Abstract
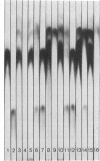
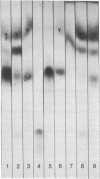
The heterogenetic Forssman antigen is a glycosphingolipid, a ceramide pentasaccharide with the structure GalNAcα1→3GalNAcβ1→3Galα1→4Galβ1→4Glc→ceramide. Forssman-positive animals are capable of synthesizing this compound in tissues or in erythrocytes, in contrast to the Forssman-negative species, including humans, which are incapable of adding the last carbohydrate in the sequence of the Forssman antigen, namely αGalNAc. The Forssman glycolipid and its precursor globoside were examined in twenty-one samples of surgically extirpated gastrointestinal mucosa and tumors derived therefrom. The results revealed that a few patients had chemically and immunologically detectable levels of the Forssman glycolipid as a normal component of their gastrointestinal mucosa (F+ population); in contrast, the majority of patients did not contain this glycolipid in their normal mucosa (F- population). Whereas the F- population included blood groups A, B, and O, the F+ population did not correspond to blood group A. The Forssman status in tumors taken from the F+ or F- population showed the following striking features: (i) all tumors derived from F- mucosa possessed Forssman glycolipid, whereas (ii) none of the tumors originating in F+ mucosa contained Forssman glycolipid. Globoside, the immediate precursor of Forssman antigen, was distributed equally among F+ and F- mucosa and the tumors derived therefrom. Thus, the expression of Forssman antigen in gastrointestinal mucosa appears akin to that of an isoantigen. Furthermore, the Forssman antigen that appears in tumors of the F- population could represent a human tumor-associated antigen. In view of the strong crossreactivity of Forssman antigen with blood group A determinants, the appearance of Forssman antigen in human tumors could be related to the “A-like antigen” (or “neo-A antigen”) of human tumors reported previously [Hakomori, S., Koscielak, J., Black, K. J. & Jeanloz, R. W. (1967) J. Immunol. 98, 31-38; Häkkinen, I. (1970) J. Natl. Cancer Inst. 44, 1183-1193].
Keywords: Forssman-positive and Forssman-negative populations, tumor, globoside, blood group
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Joseph K. C., Wistar R. Influence of membrane composition on the interaction of a human monoclonal "anti-Forssman" immunoglobulin with liposomes. Biochemistry. 1974 Nov 5;13(23):4818–4824. doi: 10.1021/bi00720a021. [DOI] [PubMed] [Google Scholar]
- Ando S., Isobe M., Nagai Y. High performance preparative column chromatography of lipids using a new porous silica, Iatrobeads. I. Separation of molecular species of sphingoglycolipids. Biochim Biophys Acta. 1976 Jan 22;424(1):98–105. [PubMed] [Google Scholar]
- Coligan J. E., Fraser B. A., Kindt T. J. A disaccharide hapten from streptococcal group C carbohydrate that cross-reacts with the Forssman glycolipid. J Immunol. 1977 Jan;118(1):6–11. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. Surface carbohydrates of hamster fibroblasts. I. Chemical characterization of surface-labeled glycosphingolipids and aspecific ceramide tetrasaccharide for transformants. J Biol Chem. 1975 Apr 10;250(7):2438–2446. [PubMed] [Google Scholar]
- Gold P., Freedman S. O. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965 Sep 1;122(3):467–481. doi: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hakomori S. I., Koscielak J., Bloch K. J., Jeanloz R. W. Immunologic relationship between blood group substances and a fucose-containing glycolipid of human adenocarcinoma. J Immunol. 1967 Jan;98(1):31–38. [PubMed] [Google Scholar]
- Hakomori S. Glycolipids of tumor cell membrane. Adv Cancer Res. 1973;18:265–315. doi: 10.1016/s0065-230x(08)60755-1. [DOI] [PubMed] [Google Scholar]
- Häkkinen I. A-like blood group antigen in gastric cancer cells of patients in blood groups Q or B. J Natl Cancer Inst. 1970 May;44(5):1183–1193. [PubMed] [Google Scholar]
- Karlsson K. A., Pascher I., Pimlott W., Samuelsson B. E. Use of mass spectrometry for the carbohydrate composition and sequence analysis of glycosphingolipids. Biomed Mass Spectrom. 1974 Feb;1(1):49–56. doi: 10.1002/bms.1200010111. [DOI] [PubMed] [Google Scholar]
- Kawanami J. The appearance of Forssman hapten in human tumor. J Biochem. 1972 Sep;72(3):783–785. doi: 10.1093/oxfordjournals.jbchem.a129960. [DOI] [PubMed] [Google Scholar]
- LEVINE P., BOBBITT O. B., WALLER R. K., KUHMICHEL A. Isoimmunization by a new blood factor in tumor cells. Proc Soc Exp Biol Med. 1951 Jul;77(3):403–405. doi: 10.3181/00379727-77-18794. [DOI] [PubMed] [Google Scholar]
- Levine P. Illegitimate blood group antigens P1, A, and MN (T) in malignancy-a possible therapeutic approach with anti-Tja, anti-A, and anti-T. Ann N Y Acad Sci. 1976;277(00):428–435. doi: 10.1111/j.1749-6632.1976.tb41719.x. [DOI] [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Sakiyama H., Gross S. K., Robbins P. W. Glycolipid synthesis in normal and virus-transformed hamster cell lines. Proc Natl Acad Sci U S A. 1972 Apr;69(4):872–876. doi: 10.1073/pnas.69.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui B., Hakomori S. A revised structure for the Forssman glycolipid hapten. J Biol Chem. 1971 Sep 25;246(18):5766–5769. [PubMed] [Google Scholar]
- Six H. R., Young W. W., Jr, Uemura K., Kinsky S. C. Effect of antibody-complement on multiple vs. single compartment liposomes. Application of a fluorometric assay for following changes in liposomal permeability. Biochemistry. 1974 Sep 10;13(19):4050–4058. doi: 10.1021/bi00716a037. [DOI] [PubMed] [Google Scholar]
- Stein-Douglas K., Schwarting G. A., Naiki M., Marcus D. M. Gangliosides as markers for murine lymphocyte subpopulations. J Exp Med. 1976 Apr 1;143(4):822–832. doi: 10.1084/jem.143.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellner K., Saito H., Hakomori S. I. Determination of aminosugar linkages in glycolipids by methylation. Aminosugar linkages of ceramide pentasaccharides of rabbit erythrocytes and of Forssman antigen. Arch Biochem Biophys. 1973 Apr;155(2):464–472. doi: 10.1016/0003-9861(73)90138-0. [DOI] [PubMed] [Google Scholar]
- Stellner K., Watanabe K., Hakomori S. Isolation and characterization of glycosphingolipids with blood group H specificity from membranes of human erythrocytes. Biochemistry. 1973 Feb;12(4):656–661. doi: 10.1021/bi00728a014. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Esselman W. J., Sweeley C. C. Structure of a pentahexosylceramide (Forssman hapten) from canine intestine and kidney. J Biol Chem. 1973 Sep 25;248(18):6528–6533. [PubMed] [Google Scholar]
- Taketomi T., Hara A., Kawamura N., Hayashi M. Further investigations on the chemical structure of Forssman globoside obtained from caprine erythrocyte stroma. J Biochem. 1974 Jan;75(1):197–199. doi: 10.1093/oxfordjournals.jbchem.a130376. [DOI] [PubMed] [Google Scholar]
- Weissmann B., Hinrichsen D. F. Mammalian alpha-acetylgalactosaminidase. Occurrence, partial purification, and action on linkages in submaxillary mucins. Biochemistry. 1969 May;8(5):2034–2043. doi: 10.1021/bi00833a038. [DOI] [PubMed] [Google Scholar]
- Yu R. K., Ledeen R. W. Gangliosides of human, bovine, and rabbit plasma. J Lipid Res. 1972 Sep;13(5):680–686. [PubMed] [Google Scholar]
- Ziolkowski C. H., Fraser B. A., Mallette M. F. Sheep erythrocyte Forssman hapten, an isohapten system: composition of the ceramide. Immunochemistry. 1975 Apr;12(4):297–302. doi: 10.1016/0019-2791(75)90179-2. [DOI] [PubMed] [Google Scholar]