1. Background
Sialic acid (Sia) linked glycoproteins are the classical influenza receptors for influenza virus haemagglutinin to bind. The distribution of Sia on cell surfaces is one of the determinants of host tropism and understanding its expression on human cells and tissues is important for understanding influenza pathogenesis. Previous research has shown the differences in apical versus basolateral infection and release of different influenza virus from polarized epithelial cells [1] and correlated this with sialic acid distribution in the human respiratory tract. Moreover, mass spectrometric analysis was recently employed to elucidate the glycans present in the tissue in a higher resolution in human lung [2]. The objective of this study was to examine in detail the distribution of these Sia-linked glycans at the cellular level by the use of confocal microscopy.
2. Materials and methods
Human primary type I-like and type II pneumocytes were isolated from human non-tumor lung tissue by tissue fragmentation, percoll density gradient centrifugation and magnetic cell sorting [1]. The cells were seeded on coverslips and maintained in small airway growth medium. When confluence was reached, cell monolayers were fixed with 4% paraformaldehyde. We used the plant lectins, Sambucus nigra agglutnin (SNA) from Roche which binds to Siaα2-6Gal, Maackia amurensis agglutinin (MAA)I and MAAII from Vector Lab which bind the Siaα2-3Gal linked glycans using Vector Red as fluorescent chromogen. The cells were counter-stained with DAPI and either with FITC-conjugated antibody against endoplasmic recticulum (Protein Disulfide-Isomerase PDI). The cells were imaged with multi-photon excitation laser scanning microscopy using Zeiss 510 LSM. The optical cross-section pictures were reconstructed by Zeiss LSM510 META.
3. Results
We found that there was more binding of MAAI and MAAII to type II pneumocytes than type I-like pneumocytes and more overall binding of these lectins than binding of SNA (Fig.1.). In keeping with results from other polarized cells there was more binding to the apical than basolateral aspect thus explaining the previously published data on apical versus basolateral infection [1]. As sialic acid has been implicated in the targeting of proteins to the surface, the relative lack of sialic acid on the basolateral aspect can explain why there is little seasonal influenza virus dissemination to the systemic circulation in human infections. Furthermore, though there was little binding of SNA to the apical or basolateral aspects of the pneumocytes, the experimental findings of infection by influenza H3N2 virus that has a strict Siaα2-6Gal tropism [3] suggests that there are Siaα2-6Gal glycans present which are not readily bound by the lectin SNA.
Figure 1.
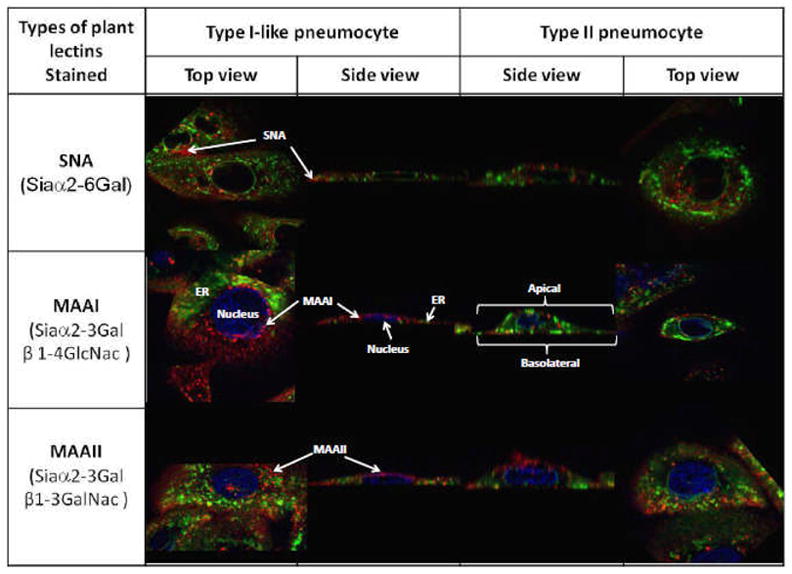
Primary human type I-like and type II pneumocytes stained with lecins (red), pdi (green) and DAPI (blue) and imaged captured with confocal microscope
4. Discussion
The in vitro model of primary human type I-like and type II pneumocytes system formed a polarized epithelium that has a similar lectin distribution to human alveoli in vivo which demonstrated that it is a physiologically relevant model to study the tropism and pathogenesis of influenza A virus.
5. References
- 1.Chan MC, Chan RW, Yu WC, Ho CC, Chui WH, Lo CK, Yuen KM, Guan YI, Nicholls JM, Peiris JS. Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells. Respir Res. 2009;10:102. doi: 10.1186/1465-9921-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan RW, Chan MC, Wong AC, Karamanska R, Dell A, Haslam SM, Sihoe AD, Chui WH, Triana-Baltzer G, Li Q, Peiris JS, Fang F, Nicholls JM. DAS181 inhibits H5N1 influenza virus infection of human lung tissues. Antimicrob Agents Chemother. 2009;53(9):3935–41. doi: 10.1128/AAC.00389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumari Kshama, Gulati Shelly, Smith David F, Gulati Upma, Cummings Richard D, Air Gillian M. Receptor binding specificity of recent human H3N2 influenza viruses. Virology Journal. 2007;4:42. doi: 10.1186/1743-422X-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]


