Abstract
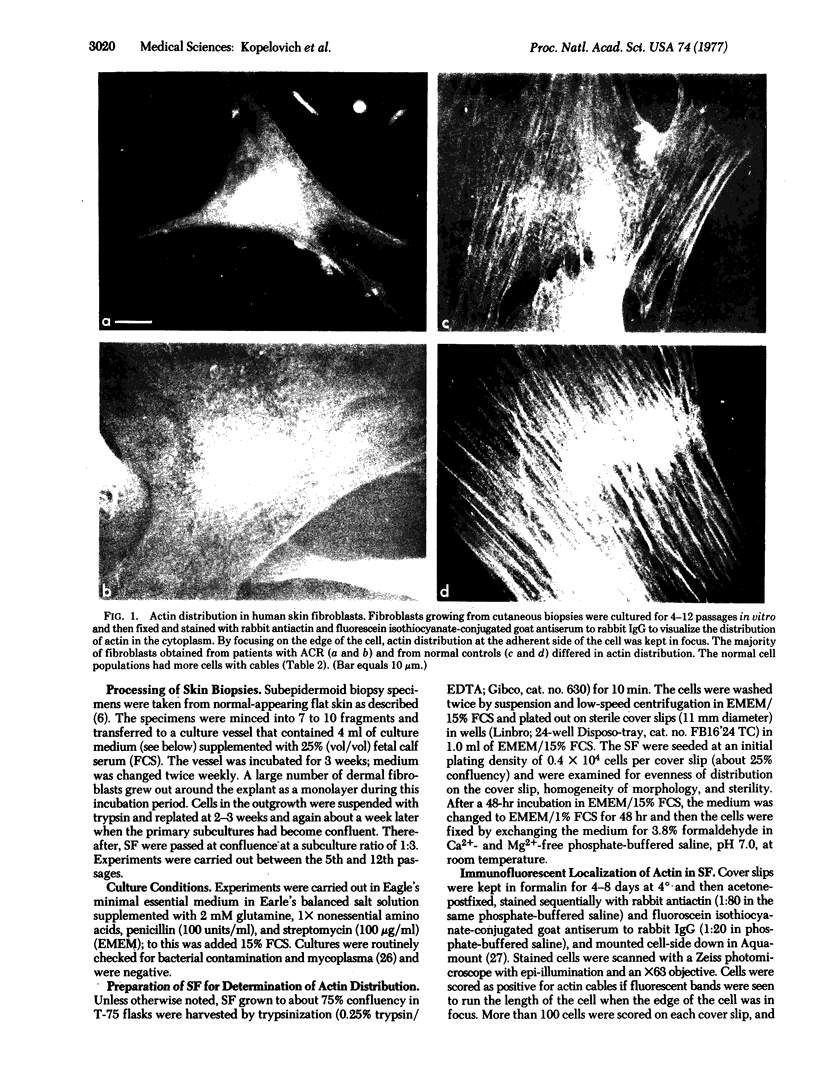
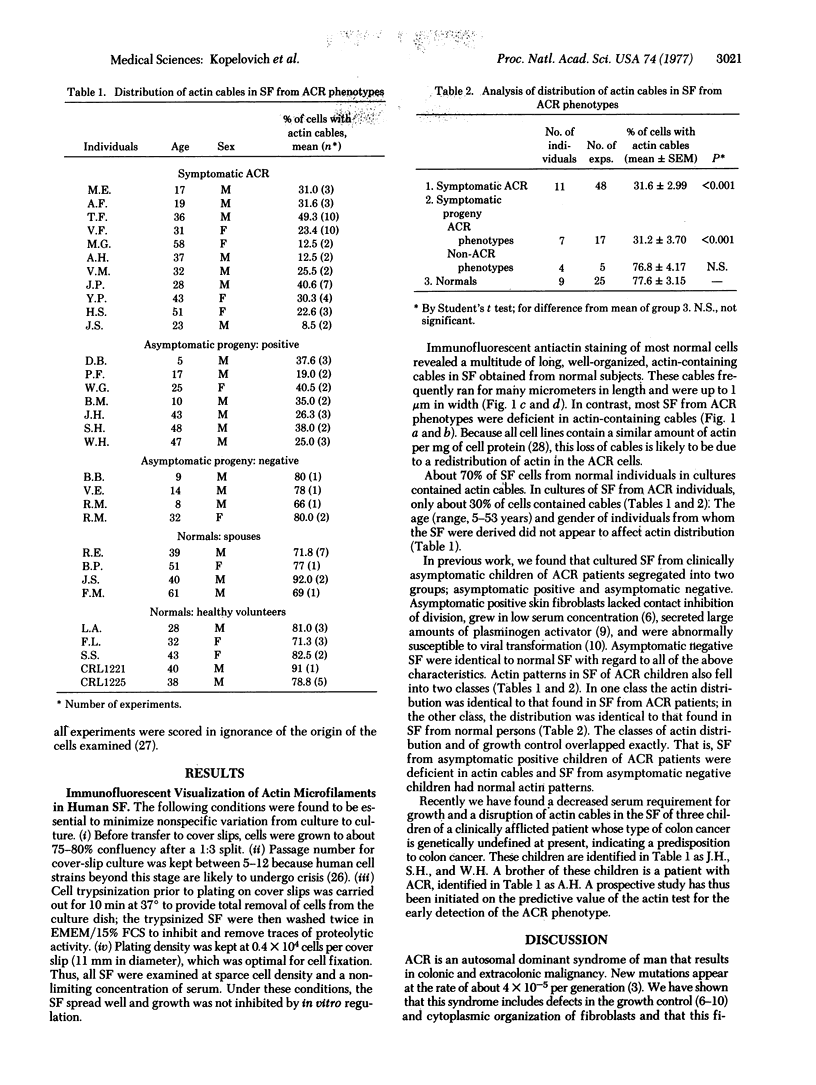
In the cytoplasm of well-spread cultured normal fibroblasts, actin is organized into a network of cables that run the length of the cell just inside the adherent cell membrane. A diffuse matrix replaces the cables in fibroblasts that have become tumorigenic as a result of oncogenic transformation. We have found a similar disruption in actin organization in cultured skin fibroblasts (passage 6-10) obtained by biopsy from patients with the inherited colonic cancer, adenomatosis of the colon and rectum (ACR). Because ACR is inherited as an autosomal dominant trait, about half the children of ACR patients will develop colon cancer, but they typically remain asymptomatic until at least the second decade of life. Actin distribution within cultured cells from children of ACR patients was identical either to that seen in cultured cells from normal persons or to that seen in cultured cells from ACR patients. The two different patterns were independent of age, sex, drug treatment, or infections of the donors. Apparently, this class of colonic carcinoma is accompanied by a systemic aberration in the organization of fibroblast cytoplasm, and this aberration can be detected by immunofluorescent localization of actin within cultured skin fibroblasts, prior to manifestation of any colonic symptoms.
Keywords: adenomatosis of colon and rectum, cytoskeleton, cancer detection, immunofluorescence, inherited disease
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley I. K., Porter K. R. Cytoplasmic fibrils in living cultured cells. A light and electron microscope study. Protoplasma. 1967;64(4):349–380. doi: 10.1007/BF01666538. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Gallimore P. H., McDougall J. K. Correlation between tumor induction and the large external transformation sensitive protein on the cell surface. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3570–3574. doi: 10.1073/pnas.73.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDNER E. J., RICHARDS R. C. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet. 1953 Jun;5(2):139–147. [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D., Lazarides E., Pollack R., Weber K. The distribution of actin in non-muscle cells. The use of actin antibody in the localization of actin within the microfilament bundles of mouse 3T3 cells. Exp Cell Res. 1975 Feb;90(2):333–344. doi: 10.1016/0014-4827(75)90323-7. [DOI] [PubMed] [Google Scholar]
- Kopelovich L., Pfeffer L., Lipkin M. Recent studies on the identification of proliferative abnormalities and of oncogenic potential of cutaneous cells in individuals at increased risk of colon cancer. Semin Oncol. 1976 Dec;3(4):369–372. [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J Cell Biol. 1973 Feb;56(2):412–428. doi: 10.1083/jcb.56.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Nelson-Rees W. A., Springer E. L. Epithelial cell cultures from normal and cancerous human tissues. J Natl Cancer Inst. 1976 Apr;56(4):843–849. doi: 10.1093/jnci/56.4.843. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Kopelovich L. Differential genetic susceptibility of cultured human skin fibroblasts to transformation by Kirsten murine sarcoma virus. Cell. 1977 Feb;10(2):313–320. doi: 10.1016/0092-8674(77)90225-2. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Risser R., Conlon S., Rifkin D. Plasminogen activator production accompanies loss of anchorage regulation in transformation of primary rat embryo cells by simian virus 40. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4792–4796. doi: 10.1073/pnas.71.12.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Risser R., Rifkin D., Pollack R. The stable classes of transformed cells induced by SV40 infection of established 3T3 cells and primary rat embryonic cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):317–324. doi: 10.1101/sqb.1974.039.01.042. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Owens R. B., Hiller A. J., Nelson-Rees W. A., Johnston J. O. The biology of human cells in tissue culture. I. Characterization of cells derived from osteogenic sarcomas. Int J Cancer. 1976 Feb 15;17(2):219–234. doi: 10.1002/ijc.2910170211. [DOI] [PubMed] [Google Scholar]
- Weber K., Lazarides E., Goldman R. D., Vogel A., Pollack R. Localization and distribution of actin fibers in normal transformed and revertant cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):363–369. doi: 10.1101/sqb.1974.039.01.047. [DOI] [PubMed] [Google Scholar]