Abstract
The nasal region of the skull has undergone dramatic changes during the course of cetacean evolution. In particular, mysticetes (baleen whales) conserve the nasal mammalian pattern associated with the secondary function of olfaction, and lack the sound-producing specializations present in odontocetes (toothed whales, dolphins and porpoises). To improve our understanding of the morphology of the nasal region of mysticetes, we investigate the nasal anatomy, osteology and myology of the southern right whale, Eubalaena australis, and make comparisons with other mysticetes. In E. australis external deflection surfaces around the blowholes appear to divert water off the head, and differ in appearance from those observed in balaenopterids, eschrichtiids and cetotherids. In E. australis the blowholes are placed above hypertrophied nasal soft tissues formed by fat and nasal muscles, a pattern also observed in balaenopterids (rorqual mysticetes) and a cetotherid (pygmy right whale, Caperea marginata). Blowhole movements are due to the action of five nasofacial muscles: dilator naris superficialis, dilator naris profundus, depressor alae nasi, constrictor naris, and retractor alae nasi. The dilator naris profundus found in E. australis has not been previously reported in balaenopterids. The other nasofacial muscles have a similar arrangement in balaenopterids, with minor differences. A novel structure, not reported previously in any mysticete, is the presence of a vascular tissue (rete mirabile) covering the lower nasal passage. This vascular tissue could play a role in warming inspired air, or may engorge to accommodate loss of respiratory space volume due to gas compression from increased pressure during diving.
Keywords: anatomy, blowhole, Eubalaena australis, mysticete, nasal muscle, nasal plug, right whale
Introduction
The nasal region of the cetacean skull has changed dramatically during the course of evolution. In the transition from land to water, the fossil record documents movement of the external bony nares from the rostral to the caudo-dorsal region of the skull, and the bones of the dorsal aspect of the skull exhibit overlapping layers (a condition known as ‘telescoping’) (Miller, 1923; Fordyce & de Muizon, 2001; Thewissen & Bajpai, 2001; Thewissen et al. 2009). These changes probably reduced the scale of head movements while breathing, resulting in more hydrodynamically efficient swimming motion at the water surface.
The two groups of modern cetaceans, toothed whales, dolphins and porpoises (Odontoceti) and baleen whales (Mysticeti), exhibit quite different configurations of the epicranial narial passages in response to different functions. Mysticetes retain two separate nostrils as paired blowholes in mysticetes, but odontocetes have a single blowhole. Major differences also exist in the internal nasal anatomy between the two cetacean groups.
In odontocetes, the nasal region is highly modified into a complex of tissues capable of producing and transmitting echolocation and communication sounds (Norris, 1968; Cranford et al. 1996; Cranford, 2000; Cranford & Amundin, 2004). The vibrating tissues of the ‘monkey-lip-dorsal-bursae complex’ (Amundin & Andersen, 1983; Amundin & Cranford, 1990; Cranford et al. 1996) surround the nasal passages and generate sounds. A specialized fat structure (melon) appears to focus and transfer the sounds to water (Norris, 1968, 1969). Air sacs that develop from the nasal passageways likely serve both to prevent pressurization of the system (expanding sacs allow controlled airflow) and to capture and recycle air for use in the next vocalization (Reidenberg & Laitman, 2008). The facial muscles surrounding this region are hypertrophied, and they likely function in the control of air sac dimensions, nasal passage diameter, melon shape, tension of vibrating elements, as well as closure of the nasal passageways through movement of the nasal plugs (Lawrence & Schevill, 1956; Mead, 1975).
Mysticetes lack the sound-producing nasofacial specializations present in toothed whales (Eschricht & Reinhardt, 1866; Carte & Macalister, 1868; Schulte, 1916; Lawrence & Schevill, 1956; Henry et al. 1983; Cave, 1988; Heyning & Mead, 1990). The mysticete nasal passageways are comparatively simple, and appear to conserve the nasal mammalian pattern associated primarily with conducting air for breathing, perhaps with a secondary function of olfaction (Thewissen et al. 2011; Godfrey et al. 2013). In contrast to odontocetes, there is limited information about the nasal morphology of mysticetes, particularly the myology. The goal of this study is to analyze nasal anatomy, osteology and myology of the southern right whale, Eubalaena australis (Desmoulins, 1822) and compare this information with published anatomical descriptions of other mysticetes.
Material and methods
Two calf specimens of southern right whales (Eubalaena australis) that stranded near the nursery grounds at Península Valdés, Argentina (42°30′S, 64°10′W), were dissected to analyze the nasal soft tissues (Table 1). Three E. australis dry skulls were used to describe the osteology of the nasal region (CNPMAMM748 neonate, USNM 267612 adult, MLP 1508 adult). A calf specimen of pygmy right whale (Caperea marginata) (MM2904) stranded in Karekare Peninsula, Northland, New Zealand, was dissected for the comparatives studies (Table 1). The osteological anatomical terminology follows Mead & Fordyce (2009).
Table 1.
Biological data of the specimens of Eubalaena australis and Caperea marginata dissected
| Species | Specimen number | Sex | Body length (m) | Age | Date of stranding | Locality |
|---|---|---|---|---|---|---|
| Eubalaena australis | 04-10 | Female | 4.90 | Calf | 23 August 2010 | El Doradillo Beach, Golfo Nuevo, Península Valdés, Chubut, Argentina |
| 03-11 | Male | 3.64 | Neonate | 14 August 2011 | Las Canteras Beach, Golfo Nuevo, Península Valdés, Chubut, Argentina | |
| Caperea marginata | MM2904 | Female | 2.96 | Calf | 4 September 2010 | Karekare Peninsula, Northland, New Zealand |
Dissections were conducted in the field and in the lab of the Centro Nacional Patagónico (Argentina) and Museum of New Zealand Te Papa Tongarewa (New Zealand) and were documented by digital photography, video recordings, physical notes, and drawings. The nasal muscles were identified and named based on topological criteria (i.e. correspondence of the relative positions of the muscles) following the description of Carte & Macalister (1868). The definitions and referenced source of the anatomical names used for cetacean nasal structures in this work are given in Table 2.
Table 2.
Principal anatomical terms of the nasal region of cetaceans used in the present work
| Term used | Synonyms | Definition | References |
|---|---|---|---|
| Nasal passage | The whole passageway for air between the blowhole and the internal bony nares including vestibule and nasal chamber. This can be divided into upper and lower nasal passage | This contribution | |
| Upper nasal passage | The portion of the nasal passage which includes the blowhole, vestibule, external bony nares and nasal fossa | This contribution | |
| Lower nasal passage | The portion of the nasal passage which includes the nasal chamber and the internal bony nares | This contribution | |
| Blowhole | Fleshy nostril | External opening of the respiratory apparatus in the skin formed by an external and inner margin | Mead & Fordyce (2009, pp. 156) and Witmer (2001) |
| Vestibule | Vestibule | Cavity generated with the opening of the blowhole for the passage of the air placed immediately below the blowhole | Henry et al. (1983) and this contribution |
| Nasal chamber | Olfactory chamber, nasal cavity | Recess of the nasal passage which contains the turbinates | Thewissen et al. (2011, pp. 5) |
| Internal bony nares | Internal nares, choanae, posterior nares | Internal or posterior opening of the nasal passage, formed by the pterygoid and vomer | Mead & Fordyce (2009) |
| Nasal plug | Soft structure, valve-like, formed by connective and adipose tissue and nasal muscles which occlude the nasal passage | Heyning & Mead (1990, pp. 69) | |
| Nassal fossa | Bony fossa on the dorsal surface of the skull formed by the premaxillae, vomer, nasal and nasal septum which hold the soft structures of the upper nasal passage (the principal are the nasal muscles), epithelium, blowhole and vestibule | This contribution | |
| External bony nares | Distal or external bony opening of the respiratory apparatus associated with the nasals and premaxillae | Mead & Fordyce (2009) |
Acronyms
CNPMAMM: Laboratorio de Mamíferos Marinos, Centro Nacional Patagónico, Puerto Madryn, Argentina. MLP: Museo de La Plata, La Plata, Argentina. MM: Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand. USNM: National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
Results
The paired nasal passages are defined as the contiguous pathway for air between the blowholes and the internal bony nares, and can be divided into upper and a lower nasal passage which includes bony and soft structures. The upper nasal passage includes the blowhole, vestibule, nasal plug, external bony nares and nasal fossa while the lower nasal passage includes the nasal chamber and the internal bony nares (Table 2). These structures will be described in the following sections.
Osteology and cartilages of nasal complex
The external bony nares form the external bony opening of the respiratory apparatus associated with the nasal and premaxillary bones. In E. australis they are located on the dorsal surface of the skull between the midpoint of rostrum (half way between the tip and the base of the rostrum, the latter defined as the level of the antorbital notches) and the rostral margin of the nasals. The nasal fossa, which holds the nasal muscles, cartilages and the nasal plugs, is a deep and oval-shaped excavation located on the dorsal surface of the skull. The nasal fossa is bordered latero-ventrally by the premaxillae and maxillae, ventrally in the midline by the vomer, and caudally by the nasals (Fig. 1). At the rostral aspect of the nasal fossa, the medial surfaces of both premaxillae meet in the midline of the skull, forming the roof of the enclosed mesorostral canal. The nasal fossa is divided in the midline by the nasal septum (comprising vomer, perpendicular plate of ethmoid, and mesorostral cartilage). The nasal septum forms the medial wall of the nasal fossa and vertically separates the right and left nasal passages. The cartilaginous portion of the nasal septum (mesorostral cartilage) extends above the skull as a flat vertical plate, reaching superiorly to the blowholes. It also extends forward in the midline to the tip of the rostrum as a cylindrical structure narrowing to a cone that fills the mesorostral canal formed by the vomer and premaxillae (Fig. 2A). This mesorostral cartilage appears to remain cartilaginous in adult specimens, as only a hollow midline canal was found in the rostrum with no ossified mesorostral structure in all adult dry skulls. The nasal fossa passes caudo-ventrally as paired nasal passages that are lined by the maxillae, premaxillae, vomer, palatines and pterygoids. The nasal passage terminates at the paired internal bony nares (choanae) in the caudoventral region of the skull, lined by the palatines and pterygoids latero-ventrally, and the vomer dorsally (Fig. 2B).
Fig. 1.
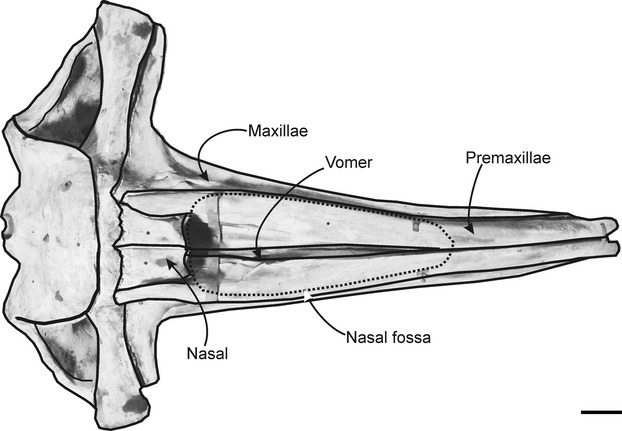
Dorsal view of the skull of Eubalaena australis showing the position and shape of the nasal fossa. Scale bar: 20 cm.
Fig. 2.
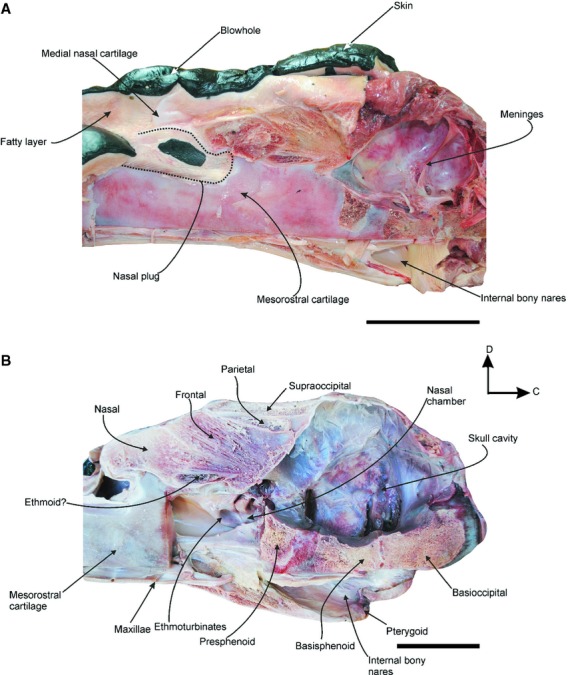
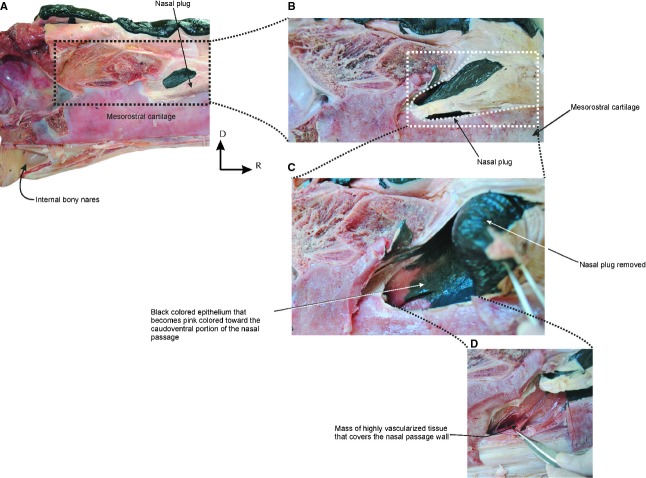
Caudal sagittal section of the head of a neonate specimen of Eubalaena australis (CNPMAMM748) showing the anatomy of the nasal region: (A) head with nasal soft tissues in situ showing the position of the nasal plug, the medial nasal cartilage and the blowhole; (B) dry skull showing the osteological components of the nasal passage. C, caudal; D, dorsal. Scale bar: 10 cm.
A midsagittal section, with the mesorostral cartilage removed, reveals the nasal chamber, a paired recess of the nasal passage that contains the turbinates. The nasal chamber is the bony portion of the nasal passage, beginning immediately below the nasal and frontal bones. This chamber holds three or four longitudinally folded and thick cartilages projected rostrally from the ethmoid, that correspond to ethmoturbinates (Fig. 2B). The ethmoturbinates are covered by a thin, delicate and highly vascularized epithelium. A portion of the caudodorsal wall of the nasal chamber is formed by the cribriform plate of the ethmoid bone. This plate is a small lamina that separates the rostral cranial fossa from the nasal chamber, and is perforated by many foramina that appear to transmit the olfactory nerves to the olfactory bulb (cranial nerve I).
The nasal fossa contains a complex of two paired cartilages that are located vertically in the caudal and medial wall of each blowhole, associated with the inner margin of the blowhole (Fig. 2A). The medial nasal cartilage is found near the midline, between the left and right nasal fossa. It articulates with the lateral nasal cartilage, which is placed in the caudal wall of the blowhole. These cartilages are the site of insertion of some nasal muscles (see below) and support the inner margin of the blowhole, maintaining it as a stable surface for muscle origins.
Soft tissues of the nasal complex
The blowholes are a pair of nostrils, and are symmetrically placed on either side of the sagittal midline of the dorsum of the head, beginning 440 and 630 mm behind the tip of the rostrum in the two calves examined (Fig. 3A). The blowholes are semicircular in shape with the concavities facing laterally, and are separated medially from each other by a groove in the skin, the median sulcus (sensu Carte & Macalister, 1868). Vibrissae are found caudal to the blowhole in some specimens (Fig. 3B). Below the integument, there is a thick blubber layer placed adjacent to the rostral and lateral margins of the blowholes. The blubber of this region is tougher and firmer than the blubber layer that covers the rest of the body. It forms a rounded protuberance on the dorsal surface of the head, and projects dorsally above the leading edges of the blowholes (Fig. 4). Each blowhole widens into a vestibule. The vestibules are paired spaces that channel the passage of the air caudo-ventrally from the blowholes into the paired external bony nares of the skull. The internal surface of the vestibule is covered by a thin, wrinkled and black pigmented epithelium that becomes pink toward the caudoventral portion of the nasal passage. The lumen of each vestibule is occupied by a mass of tissue called the nasal plug. The paired nasal plugs, one for each nasal opening, are composed of connective tissue, fat and muscle. They are covered by a black pigmented epithelium that is continuous with the ventral floor and lateral walls of the nasal passages (Fig. 5).
Fig. 3.
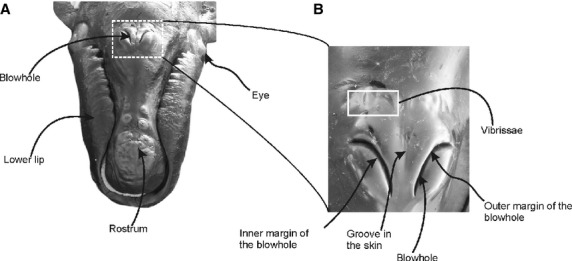
Position of the blowhole in the head of Eubalaena australis: (A) rostro-dorsal view of the head of a neonate specimen showing the position of the blowhole on the dorsum of the head; (B) dorsal view of the head of a calf specimen exhibiting a detail of the blowhole region.
Fig. 4.
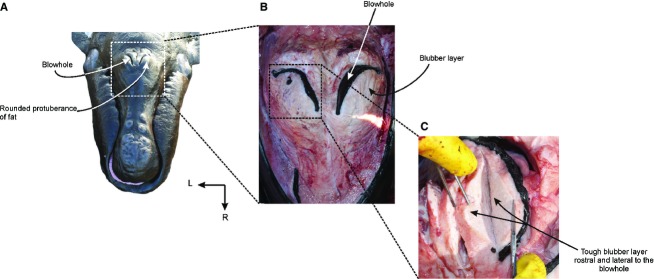
Soft tissue structures associated with the blowhole: (A) rostro-dorsal view of the head of a neonate specimen of Eubalaena australis showing the position of the blowholes and the rounded protuberance rostral to the blowholes; (B) dorsal view of the blowhole region with the integument removed exhibiting the fat tissue around the blowholes; (C) detail of the fat tissue structure around the right blowhole.
Fig. 5.
Vascularized tissue in the nasal passage of Eubalaena australis: (A) caudal sagittal section of the head of a neonate specimen; (B-D) detail of the nasal plug area showing the vascularized tissue that covers the caudo-ventral portion of the nasal passage. C, caudal; D, dorsal.
At the caudal region of the nasal passage, there is a mass of highly vascularized tissue that covers the nasal passage wall (Fig. 5D). Macroscopically, this tissue resembles the ophthalmic rete present in the eye of E. australis (Buono et al. 2012). Histologically, this tissue is composed of a complex network of abundant arteries, arterioles and capillaries (Fig. 6).
Fig. 6.
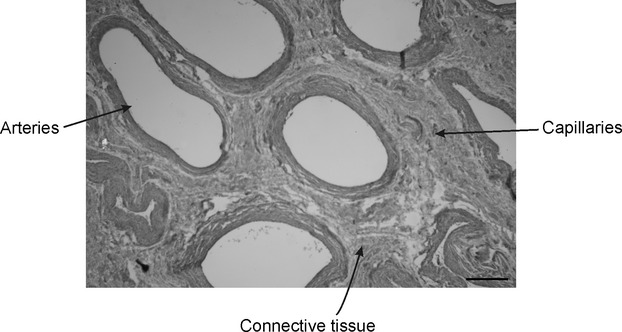
Histology of the vascularized tissue found in the caudo-ventral portion of the nasal passage of Eubalaena australis. Note the abundant arteries and capillaries. H&E stain. Scale bar: 10 μm.
Nasofacial musculature
Five nasal muscles (m. dilator naris superficialis, m. dilator naris profundus, m. constrictor naris, m. retractor alae nasi and m. depressor alae nasi) are present in E. australis (Figs 9). M. dilator naris profundus has not been reported previously for any mysticete, and is identified and named here for the first time.
Fig. 9.
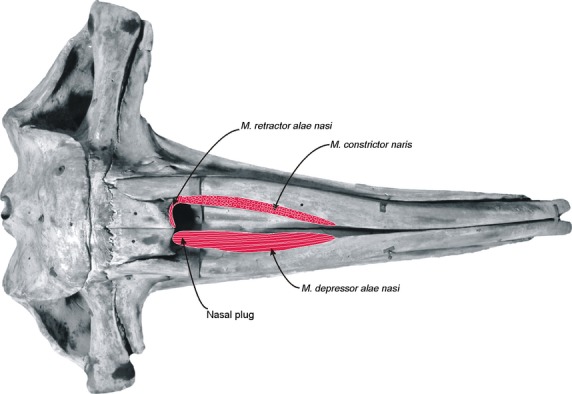
Schematic reconstruction of the nasal muscles of Eubalaena australis (skull in dorsal view). In the left side of the skull are represented the attachments of the most superficial nasal muscles, the m. constrictor naris and m. retractor alae nasi. Note that the fibers of the m. constrictor naris are sectioned in a horizontal plane with respect to the sagittal plane of the skull. In the right side of the skull is represented the attachment of the deepest nasal muscle, the m. depressor alae nasi, along the whole ventral surface of the nasal fossa.
Musculus dilator naris superficialis (Figs 7A and 8A,B) is the most superficial of the facial muscles and is found below the blubber layer. It attaches from the rostral surface of the descending process of the maxilla and extends rostrally along the lateral surface of the maxilla through a strong aponeurosis. The medial fibers insert onto the premaxilla, in the median raphe, rostral to the blowhole. The rostral-most fibers attach onto the rostral tip of the premaxilla along the midline. The fibers of this muscle have an irregular arrangement and the muscle is very thin.
Fig. 7.
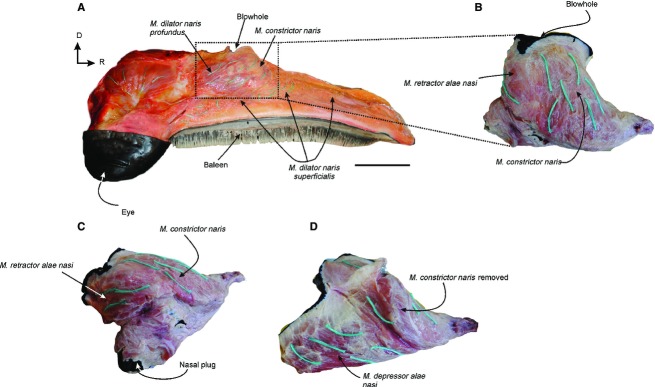
Nasal muscles of Eubalaena australis: (A) right lateral view of the head of a neonate specimen showing the most superficial nasal muscles in situ; (B) lateral and (C,D) caudo-lateral view of the nasal muscles removed from the nasal fossa showing the fibers directions (green threads) of the m. contrictor naris m. retractor alae nasi and m. depressor alae nasi. D, dorsal; R: rostral. Scale bar: 15 cm (A).
Fig. 8.
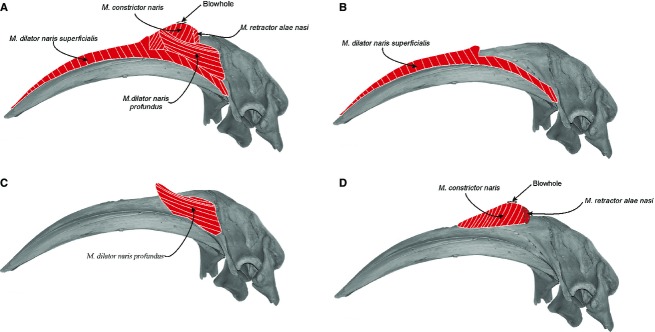
Schematic reconstruction of the nasal muscles in the skull of Eubalaena australis in lateral view: (A) four of the nasal muscles described; (B,C) detail of each nasal muscles from the most superficial (m. dilator naris superficialis and m. dilator naris profundus); (D) to the most profundus (m. constrictor naris and m. retractor alae nasi).
Musculus dilator naris profundus (Figs 7A and 8A,C) is a thin muscle found immediately deep to the m. dilator naris superficialis. It is located in the medial plane, and originates from the caudodorsal region of the maxilla and premaxilla. Its fibers run in a rostro-dorsal direction and insert into an aponeurosis in the blubber layer, in a position rostral to the blowhole.
Musculus constrictor naris (Figs 7, 8A,D and 9) is found immediately deep to the m. dilator naris profundus, and originates from the whole lateral margin of the nasal fossa formed by the premaxilla. Its fibers run dorsally toward the rostral and lateral margin of the blowhole and insert into an aponeurosis attached to the blubber layer of the outer margin of the blowhole. The dorso-ventral orientation of this muscle contributes bulk to the blowholes, resulting in a high profile above the dorsal surface of the rostrum. The separation between this muscle and the deeper m. depressor alae nasi is not clear because their fibers are interdigitated.
Musculus retractor alae nasi (Figs 7A-C, 8 and 9) is a small muscle situated at the caudal margin of the blowhole and the fibers run obliquely. The muscle arises medially from the caudal region of the medial nasal cartilage. It runs diagonally (dorso-laterally) to attach at the caudal surface of the lateral nasal cartilage, and then continues laterally into an aponeurosis attached to the caudal margin of the m. constrictor naris.
Musculus depressor alae nasi (Figs 7D and 9) is the largest and deepest muscle of the nasal region and occupies the whole ventral surface of the nasal fossa. It arises from the rostral-most end of the nasal fossa in the medial and ventral surfaces of the premaxilla. The fibers run caudally and are obliquely oriented in a rostro-dorsal direction. They insert medially into an aponeurosis attached to the medial nasal cartilage, and caudally into an aponeurosis attached to the epidermis of the nasal plug. The separation between this muscle and the m. constrictor naris is difficult because the fibers are interdigitated.
Discussion
One of the main challenges to cetaceans during breathing is keeping water out of the respiratory tract while allowing fast and efficient respiration via a blowhole region that is suitably streamlined for swimming. Cetaceans evolved external protection from incursions of water through the development of a valve-like structure that seals the blowholes. Mysticetes evolved additionally a complex of external deflection surfaces, ridges and grooves around the blowholes that divert water off the region of the blowholes. These water-deflecting tissues show different morphological patterns in the extant representatives of balaenids (bowhead and right whales), balaenopterids (rorqual whales such as humpback, minke, fin, sei, Bryde's and blue whales), cetotheriids (pygmy right whale) and eschrichtiids (gray whales). In balaenids, water is excluded from the blowholes due to their high position on the head, which results from a combination of the highly arched skull and hypertrophy of the soft tissues under the blowholes. In addition, balaenids have a medial sulcus between both blowholes and a rostral ridge that also helps to shed the water. The water deflection surfaces observed in balaenids differ from those observed in balaenopterids. Balaenopterids have a straight rostrum that facilitates water accumulating on the flattened dorsal surface of the head, in contrast to the water-shedding arched rostrum of the right whales. Unlike right whales, however, balaenopterids have V-shaped ridges in front of the blowholes that exclude water efficiently from the dorsal surface of the head. The blowholes are also raised above the flat dorsal surface of the head, although not as high as those of balaenids. The cetotherid Caperea has a moderately arched rostrum and the blowholes are positioned above a mass of fat and muscle markedly lower than those observed in balaenids and balaenopterids. The body profile in lateral view shows that the nares in MM2904 are not elevated above the smooth convex dorsal profile of the rostrum. There is some evidence from photographs of adult animals in life (e.g. Baker, 1985: fig. 2; Best, 2007: fig. 11, Kemper, 2009: fig. 2) and from articulating dried skulls with the vertebral column that the head is sometimes elevated to place the blowholes at or near the dorsal highest point on the rostrum. Underwater photos do not show the blowholes elevated (Ross et al. 1975: figs 1–5). In juvenile Caperea, the blowhole is not obviously raised above the profile of the head, as can be seen in lateral view (Leeney et al. 2013: figs 2 and 4; Fig. S1). The rostrum has a low median ridge rostral to the nares which bifurcates (about 32 mm before the blowholes) into V-shaped ridges that diverge steeply caudally, lateral to each blowhole (DSC5216) (Supporting Information Fig. S1), similar to the condition observed in balaenopterids. A prominent median sulcus lies between the blowholes, similar to the structure found in balaenids. In eschrichtiids the blowholes appear to be positioned above the same nasal soft tissue (fat and muscles) described for balaenids, balaenopterids and Caperea; however, this should be confirmed by dissections of this species (Andrews, 1914). The common pattern of all these groups appears to be that the blowholes are positioned above hypertrophied nasal soft tissues (fat and muscles).
During breathing, each of the paired blowholes changes from a slit-like shape when closed to an oval-shape when open. The inner margin of the blowhole remains rigid and unmovable in E. australis due to the presence of the medial and caudal nasal cartilages and the firmer blubber layer, while the outer margin moves freely to open and close the blowhole. The tougher and firmer fibrous-fatty layer of the outer margin supports its shape, allowing the attached nasal muscles to generate movements during the respiration cycles.
Blowhole movements are due to the action of five nasal muscles in E. australis. The muscles that open of the dorsal part of the nasal passage (blowhole and vestibule) are the dilator naris superficialis, the depressor alae nasi and the dilator naris profundus. The dilator naris superficialis and profundus muscles pull the outer lip of the blowhole latero-ventrally. The deeper, more ventral, portion of the nasal passage is opened by the nasal plug muscle (depressor alae nasi), which pulls the nasal plug tissue latero-rostrally. The simultaneous action of these three muscles should dilate the lumen of the nasal passage during the inhalation and exhalation cycles. Closing the nasal passage is accomplished by relaxation of the nasal plug muscle (depressor alae nasi) and by contraction of two other muscles, constrictor naris and retractor alae nasi. The relaxation of the depressor alae nasi should cause the nasal plug to return back into the lumen of the nasal passage. The fibrous-fatty structure of the nasal plugs, additionally, may give a spring-like action that helps the plugs to return. In addition, the simultaneous contraction of the constrictor and the retractor alae nasi may compress the outer and inner margins of the blowhole, closing the nasal passage and sealing the blowhole from influx of water when submerged.
Despite similarities in the overall organization of their nasal muscles, there are some differences between balaenids and balaenopterids in their specific muscle attachments (Table 3). In Balaenoptera acutorostrata, the constrictor naris muscle arises from the rostral ‘edge of the temporal fossa’ (Carte & Macalister, 1868, pp. 239; interpreted here as probably the crest on the maxilla dorsal to the posterior maxillary concavity) and from the dorsal surface of the maxilla (probably the ascending process), and inserts rostrally into the dorsal surface of the mesorostral cartilage, and caudally into the outer margin of the blowhole and into the dorsal surface of the maxilla (Carte & Macalister, 1868). In E. australis the origin of this muscle is restricted to the premaxilla and its insertion is in the outer margin of the blowhole. In addition, the principal direction of this muscle is dorso-ventral, and its mass contributes to higher profile of the blowholes above the dorsal surface of the rostrum. In B. acutorostrata, the constrictor naris muscle is rostro-caudally oriented, located lateral to the blowhole, and forms an arched structure, with the concavity facing medially, in dorsal view. Unlike E. australis, it does not run dorso-ventrally and therefore does not contribute to the higher profile of the blowholes as in E. australis. In B. acutorostrata the retractor alae nasi muscle arises from the rostro-lateral portion of frontal, the fibers run rostrally and insert into the medial and caudal nasal cartilages. This condition differs from E. australis, where this muscle is restricted to the nasal cartilages and never inserts into the frontal bone. In B. acutorostrata, the dilator naris superficialis and depressor alae nasi muscles have similar arrangements to those of E. australis.
Table 3.
Comparative table of the nasal muscles attachment of Eubalaena australis and Balaenoptera acutorostrata (according to Carte & MacAlister, as tabulated by Allen, 1916: 264)
|
Eubalaena australis |
Balaenoptera acutorostrata |
|||
|---|---|---|---|---|
| Muscle | Origin | Insertion | Origin | Insertion |
| M. dilator naris superficialis | Rostral surface of the descending process of the maxilla and lateral surface of the maxilla | Median raphe of premaxilla and rostral tip of the premaxilla along the midline | Rostral border of maxilla from tip to jugal | Outer lip of nares and median raphe of rostrum |
| M. dilator naris profundus | Caudodorsal region of the maxilla and premaxilla | Rostral to the blowhole | Not reported | Not reported |
| M. constrictor naris | Lateral margin of the nasal fossa formed by the premaxilla | Outer margin of the blowhole | Rostral ‘edge of temporal fossa’, here interpreted as probably the crest on the maxilla dorsal to the posterior maxillary concavity | Outer lip of nares and median raphe of rostrum |
| M. retractor alae nasi | Caudal region of the medial nasal cartilage | Caudal surface of the lateral nasal cartilage, and caudal margin of the m. constrictor naris | Rostro-lateral portion of frontal | Cartilaginous lateral and caudal lip of nares |
| M. depressor alae nasi | Rostral-most end of the nasal fossa in the medial and ventral surfaces of the premaxilla | Medial nasal cartilage, and the epidermis of the nasal plug | Premaxilla and median raphe of rostrum | Outer margin of nares |
The different muscular attachment patterns observed in the blowhole region between balaenids and balaenopterids could be related to the different anatomy of the skull of these groups. For example, in balaenopterids the rostrum is straight rather than strongly arched in the sagittal plane, the maxilla is wide, and the ascending process of the maxilla is well developed, all of which provide a wide insertion surface for the constrictor naris muscle. In balaenids, the rostrum is transversally compressed and highly arched, the maxilla is very narrow, and the ascending process is reduced; in this case the premaxilla gives the surface area for the attachment of the constrictor naris muscle.
The dilator naris profundus muscle identified in E. australis has not previously reported for B. acutorostrata. The latter could be related to the lack of identification of this muscle in the previous studies (perhaps overlooked because this muscle is very thin), or because this muscle is not present in balaenopterids. It should be noted that the dilator naris superficialis of E. australis is homologous to the dilator naris of B. acutorostrata as reported by Carte & Macalister (1868) but it has been specifically identified as superficialis here to distinguish it from the profundus recognized in this study; these names reflect the topological position of both muscles. Additional studies of the musculature of the nasal region of balaenopterids should help to clarify this issue.
In Caperea the dilator naris superficialis, dilator naris profundus, constrictor naris muscle and depressor alae nasi muscles were identified with a similar arrangement to that of E. australis (Supporting Information Fig. S2). The most conspicuous difference was observed in the dilator naris superficialis. In Caperea, this muscle presents two layers: a superficial layer which arises from the lateral surface of the descending process of the maxilla and attaches to the dorso-lateral surface of the maxilla; and a deep layer which arises from the rostral margin of the blowhole and extends rostrally along the dorso-lateral surface of the premaxilla. In E. australis all the fibers attached to the dorsal surface of the premaxilla were identified as dilator naris superficialis muscle, and a clear separation between a superficial and deep layer was not observed, which could be related to the neonate condition of the specimen dissected. Identification of the retractor alae nasi muscle is pending a more carefully dissection of the nasal region of Caperea.
The homologies of nasofacial muscles between cetaceans and land mammals have been unclear. In odontocetes, the upper respiratory tract is highly modified for sound production and the associated facial musculature is therefore complex (Lawrence & Schevill, 1956; Mead, 1975; Huggenberger et al. 2009). Unlike mysticetes, most of the odontocete nasofacial muscles arise from the facial fossa on the maxilla and premaxilla, insert into soft tissue structures associated with sound generation/transmission (e.g. melon, phonic lips, blowhole ligament, accessory air sacs). It has been suggested that this specialized nasofacial musculature is derived from the m. maxillonasolabialis of terrestrial mammals (Huber, 1934). However, due to the clearly divergent morphology in the nasal region of odontocetes and mysticetes, it is difficult to establish homologies between these two cetacean groups. Howell (1927), for example, discussed muscles other than the m. maxillonasolabialis that might be present on the odontocete face. In addition, Huber (1934) and previous studies (e.g. Lawrence & Schevill, 1956; Mead, 1975; Huggenberger et al. 2009) identify and name nasofacial muscles based on topological criteria. However, future studies should also consider embryonic development, innervation, and vascular patterns to identify and confirm or refute proposed nasofacial muscle homologies between cetaceans and terrestrial mammals, as well as between mysticetes and odontocetes.
The development of retia mirabilia in cetaceans has been extensively reported in the literature (e.g. Ommanney, 1932; Nagel et al. 1968; Vogl & Fisher, 1981, 1982; Pfeiffer & Kinkead, 1990). The retia are anastomotic networks of arteries, veins or mixed composition present around the orbit, skull base, dura, mandible, pterygoid air sac/ear, vertebral column, and thorax (Nagel et al. 1968; Pfeiffer & Kinkead, 1990; Geisler & Luo, 1998; Buono et al. 2012; Ford et al. 2013). It has been suggested that these retia play a role in modulating and dampening fluctuations in the flow of blood to the central nervous system, providing oxygen storage, and regulating body temperature (Geisler & Luo, 1998; Ninomiya & Yoshida, 2007). Our results show the presence of a highly vascularized tissue covering the lower nasal passage that macroscopically and microscopically resembles a rete mirabile. Considering that in mysticetes the ethmoturbinates are reduced in comparison with land mammals, in spite of the much larger sizes of mysticetes, it is likely that the ethmoturbinates do not function to warm inspired air. Therefore, it is possible that this vascular tissue evolved initially to serve this role of warming of the inspired air but, like other retes, it may now serve other functions. We propose that it may engorge to accommodate loss of respiratory space gas volume due to increased ambient pressure during diving. This issue merits further exploration to determine whether highly vascularized nasal tissue is restricted to E. australis or is present in other diving mammals, particularly cetaceans, thus indicating a pressure-accommodating function during diving.
Concluding remarks
Our study shows that in E. australis, as well as in balaenopterids and C. marginata, the common pattern appear to be that the blowholes are positioned above hypertrophied nasal soft tissues (fat and muscles). There is a rounded protuberance of fat tissue that projects dorsally above the leading edges of the blowholes. This tough and firm fatty layer supports the shape of the outer margin of the blowhole, allowing the associated nasal muscles to generate movements during the respiration cycles. In E. australis there are five nasofacial muscles which open and close the dorsal part of the nasal passage (blowhole and vestibule). The muscles that open the dorsal part of the nasal passage are the dilator naris superficialis, the depressor alae nasi and the dilator naris profundus. Closing the nasal passage is accomplished by relaxation of the depressor alae nasi and by contraction of constrictor naris and retractor alae nasi. The overall organization of the nasal muscles is similar between balaenids and balaenopterids, which can be related to the lack of specialization in nasal muscles mysticetes in general. However, there are some differences in their specific muscle attachments which could be related to the different anatomy of the skull, and different lifestyles, of these groups. In addition, the dilator naris profundus muscle has not been identified in B. acutorostrata.
External ridges or raised areas around the blowholes are interpreted as deflection surfaces to divert water off the head. These water-deflecting tissues show different morphological patterns in the extant representatives of balaenids, balaenopterids, eschrichtiids and cetotherids (pygmy right whale).
Finally, a novel structure, not reported previously in any mysticete, is a vascular rete mirabile covering the lower nasal passage. This vascular tissue could play a role in warming inspired air, or may engorge to accommodate loss of respiratory space volume due to gas compression from increased pressure during diving.
Acknowledgments
We are extremely grateful to the personnel and volunteers of the Stranding Network at Península Valdés (V. Rowntree, M. Sironi, M. Uhart, A. Chirife, M. Di Martino), H. Ruiz, L. F. Britos, N. V. Barloa, J. Briguglio, N. García for their valuable help, support, and encouragement during dissections in the field and in the lab. We would like to thank A. van Helden (Museum of New Zealand Te Papa Tongarewa) for allowing R. Ewan Fordyce and Mónica R. Buono to participate in the dissection of a Caperea specimen in New Zealand. We thank N. Luján from the histological service of Centro Nacional Patagónico (CENPAT-CONICET) for the preparation of the sample tissue for the histological studies and C. Pastor (CENPAT-CONICET) for the histological photographs. Thanks also to F. Marx (National Museum of Nature and Science of Japan) for providing the photograph in Fig. 1. We thank E. Crespo and N. Garcia (Centro Nacional Patagónico), and C. W. Potter and N. D. Pyenson (National Museum of Natural History) for allowing M. R. Buono to examine specimens in their care and for assistance during her visits. M. T. Dozo (CENPAT-CONICET) is thanked for her assistance during this study. Field dissections were conducted under permits from Dirección de Fauna y Flora Silvestre and Secretaria de Turismo y Areas Protegidas del Organismo Provincial de Turismo, Chubut Province. This research has been supported by the following grants: Cetacean Society International (CSI), American Museum of Natural History (Lerner Gray Fund for Marine Research), Society for Marine Mammalogy (Grant In Aid of Research) and Smithsonian Institution (Remington Kellogg Fund) to M.R.B.; Consejo Nacional de Investigaciones Científicas y Tecnológicas (grant number PIP 0433); Agencia Nacional de promoción Científica y Tecnológica (grant number PICT 0748) to M.S.F. This article is part of doctoral research of Mónica R. Buono at Universidad de La Plata and Centro Nacional Patagónico (CENPAT-CONICET).
Author contributions
M.R.B. performed the dissections and collected the data of E. australis; R.E.F performed the dissections and collected the data of C. marginata; M.R.B., J.S.R. M.S.F. and R.E.F discussed the results and wrote the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Blowhole anatomy of a calf specimen of Caperea marginata (MM2904).
Fig. S2. Nasal muscles of Caperea marginata (MM2904).
References
- Amundin M, Andersen SH. Bony nares air pressure and nasal plug muscle activity during click production in the harbour porpoise, Phocoena phocoena, and the bottlenosed dolphin, Tursiops truncatus. J Exp Biol. 1983;105:275–282. [Google Scholar]
- Amundin M, Cranford TW. Forehead anatomy of Phocoena phocoena and Cephalorhynchus commersonii: 3-dimensional computer generated reconstructions with emphasis on the nasal diverticula. In: Thomas J, Kastelein RA, editors. Sensory Abilities of Cetaceans. New York: Plenum Press; 1990. pp. 1–18. [Google Scholar]
- Baker AN. Pygmy right whale. Caperea marginata (Gray, 1846) In: Ridgway SH, Harrison RJ, editors. Handbook of Marine Mammals, Volume 3. The Sirenians and Baleen Whales. London: Academic Press; 1985. pp. 345–354. [Google Scholar]
- Best PB. Whales and Dolphins of the Southern African Subregion. New York: Cambridge University Press; 2007. [Google Scholar]
- Buono MR, Fernández MS, Herrera Y. Morphology of the eye of the southern right whales (Eubalaena australis. Anat Rec. 2012;295:355–368. doi: 10.1002/ar.21541. [DOI] [PubMed] [Google Scholar]
- Carte E, Macalister A. On the anatomy of Balaenoptera rostrata. Philos Trans R Soc Lond B. 1868;158:210–261. [Google Scholar]
- Cave AJE. Note on olfactory activity in mysticetes. J Zool Lond. 1988;214:307–311. [Google Scholar]
- Cranford TW. In search of impulse sound sources in odontocetes. In: Au WWL, Popper AN, Fay RR, editors. Hearing by Whales and Dolphins. New York: Springer; 2000. pp. 109–155. [Google Scholar]
- Cranford TW, Amundin M. Biosonar pulse production in odontocetes: the state of our knowledge. In: Thomas JA, Moss C, Vater M, editors. Echolocation in Bats and Dolphins. Chicago: University Press; 2004. pp. 26–59. [Google Scholar]
- Cranford TW, Amundin M, Norris KS. Functional morphology and homology in the odontocete nasal complex: implications for sound generation. J Morphol. 1996;228:223–285. doi: 10.1002/(SICI)1097-4687(199606)228:3<223::AID-JMOR1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Desmoulins A. Baleine. Dictionnaire Classique d'Histoire Naturelle 2. Paris: Rey et Gravier; 1822. [Google Scholar]
- Eschricht DF, Reinhardt J. On the Greenland right whale (Balaena mysticetus. In: Flower WH, editor. Recent Memoirs on the Cetacea. London: Ray Society; 1866. pp. 1–143. [Google Scholar]
- Ford TJ, Werth AJ, George JC. An intraoral thermoregulatory organ in the bowhead whale (Balaena mysticetus), the corpus cavernosum maxillaris. Anat Rec. 2013;296:701–708. doi: 10.1002/ar.22681. [DOI] [PubMed] [Google Scholar]
- Fordyce RE, de Muizon C. Evolutionary history of cetaceans: a review. In: Mazin J-M, de Buffrenil V, editors. Secondary Adaptation of Tetrapods to Life in Water. München: Verlag Dr. Friedrich Pfeil; 2001. pp. 169–233. [Google Scholar]
- Geisler JH, Luo Z. Relationships of Cetacea to terrestrial Ungulates and the evolution of cranial vasculature in Cete. In: Thewissen JGM, editor. The Emergence of the Whales. Evolutionary Patterns in the Origin of Cetacea. New York: Plenum Press; 1998. pp. 163–212. [Google Scholar]
- Godfrey SJ, Geisler J, Fitzgerald EMG. On the olfactory anatomy in an archaic whale (Protocetidae, Cetacea) and the minke whale Balaenoptera acutorostrata (Balaenopteridae, Cetacea) Anat Rec. 2013;296:257–272. doi: 10.1002/ar.22637. [DOI] [PubMed] [Google Scholar]
- Henry RW, Haldiman JT, Albert TF, et al. Gross anatomy of the respiratoy system of the bowhead whale, Balaena mysticetus. Anat Rec. 1983;207:435–449. doi: 10.1002/ar.1092070306. [DOI] [PubMed] [Google Scholar]
- Heyning JE, Mead JG. Evolution of the nasal anatomy of cetaceans. In: Thomas JA, Kastelein RA, editors. Sensory Abilities of Cetaceans: Laboratory and Field Evidence. New York: Plenum; 1990. pp. 67–80. [Google Scholar]
- Howell AB. Contribution to the anatomy of the Chinese finless porpoise Neomeris phocaenoides. Proc U S Nat Mus. 1927;70:1–43. [Google Scholar]
- Huber E. Anatomical notes on pinnipedia and cetacea. Contrib Palaeontol. 1934;447:105–136. [Google Scholar]
- Huggenberger S, Rauschmann MA, Vogl TJ, et al. Functional morphology of the nasal complex in the harbor porpoise (Phocoena phocoena L.) Anat Rec. 2009;292:902–920. doi: 10.1002/ar.20854. [DOI] [PubMed] [Google Scholar]
- Kemper CM. Pygmy right whale Caperea marginata. In: Perrin WF, Wuersig B, Thewissen JGM, editors. Encyclopedia of Marine Mammals. 2nd edn. London: Academic Press; 2009. pp. 939–941. [Google Scholar]
- Lawrence B, Schevill WE. The functional anatomy of the delphinid nose. Bull Mus Comp Zool. 1956;114:103–151. [Google Scholar]
- Leeney RH, Post K, Best PB, et al. Pygmy right whale Caperea marginata records from Namibia. Afr J Mar Sci. 2013;35:133–139. [Google Scholar]
- Mead JG. Anatomy of the external nasal passages and facial complex in the Delphinidae (Mammalia: Cetacea) Smithsonian Contrib Zool. 1975;207:1–72. [Google Scholar]
- Mead JG, Fordyce RE. The therian skull. A lexicon with emphasis on the odontocetes. Smithsonian Contrib Zool. 2009;627:1–248. [Google Scholar]
- Miller GS. The telescoping of the cetacean skull. Smithsonian Miscellaneous Coll. 1923;76:1–70. 8 pls. [Google Scholar]
- Nagel EL, Morgane PJ, McFarland WL, et al. Rete mirabile of dolphin: its pressure-dampening effect on cerebral circulation. Science. 1968;161:898–900. doi: 10.1126/science.161.3844.898. [DOI] [PubMed] [Google Scholar]
- Ninomiya H, Yoshida E. Functional anatomy of the ocular circulatory system: vascular corrosion casts of the cetacean eye. Vet Ophthalmol. 2007;10:231–238. doi: 10.1111/j.1463-5224.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Norris KS. The evolution of acoustic mechanisms in odontocete cetaceans. In: Drake ET, editor. Evolution and Environment. New Haven: Yale University Press; 1968. pp. 297–324. [Google Scholar]
- Norris KS. The echolocation of marine mammals. In: Andersen HT, editor. The Biology of Marine Mammals. New York: Academic Press; 1969. pp. 391–423. [Google Scholar]
- Ommanney FD. The vascular networks (retia mirabilia) of the fin whale (Balaenoptera physalus. Discovery Rep. 1932;5:327–362. [Google Scholar]
- Pfeiffer CJ, Kinkead TP. Microanatomy of retia mirabilia bowhead whale foramen magnum and mandibular foramen. Acta Anat. 1990;139:141–150. doi: 10.1159/000146990. [DOI] [PubMed] [Google Scholar]
- Reidenberg JS, Laitman JT. Sisters of the sinuses: cetacean air sacs. Anat Rec. 2008;291:1389–1396. doi: 10.1002/ar.20792. [DOI] [PubMed] [Google Scholar]
- Ross GJB, Best PB, Donelly BG. New records of the pygmy right whale (Caperea marginata) from South Africa, with comments on distribution, migration, appearance, and behavior. J Fish Res Board Can. 1975;32:1005–1017. [Google Scholar]
- Schulte HVW. Monographs of the Pacific Cetacea. The Sei whale (Balaenoptera borealis Lesson). Part 2: anatomy of a foetus of Balaenoptera borealis. Memoirs of the American Museum of Natural History. 1916;6:391–502. [Google Scholar]
- Thewissen JGM, Bajpai S. Whale origins as poster child for macroevolution. BioScience. 2001;51:1037–1049. [Google Scholar]
- Thewissen JGM, Cooper LN, George JC, et al. From land to water: the origin of whales, dolphins, and porpoises. Evo Edu Outreach. 2009;2:272–288. [Google Scholar]
- Thewissen JGM, George J, Rosa C, et al. Olfaction and brain size in the bowhead whale (Balaena mysticetus. Mar Mamm Sci. 2011;27:282–294. [Google Scholar]
- Vogl AW, Fisher HD. The internal carotid artery does not directly supply the brain in the Monodontidae (order Cetacea) J Morphol. 1981;170:207–214. doi: 10.1002/jmor.1051700207. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Fisher HD. Arterial retia related to supply of the central nervous system in two small toothed whales – narwhal (Monodon monoceros) and beluga (Delphinapterus leucas. J Morphol. 1982;174:41–56. doi: 10.1002/jmor.1051740105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Blowhole anatomy of a calf specimen of Caperea marginata (MM2904).
Fig. S2. Nasal muscles of Caperea marginata (MM2904).