gyrus; OR, optic radiation; SLF, superior longitudinal fascicle; SS, stratum sagittalis.
After this step, the insular grey matter and the insulo- and claustro-opercular fibres are removed, in order to demonstrate the ventral and dorsal claustrum, the fibres of the external capsule (EC) and TS. In this way it is also possible to follow the most anterior course of the OR and IFOF within the temporal lobe (Fig. 6A,B). The IFOF is definitely the bundle most parallel and overlapping the OR. Over its entire course, in fact, from the posterior terminations (i.e. occipital striate and extra-striate cortices and parietal lobe) to the anterior compact stem at the level of the more ventral and anterior EC (Martino et al. 2010b; Sarubbo et al. 2013), the IFOF fibres have a parallel course and cover the lateral part of the OR and most of the posterior thalamic radiation (Fig. 6B). Essentially, the OR represents the inferior and ventro-basal portion of the posterior thalamic radiation and it is identified following the fibres terminating within the inferior and superior cortices of the calcarine scissure (Figs 7 and 8). The OR fibres coming from the superior and inferior calcarine cortices cover the lateral wall of the ventricular trigone and the occipital horn (at the level of the occipital and parietal WM). Immediately superficial to the OR, we found the IFOF fibres, which have, in this region, the most fanned distribution of both the occipital and parietal components and completely overlap the superior portion of the OR, coming from the superior calcarine cortex (i.e. inferior contralateral visual quadrant), and the two-thirds of the inferior portion of the OR, coming from the inferior calcarine cortex (i.e. superior contralateral visual quadrant; Fig. 7B).
Fig. 6.
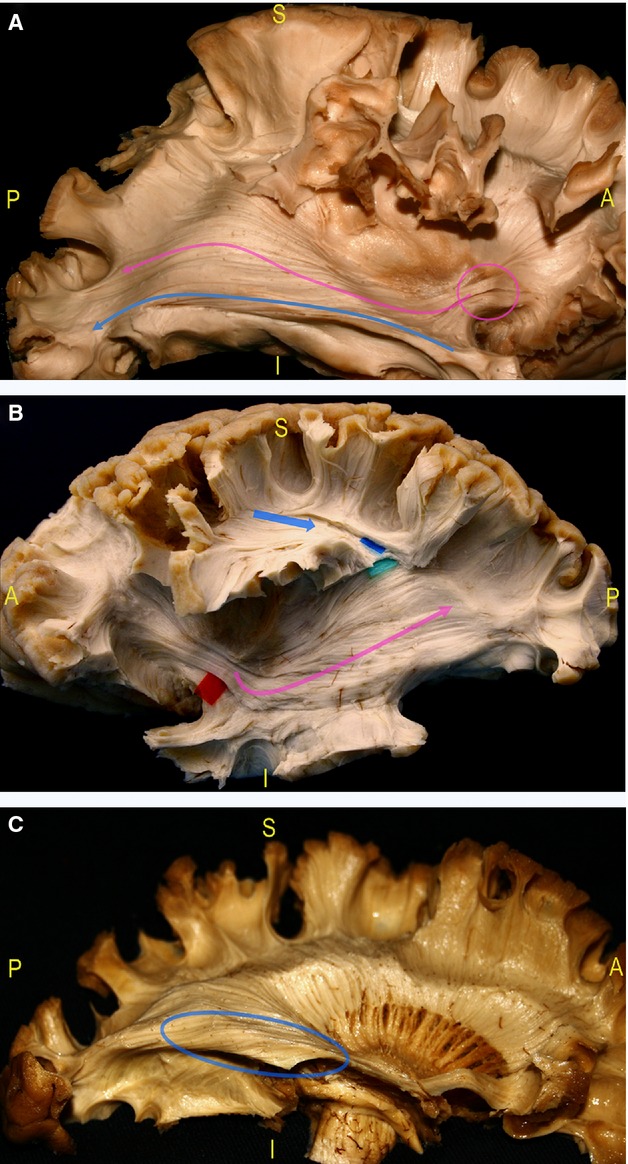
(A) After the removal of the insula and of the claustro-opercular fibres, the dissection study demonstrated the parallel course of the IFOF (pink arrow) and OR (blue arrow) from the occipital lobe to the TS, up to the IFOF fan-shaped stem at the level of the ventral EC before it enters within the frontal lobe (pink circle). (B) The IFOF and OR run deeper in the posterior and middle SS with respect to the AF (blue arrow), and lateral along to the occipital horn, the trigone and the temporal horn (pink arrow). (C) The inferior component of the OR envelops the floor of the occipital horn, the trigone and temporal horn (blue circle). AF, arcuate fasciculus; EC, external capsule; IFOF, inferior fronto-occipital fascicle; OR, optic radiation; SS, stratum sagittalis; TS, temporal stem.
Fig. 7.
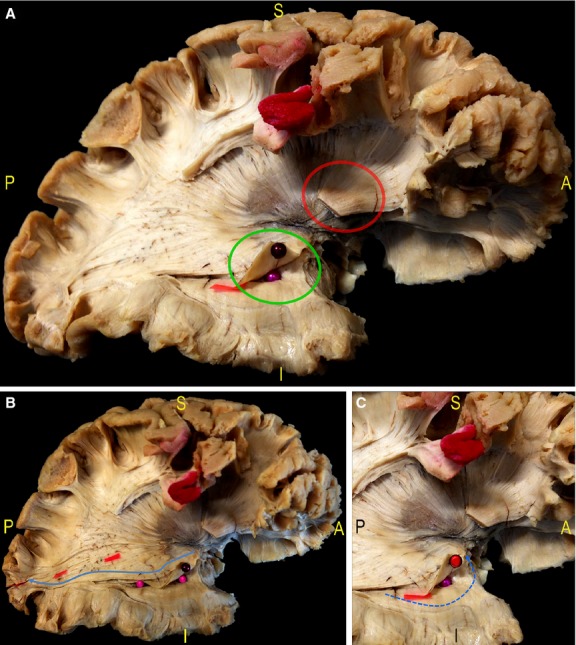
(A) After cutting the IFOF stem at the level of the ventral EC (red circle) and removal of the fibres of the occipito-temporal portion of the IFOF, the deeper putamen and the fibres of the anterior OR are shown (green circle). (B) In particular, the fibres of the inferior and superior component were separated by means of red tags, starting posteriorly from the calcarine sulcus. The inferior OR was followed on its anterior course along the temporal horn (blue arrow, pink pins) with the demonstration of the fibres Meyer's Loop, enveloping in an antero-medial direction the tip of the temporal horn. (C) The dissection allowed to lift up the main component of the Meyer's Loop without cutting in order better demonstrate its relationships with the tip of the temporal horn (red pin, blue arrow). EC, external capsule; IFOF, inferior-fronto-occipital fascicle; OR, optic radiation.
Fig. 8.
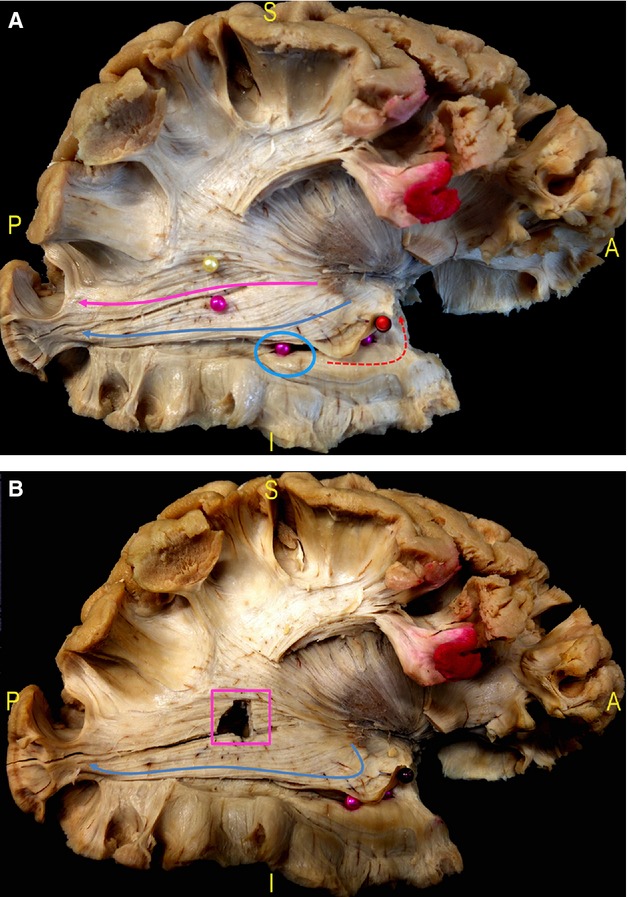
(A) The border between inferior and superior OR is highlighted starting posteriorly from the calcarine sulcus and it corresponds to the inferior margin of the lateral wall of the trigone (pink pin). The superior OR completely covers the lateral wall of the trigone and then courses antero-inferiorly within the posterior thalamic radiation (pink arrow). The inferior OR has a more inferior course along the lateral and inferior wall of the ventricle (blue circle, inferior intra-ventricular pink pins) up to the enlarge in an antero-medial direction (blue arrow) around the tip of the temporal horn (Meyer's Loop, lifted up by a red pin). (B) After cutting the fibres between the two pins in picture (A), the trigone is opened (pink square), demonstrating the course of the inferior OR at its inferior margin (blue arrow). OR, optic radiation.
On the other hand, the inferior portion of the OR envelopes also the infero-basal portion of the lateral wall and the floor of the ventricle, and it is more ventral and basal with respect to the IFOF (Fig. 6C). Posteriorly, at the level of the occipital horn, the superior and inferior OR fibres have a more compact and mild inferior oriented course (directed to the calcarine cortices) lateral to the superior and inferior half of the lateral wall of the occipital horn, respectively. More anteriorly, in the context of the WM of the stratum sagittalis (SS), the anatomical limit between the inferior and superior components of the OR was demonstrated in all the specimens at the level of the inferior margin of the lateral wall of the ventricular trigone (Fig. 8 and 9). In the middle third of the temporal WM the inferior fibres of the OR have a more inferior and basal course, covering the lateral and inferior wall of the ventricle.
Fig. 9.
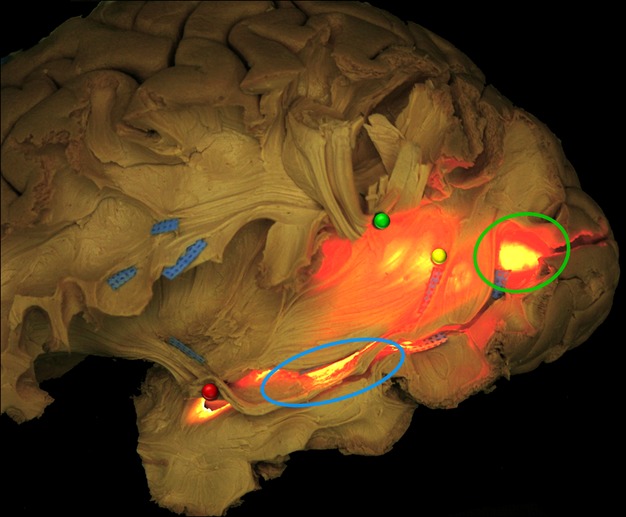
In this figure, a transventricular illumination is applied from the mesial side of a left hemisphere. It allowed to better show the relationships of WM fibres isolated during the dissection with the ventricular structures. The more superficial WM fibres, particularly AF and ILF, are, respectively, cut and lifted up (green pin) and lifted ‘in situ’ highlighted with blue tag. The more posterior portions of IFOF and OR before the occipital terminations are shown overlapping the occipital horn (green circle) and running anteriorly. The inferior and superior components of OR and IFOF course all along the lateral ventricle (yellow pin), crossing the ILF main stem (blue tags), up to the tip of the temporal horn (blue circle) where the fibres of the Meyer's Loop are lifted up with a red pin. AF, arcuate fasciculus; IFOF, inferior fronto-occipital fascicle; ILF, inferior longitudinal fascicle; OR, optic radiation; WM, white matter.
The superior component has a more superior course on the latero-superior margin of the lateral wall and on the roof of the ventricle, deeper in respect to the IFOF fibres, and run anteriorly turning progressively in a medial direction. The inferior component has a different course at the level of the middle and anterior thirds of the temporal WM (Figs 7 and 8). These fibres, in fact, enlarge running anteriorly along the lateral wall of the temporal horn and turn around to the tip and to the basal part of the most anterior portion of the temporal horn running in a medial and posterior direction up to lateral geniculate body, forming the so-called Meyer's Loop (Figs 7 and 8). The Meyer's Loop completely envelopes the anterior portion of the temporal horn, just posterior to the main course of the uncinate fascicle, inferior to the IFOF stem and lateral to the head of the hippocampus (Fig. 7 and 8). These fibres are competent for the contralateral upper quadrant of the visual field and are not overlapped by the IFOF fibres.
Surgical cases
Case 1
The resection started from the posterior third of the inferior temporal gyrus (ITG) of the right non-dominant hemisphere that was infiltrated by the tumour, according to the pre-operative MRI (Fig. 1A). The subpial dissection continued in a medial direction below the floor of the ventricle and then anteriorly, following the lateral wall of the ventricle, up to the tip of the temporal horn. At this level, the temporo-mesial structures were removed up the identification of the arachnoid and vessels of the basal cisterns (Fig. 1B). Proceeding superiorly along the lateral wall of the ventricle, from a posterior to an anterior direction, we stimulated the WM during the task execution and we stopped the resection when flashes visualization at the limit between the inferior and superior contralateral quadrants of visual field was elicited by DES. We followed this functional limit all along the ventricle, up to the tip of the temporal horn and the resection was stopped at this stage.
In this case the stimulation of the ILF fibres did not produce any problems during both denomination and visual recognition tasks, and it was completely resected without any post-operative deficit. On the contrary, at the level of the inferior margin of the lateral wall of the ventricular trigone, DES induced systematically visual recognition errors, associated with flashes at the stimulation of the most inferior overlapping point between the ventral fibres of the superior OR and the inferior fibres of the IFOF (Fig. 1B). The resection was stopped and the post-operative MRI demonstrated the complete resection of the lesion (Fig. 1C). The post-operative visual field digital examination revealed the selective post-operative superior contralateral quadrantanopia (Fig. 1D).
Case 2
The resection started from the posterior third of the ITG, just anteriorly to the Labbé vein in the left-dominant hemisphere. The cortical mapping did not produce any visual and/or language disturbances during the task execution in the region of cortical approach, but semantic paraphasias (tag 3) were elicited anteriorly, at the level of the middle third of the ITG and MTG (Fig. 2B). The subpial dissection started in the medial and anterior directions, below the floor of the ventricle at the temporo-parieto-occipital (TPO) junction, up to the visualization of the arachnoid of basal cisterns. The resection in the superior direction along the lateral wall of the ventricle trigone was stopped after elicitation of flashes at the limit between inferior and superior contralateral visual quadrants. This critical point was located at the border between the floor and the inferior-third of the lateral wall of the trigone and at cortical level, it projects at the border between the posterior thirds of the MTG and ITG (Fig. 2B,C). In this point the patient experienced also semantic paraphasias at the DES, demonstrating the contact with the most inferior fibres of the IFOF. The resection was stopped beyond the enhancing portion of the tumour, according to the functional limits, and the anaesthesiological drugs infusions started again. The post-operative perfusion computed tomographic (CT)-scan showed the complete resection of the lesion and of the perilesional non-enhancing region (Fig. 2A). The patient reported a not complete superior contralateral quadrantanopia, and did not experience any post-operative language deficits.
Case 3
In this case the resection started with the complete removal of the anterior temporal lobe, including a portion of the WM of the TS. During resection of the temporal WM and after the visualization of the tip of the temporal horn, the subcortical DES started laterally and below the temporal horn, up to the visualization of the arachnoids of the basal cisterns medially. In the upper and posterior part of the temporal WM the resection was stopped when the DES evoked flashes at the border with the inferior left quadrant of the visual field associated with not systematic visual recognition troubles (indicating the contact with the more anterior fibres of the superior OR in proximity to the IFOF stem), on the medial-posterior roof of the temporal horn and within the WM of the TS (Fig. 3B). Finally, we removed the frontal WM invaded and the insula, via subpial combined trans-temporal and trans-pars orbitalis approach. The post-operative MRI showed the complete resection of the insula, the resection of the temporal lobe with residual tumour (5 cc) at the level of the medial and posterior WM of the TS, according to the intra-operative findings (Fig. 3A). The patient experienced an upper contralateral quadrantanopia without post-operative permanent deficits.
Discussion
The OR has been discussed since the early part of last century (Meyer, 1907), and constitutes the deepest bundle in the WM of the lateral brain surface. It extends from the occipital to the temporal lobe following the occipital horn, the lateral portion and the temporal horn of the ventricle. Over the decades, results coming from post-mortem dissections and, more recently, from DTI reconstructions allowed a better definition of the anatomical features and course of this pathway (van Buren & Baldwin, 1958; Türe et al. 2000; Rhoton, 2002).
The preservation of the OR represents a challenge during surgery of deep-sited or extensively infiltrating tumours. For this reason, many authors propose the integration of different modern anatomical and functional techniques to provide monitoring of visual functions (i.e. DTI integrated with neuronavigation, intra-operative DTI, intra-operative on-line visual-evoked potentials merged with neuronavigation and DTI; Curatolo et al. 2000; Kamada et al. 2005; Wu et al. 2007; Romano et al. 2011; Kuhnt et al. 2012). Nonetheless, all these experiences have different technical limitations and do not provide any on-line information about the functional role of the neighbouring eloquent WM structures.
Currently, subcortical DES has been proposed as an effective and safe method to navigate the real individual course of the OR with a reliable monitoring also of the functions subserved by the bundles crossing and overlapping the visual pathways. After three case reports (Duffau et al. 2004; Nguyen et al. 2011; Šteňo et al. 2012), the largest case series of awake monitoring of the visual pathways has been recently reported (Gras-Combe et al. 2012). In these patients, the direct visual mapping allowed to limit the postoperative (i.e. extensive resections of right and left LGGs) deficits to quadrantanopia. Patients with quadrantopia are generally asymptomatic (Krolak-Salmon et al. 2000), and most of countries do not consider superior quadrantanopia as a contraindication to drive (Gras-Combe et al. 2012). Thus, the concept of maximal neuroncological resection according to the functional individual limits has also been applied to lesions harbouring the OR. To this end, an accurate anatomical knowledge of the OR and its relationships with other eloquent fibres still remains crucial for surgical planning and surgical resections with or without DES.
Classically, the anatomical characterization of the OR identified three groups of fibres, anterior, central, posterior, according to their courses and relationships with the ventricle (Rhoton, 2002; Sincoff et al. 2004; Rubino et al. 2005).
In this study we provided high-quality dissections of the OR, with special emphasis on its relationships with the other eloquent WM pathways. We characterized the OR as a double-components bundle divided in an inferior and a superior portion. We started showing the posterior relationships of the superior and inferior OR with the occipital horn and the ventricular trigone. The dissections demonstrated that the fibres coming from the inferior calcarine cortex run along the inferior half of the lateral wall of the occipital horn (Figs 7, 8 and 9). Anteriorly, at the level of the ventricular lateral wall, the limit between the inferior and superior OR are at the inferior margin of the lateral wall (Fig. 8A,B). According to recent anatomical and DTI results (Martino et al. 2013; De Benedictis et al. 2014), our findings indicate that the risk to induce a contralateral homonymous hemianopia and to damage the most inferior fibres of the IFOF, which overlaps the superior OR at the level of the lateral wall of the ventricle, is significantly reduced by following an antero-posterior direction of resection during the approach to the ventricle from the cranial base to the ventricular floor (Figs 7, 8 and 9). In the two surgical cases showing a resection at this level (cases 1 and 2), DES confirmed the results of our anatomical observations (Figs 1). The subcortical mapping performed in case 1, in fact, demonstrated the limit between the superior and inferior OR just at the level of the inferior margin of the lateral wall of the ventricle. Moreover, in both cases we obtained functional responses (visual recognition troubles and semantic paraphasia, respectively) due to stimulation of the non-dominant and dominant IFOF (Figs 1A–C and 2B,C). Relationships with this complex long association pathway are very important, because of its functional implications within both the dominant and non-dominant hemispheres. The IFOF dorsal (i.e. fronto-parietal) component, for instance, is proposed to support the visuo-spatial elaboration (particularly, attention and planning of visually guided movements), by integrating information coming from the sensori-motor areas. Moreover, the IFOF is also crucially implicated in processing for the recognition of objects, places, words, faces and colours, through the connections with the occipito-temporal visual cortices (Aralasmak et al. 2006). Finally, the IFOF provides a direct connection between the posterior and frontal language cortices (Catani & Thiebaut de Schotten, 2008; Martino et al. 2010b; Sarubbo et al. 2013), representing the main ventral stream for semantic elaboration, with the support of indirect connection provided by the ILF (Mandonnet et al. 2007; Duffau et al. 2014). In our cases, the stimulation of ILF resulted silent at DES. This bundle has been proposed to provide both direct and indirect connections between the occipital visual territory and temporal limbic and memory areas, thus participating in several aspects of visual input processing (i.e. face recognition, reading, visual perception and memory; Mandonnet et al. 2007, 2009; Catani & Thiebaut de Schotten, 2008).
The most inferior overlapping point between the IFOF and OR at the level of the wall of the lateral ventricle was approximately projected on the cortical level at the junction between the posterior third of the MTG and ITG (Fig. 9). Within the temporal WM, the inferior OR runs along the floor and the lateral wall of the temporal horn, and the superior OR runs on the roof and the supero-medial side of the temporal horn, deeper with respect to the IFOF that is again completely overlapped to this portion of the OR (Figs 6 and 9). The functional mapping in surgical case 1 confirmed this course, such as the pre-operative DTI (Fig. 1). We opened the floor of the temporal horn up to the tip, preserving the superior two-thirds of the lateral wall of the temporal horn, according to the results of the subcortical DES. The post-operative visual field confirmed exactly the distribution of the VFD in the superior contralateral quadrant (Fig. 1D).
Within the anterior temporal WM, the fibres of the inferior OR enlarge anteriorly to completely envelope the tip of the temporal horn and turn it around medially and posteriorly up to the lateral geniculate body (Figs 7A–C; 8A,B and 9). This anterior course of the OR coming from the inferior calcarine cortex is known as Meyer's Loop, and it constitutes the anatomical background for VFDs during the anterior temporal lobe resection, as performed in epilepsy or tumour surgery (Winston et al. 2012). Previous DTI studies have demonstrated a considerable inter-subject variability in the anterior course of this OR portion (Nilsson et al. 2007; Yogarajah et al. 2009). The exclusive resection of the anterior arcuate fibres of the Meyer's Loop produces a partial or complete contralateral upper quadrantanopia, according also to the eventual extension of the resection in the WM of the TS (Martino et al. 2010a; Peltier et al. 2010). On the other hand, the postero-superior extension of the resection on the roof of the ventricle, where the OR fibres have a more compact distribution, also induces damage to the inferior quadrant of the visual field (Figs 7A–C and 8A) with a consequent hemianopia.
In case 3, the intra-operative mapping combined with subpial dissection starting from the temporal lobe and completed by the orbital portion of the IFG invaded by the tumour, allowed to safely approach the insula with a complete resection of this lobe and to extend the resection also to the temporal WM, posteriorly up to the limit of the superior portion of the OR (i.e. the roof of the temporal horn), and medially up to the WM of the TS and internal capsule (Figs 1A,B, 2A–C and 7A–C). We included this case in this study not to recommend the visual pathways monitoring in all the anterior temporal or insular resections, but to highlight the location and the anatomical course of the superior and inferior OR components within the TS. Moreover, we demonstrated the more postero-superior and deep subcortical limits of an extensive functional tailored temporal lobectomy also within the right non-dominant hemisphere. The lobectomy was stopped, in fact, after resection of the more dorsal and anterior WM forming the TS (including the inferior OR) up to the identification of the postero-ventral fibres of the superior OR. DES confirmed the results of our dissections, demonstrating that these fibres are continuously overlapped by the compact stem of the IFOF also at the level of the anterior roof of the temporal horn and through the parieto-temporal WM (Fig. 6), as shown also by recent studies (Martino et al. 2010a; Peltier et al. 2010).
As a consequence, preserving the IFOF stem within the anterior temporal WM, within both the dominant or non-dominant hemispheres, allows the neurosurgeon to preserve the inferior contralateral visual quadrant, so avoiding post-operative hemianopia during anterior temporal resections.
The tip of the ventricle and the anterior two-thirds of the lateral wall and floor of the temporal horn represent the anatomical landmarks for selective resection of inferior OR fibres. These anatomical and intra-operative evidences elucidate also the reason why the trans-sylvian approach, described in the literature and adopted for the temporo-mesial lesions, through the inferior limiting sulcus of the insula does not exclude the possibility of post-operative VFDs and hemianopia (Yasargil et al. 2004; Rubino et al. 2005). After sylvian fissure opening, the approach to the temporo-mesial structures should be limited to the most anterior portion of the inferior limiting sulcus, as suggested also by other authors (Rubino et al. 2005), considering the high risk of damaging the fibres of the IFOF stem and also the superior OR fibres medially directed to the lateral geniculate body (Fig. 3A,B). We argue that this anatomical background could represent a relative limitation of the transylvian approach for resection of posteriorly extended temporo-mesial lesions.
Finally, considering the supero-inferior distribution of the OR fibres during surgery within or through the SS, particularly regarding the visual pathways and the long association pathway we showed in our dissections (i.e. IFOF, ILF, AF), the surgical resection should be tailored in progressive antero-posterior steps, along the natural course of the WM of this region, and not in a supero-inferior direction. In this way, the neurosurgeon reduces the risk of inducing a complete hemianopia, as well as language deficits, due to complete disconnection of both the superior and inferior calcarine cortices produced by supero-to-inferior approaches.
In our anatomical study we did not find any substantial difference in the anatomical distribution of the posterior and middle portion of the OR. On the contrary, the anterior and lateral extension of the Meyer's Loop resulted slightly variable among the six hemispheres. Even if the anatomical variability of the anterior portion of the OR has been reported in different MR post-resection and DTI studies (Coenen et al. 2005; Nilsson et al. 2007; Jeelani et al. 2010), the intrinsic limitations of the Klingler's technique did not allow us to draw definitive conclusions due to the possible partial destruction of the thinner fibres during the layer by layer dissection.
Conclusions
The OR can be considered a double-component bundle. The superior component courses along the lateral wall of the occipital horn and the trigone, and along the roof and supero-medial wall of the temporal horn. The inferior component covers the inferior third of the occipital horn, the inferior border of the trigone and the lateral wall of the temporal horn, and enlarges anteriorly in an antero-medial direction to embrace the temporal tip.
The IFOF overlaps the superior OR all over its course, up to the TS and represents the functional limit of resection within both the dominant and non-dominant hemisphere, allowing the preservation at least of the inferior contralateral visual field. The ILF runs from the occipital to the temporal lobe in a supero-inferior direction, crossing laterally the superior and then the inferior component of the OR.
The subcortical DES confirmed the results of the dissection analysis and represents, in our experience, the most specific and sensitive tool for performing safe resections of lesions invading or adjacent to the optic pathways and to the eloquent bundles of this region, in order to reduce the risk of post-operative visual, language and cognitive deficits.
Finally, the anatomical structural and functional evidences provided by DES and dissection in this study, even if not adaptable a priori to all the patients considering the inter-individual and inter-hemispheric variability, represent a useful tool to improve the surgical ability for finer resections tailored according to a three-dimensional ‘network-based’ vision of the brain.
Conflict of interests
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethical statements and notes
The work presented in this manuscript is original and has not been published elsewhere, it is not presently under consideration of publication by any other journal, and it was not presented at Conferences or Scientific Meetings.
The Ethical Committees of the Azienda Provinciale per i Servizi Sanitari (APSS) of Trento (Italy) and of the ‘S. Anna’ University-Hospital of Ferrara (Italy) authorized the ‘post mortem’ dissections, and the integration of the data provided from the intra-operative DES during awake surgery and from the DTI reconstructions.
References
- Aralasmak A, Ulmer JL, Kocak M, et al. Association, commissural, and projection pathways and their functional deficit reported in literature. J Comput Assist Tomogr. 2006;30:695–715. doi: 10.1097/01.rct.0000226397.43235.8b. [DOI] [PubMed] [Google Scholar]
- van Buren JM, Baldwin M. The architecture of the optic radiation in the temporal lobe of man. Brain. 1958;81:2–41. doi: 10.1093/brain/81.1.15. [DOI] [PubMed] [Google Scholar]
- Catani M, Ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Coenen VA, Huber KK, Krings T, et al. Diffusion-weighted imaging-guided resection of intracerebral lesions involving the optic radiation. Neurosurg Rev. 2005;28:188–195. doi: 10.1007/s10143-005-0385-6. [DOI] [PubMed] [Google Scholar]
- Curatolo JM, Macdonell RA, Berkovic SF, et al. Intraoperative monitoring to preserve central visual fields during occipital corticectomy for epilepsy. J Clin Neurosci. 2000;7:234–237. doi: 10.1054/jocn.1999.0208. [DOI] [PubMed] [Google Scholar]
- De Benedictis A, Duffau H. Brain hodotopy: from esoteric concept to practical surgical applications. Neurosurgery. 2011;68:1709–1723. doi: 10.1227/NEU.0b013e3182124690. [DOI] [PubMed] [Google Scholar]
- De Benedictis A, Moritz-Gasser S, Duffau H. Awake mapping optimizes the extent of resection for low-grade gliomas in eloquent areas. Neurosurgery. 2010;66:1074–1084. doi: 10.1227/01.NEU.0000369514.74284.78. [DOI] [PubMed] [Google Scholar]
- De Benedictis A, Duffau H, Paradiso B, et al. Anatomo-functional study of the temporo-parieto-occipital region: dissection, tractographic and brain mapping evidence from a neurosurgical perspective. J Anat. 2014;225:132–51. doi: 10.1111/joa.12204. doi: 10.1111/joa.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H. New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity – a review. J Neurooncol. 2006;79:77–115. doi: 10.1007/s11060-005-9109-6. [DOI] [PubMed] [Google Scholar]
- Duffau H. A new concept of diffuse (low-grade) glioma surgery. Adv Tech Stand Neurosurg. 2012;38:3–27. doi: 10.1007/978-3-7091-0676-1_1. [DOI] [PubMed] [Google Scholar]
- Duffau H. Surgical neurooncology is a brain networks surgery: a “Connectomic” perspective. World Neurosurg. 2013;82:e405–e407. doi: 10.1016/j.wneu.2013.02.051. doi: 10.1016/j.wneu.2013.02.051. [DOI] [PubMed] [Google Scholar]
- Duffau H, Velut S, Mitchell MC, et al. Intra-operative mapping of the subcortical visual pathways using direct electrical stimulations. Acta Neurochir (Wien) 2004;146:265–269. doi: 10.1007/s00701-003-0199-7. [DOI] [PubMed] [Google Scholar]
- Duffau H, Moritz-Gasser S, Mandonnet E. A re-examination of neural basis of language processing: proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain Lang. 2014;131:1–10. doi: 10.1016/j.bandl.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Duncan JS. Imaging in the surgical treatment of epilepsy. Nat Rev Neurol. 2010;6:537–550. doi: 10.1038/nrneurol.2010.131. [DOI] [PubMed] [Google Scholar]
- Ffytche DH, Catani M. Beyond localization: from hodology to function. Philos Trans R Soc Lond B Biol Sci. 2005;360:767–779. doi: 10.1098/rstb.2005.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras-Combe G, Moritz-Gasser S, Herbet G, et al. Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways. J Neurosurg. 2012;117:466–473. doi: 10.3171/2012.6.JNS111981. [DOI] [PubMed] [Google Scholar]
- Jeelani NU, Jindahra P, Tamber MS, et al. Hemispherical asymmetry in the Meyer's Loop: a prospective study of visual-field deficits in 105 cases undergoing anterior temporal lobe resection for epilepsy. J Neurol Neurosurg Psychiatry. 2010;81:985–991. doi: 10.1136/jnnp.2009.182378. [DOI] [PubMed] [Google Scholar]
- Kamada K, Todo T, Morita A, et al. Functional monitoring for visual pathway using real-time visual evoked potentials and optic-radiation tractography. Neurosurgery. 2005;57:121–127. doi: 10.1227/01.neu.0000163526.60240.b6. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Guenot M, Tiliket C, et al. Anatomy of optic nerve radiations as assessed by static perimetry and MRI after tailored temporal lobectomy. Br J Ophthalmol. 2000;84:884–889. doi: 10.1136/bjo.84.8.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnt D, Bauer MH, Becker A, et al. Intraoperative visualization of fiber tracking based reconstruction of language pathways in glioma surgery. Neurosurgery. 2012;70:911–919. doi: 10.1227/NEU.0b013e318237a807. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, et al. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Gatignol P, Duffau H. Evidence for an occipito-temporal tract underlying visual recognition in picture naming. Clin Neurol Neurosurg. 2009;111:601–605. doi: 10.1016/j.clineuro.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Martino J, Vergani F, Robles SG, et al. New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery. 2010a;66:4–12. doi: 10.1227/01.NEU.0000348564.28415.FA. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, et al. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010b;46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Martino J, De Witt Hamer PC, Vergani F, et al. Cortex-sparing fiber dissection: an improved method for the study of white matter anatomy in the human brain. J Anat. 2011;219:531–541. doi: 10.1111/j.1469-7580.2011.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J, da Silva-Freitas R, Caballero H, et al. Fiber dissection and diffusion tensor imaging tractography study of the temporoparietal fiber intersection area. Neurosurgery. 2013;72:87–97. doi: 10.1227/NEU.0b013e318274294b. [DOI] [PubMed] [Google Scholar]
- Meyer A. The connections of the occipital lobes and the present status of the cerebral visual affections. Trans Assoc Am Phys. 1907;22:7–23. [Google Scholar]
- Nguyen HS, Sundaram SV, Mosier KM, et al. A method to map the visual cortex during an awake craniotomy. J Neurosurg. 2011;114:922–926. doi: 10.3171/2010.11.JNS101293. [DOI] [PubMed] [Google Scholar]
- Nilsson D, Starck G, Ljungberg M, et al. Intersubject variability in the anterior extent of the optic radiation assessed by tractography. Epilepsy Res. 2007;77:11–16. doi: 10.1016/j.eplepsyres.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Peltier J, Travers N, Destrieux C, et al. Optic radiations: a microsurgical anatomical study. J Neurosurg. 2006;105:294–300. doi: 10.3171/jns.2006.105.2.294. [DOI] [PubMed] [Google Scholar]
- Peltier J, Verclytte S, Delmaire C, et al. Microsurgical anatomy of the temporal stem: clinical relevance and correlations with diffusion tensor imaging fiber tracking. J Neurosurg. 2010;112:1033–1038. doi: 10.3171/2009.6.JNS08132. [DOI] [PubMed] [Google Scholar]
- Rhoton AL., Jr The cerebrum. Neurosurgery. 2002;51(Suppl 4):S1–S51. doi: 10.1097/00006123-200210001-00002. [DOI] [PubMed] [Google Scholar]
- Romano A, D'Andrea G, Calabria LF, et al. Pre- and intraoperative tractographic evaluation of corticospinal tract shift. Neurosurgery. 2011;69:696–704. doi: 10.1227/NEU.0b013e31821a8555. [DOI] [PubMed] [Google Scholar]
- Rubino PA, Rhoton AL, Jr, Tong X, et al. Three-dimensional relationships of the optic radiation. Neurosurgery. 2005;57:219–227. doi: 10.1227/01.neu.0000176415.83417.16. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, Latini F, Panajia A, et al. Awake surgery in low-grade gliomas harboring eloquent areas: 3-year mean follow-up. Neurol Sci. 2011;32:801–810. doi: 10.1007/s10072-011-0587-3. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, Latini F, Sette E, et al. Is the resection of gliomas in Wernicke's area reliable? Wernicke's area resection. Acta Neurochir (Wien) 2012a;154:1653–1662. doi: 10.1007/s00701-012-1416-z. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, Le Bars E, Moritz-Gasser S, et al. Complete recovery after surgical resection of left Wernicke's area in awake patient: a brain stimulation and functional MRI study. Neurosurg Rev. 2012b;35:287–292. doi: 10.1007/s10143-011-0351-4. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, De Benedictis A, Maldonado I, et al. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct. 2013;218:21–37. doi: 10.1007/s00429-011-0372-3. [DOI] [PubMed] [Google Scholar]
- Sincoff EH, Tan Y, Abdulrauf SI. White matter fiber dissection of the optic radiations of the temporal lobe and implications for surgical approaches to the temporal horn. J Neurosurg. 2004;101:739–746. doi: 10.3171/jns.2004.101.5.0739. [DOI] [PubMed] [Google Scholar]
- Šteňo A, Karlík M, Mendel P, et al. Navigated three-dimensional intraoperative ultrasound-guided awake resection of low-grade glioma partially infiltrating optic radiation. Acta Neurochir (Wien) 2012;154:1255–1262. doi: 10.1007/s00701-012-1357-6. [DOI] [PubMed] [Google Scholar]
- Türe U, Yasargil MG, Friedman AH. Fiber dissection technique: lateral aspect of the brain. Neurosurgery. 2000;47:417–427. doi: 10.1097/00006123-200008000-00028. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Winston GP, Daga P, Stretton J, et al. Optic radiation tractography and vision in anterior temporal lobe resection. Ann Neurol. 2012;71:334–341. doi: 10.1002/ana.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JS, Zhou LF, Tang WJ, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61:935–948. doi: 10.1227/01.neu.0000303189.80049.ab. [DOI] [PubMed] [Google Scholar]
- Yasargil MG, Türe U, Yasargil DC. Impact of temporal lobe surgery. J Neurosurg. 2004;101:725–738. doi: 10.3171/jns.2004.101.5.0725. [DOI] [PubMed] [Google Scholar]
- Yogarajah M, Focke NK, Bonelli S, et al. Defining Meyer's loop-temporal lobe resections, visual field deficits and diffusion tensor tractography. Brain. 2009;132:1656–1668. doi: 10.1093/brain/awp114. [DOI] [PMC free article] [PubMed] [Google Scholar]


