Abstract
A subset of patients with glioblastoma (GBM) have butterfly GBM (bGBM) that involve both cerebral hemispheres by crossing the corpus callosum. The prognoses, as well as the effectiveness of surgery and adjuvant therapy, are unclear because studies are few and limited. The goals of this study were to: (1) determine if bGBM have worse outcomes than patients with non-bGBM, (2) determine if surgery is more effective than biopsy, and (3) identify factors independently associated with improved outcomes for these patients. Adult patients who underwent surgery for a newly diagnosed primary GBM at an academic tertiary-care institution between 2007 and 2012 were retrospectively reviewed and tumors were volumetrically measured. Of the 336 patients with newly diagnosed GBM who were operated on, 48 (14 %) presented with bGBM, where 29 (60 %) and 19 (40 %) underwent surgical resection and biopsy, respectively. In multivariate analysis, a bGBM was independently associated with poorer survival [HR (95 % CI) 1.848 (1.250–2.685), p < 0.003]. In matched- pair analysis, patients who underwent surgical resection had improved median survival than biopsy patients (7.0 vs. 3.5 months, p = 0.03). In multivariate analysis, increasing percent resection [HR (95 % CI) 0.987 (0.977–0.997), p = 0.01], radiation [HR (95 % CI) 0.431 (0.225–0.812), p = 0.009], and temozolomide [HR (95 % CI) 0.413 (0.212–0. 784), p = 0.007] were each independently associated with prolonged survival among patients with bGBM. This present study shows that while patients with bGBM have poorer prognoses compared to non-bGBM, these patients can also benefit from aggressive treatments including debulking surgery, maximal safe surgical resection, temozolomide chemotherapy, and radiation therapy.
Keywords: Butterfly, Corpus callosum, GBM, Glioblastoma, Radiation, Survival, Temozolomide
Introduction
Patients with glioblastoma (GBM) are known to have poor survival [1–6]. Among these patients, it is argued that butterfly GBM (bGBM), which are those that cross the corpus callosum and involve both cerebral hemispheres, has the worst prognoses [7–9]. This assumption, however, is primarily based on case reports [8, 10–20]. Because of the relative rarity of these lesions, large volume studies do not exist (Table 1) [8, 10–20]. As a result, it remains poorly understood if patients with bGBM really do have poorer outcomes and, more importantly, if current therapies including aggressive resection, radiation, and temozolomide are effective for these lesions [7].
Table 1.
Summary of non-case report studies on patients with butterfly glioblastoma (GBM)
| Studies | Year | No. butterfly patients |
Compared butterfly vs. non-butterfly GBM |
Evaluated surgery vs. biopsy of a butterfly GBM |
Volumetric studies |
Included different grade tumors |
|---|---|---|---|---|---|---|
| Present study | 2013 | 48 | Yes | Yes | Yes | No |
| Dziurzynski et al. | 2012 | 23 | No | No | No | No |
| Ramakrishna et al. | 2010 | 10 | No | No | No | No |
| Balana et al. | 2007 | 13 | No | No | No | Yes |
| Chang et al. | 2003 | 13 | No | No | No | Yes |
| Stelzer et al. | 1997 | 32 | No | No | No | Yes |
| Devaux et al.a | 1993 | 13 | No | No | No | Yes |
Only looked at corpus callosum involvement
The goals of this study were to: (1) determine if there are clinical differences between patients with and without bGBM, (2) determine if outcomes are different for patients with bGBM who underwent needle biopsy or surgical resection, and (3) identify factors associated with improved outcomes among patients with bGBM. An understanding of these features may help guide treatment regimens aimed at optimizing outcomes for patients with this subset of GBM.
Methods
Patient selection and recorded variables
Institutional review board approval was obtained prior to the start of this study. All adult patients (>18 years) who underwent surgery for a newly diagnosed intracranial GBM from January 2007 to July 2012 were included based on an institutional brain tumor registry. Determination of a GBM was made by a senior neuropathologist according to the World Health Organization (WHO) classification system [21, 22]. Patients with prior resections, previous lower grade gliomas (based on previous surgeries with pathology revealing a lower grade glioma as well as lesions that underwent contrast enhancement changes while being monitored with serial radiographic imaging), multifocal lesions, and infratentorial lesions were excluded.
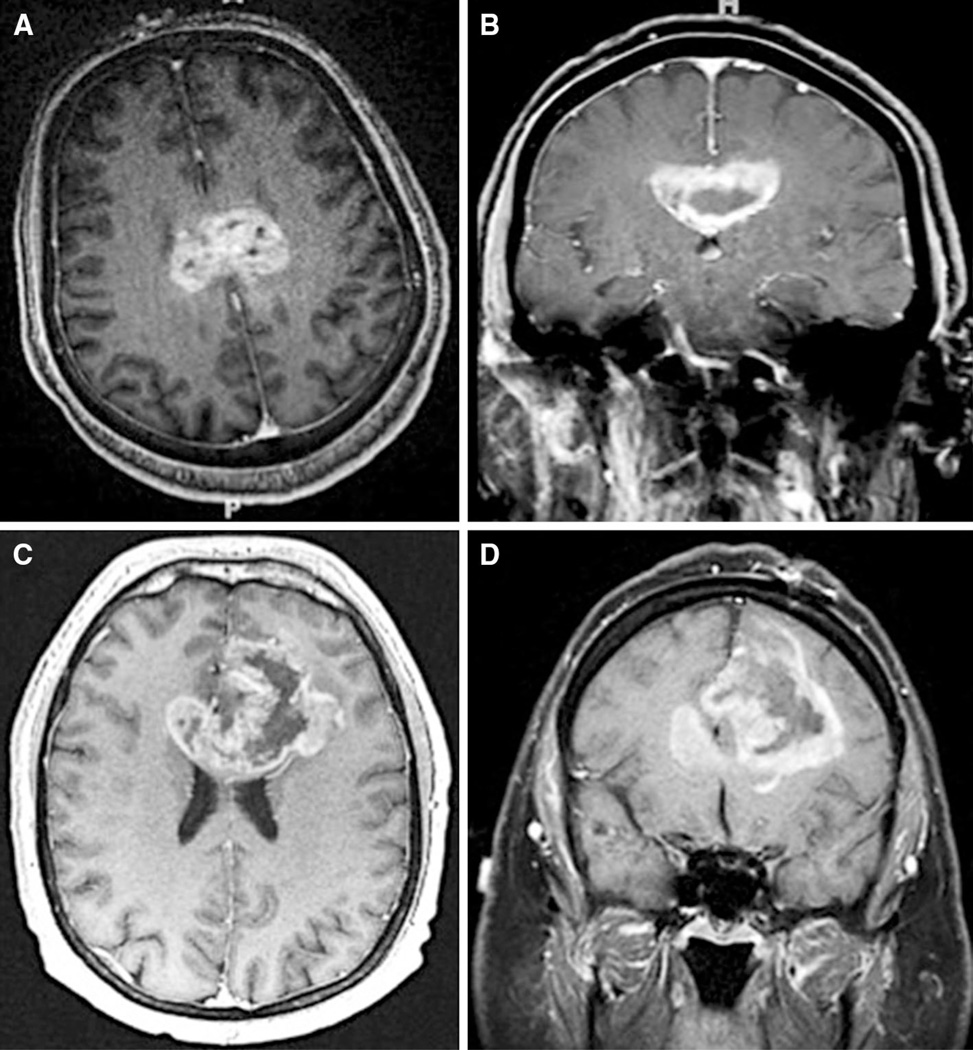
The clinical records of all included patients were retrospectively reviewed using an institutional electronic patient record database. A bGBM was defined as a contrast- enhancing lesion spanning both hemispheres via the corpus callosum on radiographic imaging (Fig. 1). Tumors with edema but not contrast-enhanced tumor crossing the corpus callosum were not classified as a bGBM. With regard to radiographic characteristics, the pre and postoperative MRIs were obtained and reviewed for each patient. The pre and postoperative tumor volumes were measured using T1-weighted gadolinium-enhanced MRI (1.5–3 mm axial cuts) obtained within 48 h of surgery (OsiriX, Los Angeles, CA, USA) as previously described [23, 24]. For butterfly lesions, tumor volumes in the right and left hemispheres were also measured. The volume of blood products rather than residual tumor on postoperative imaging was confirmed by comparing T1-weighted gadolinium- enhanced and non-enhanced MRIs. Percent resection was calculated using the following formula: (preoperative − postoperative tumor volume)/preoperative tumor volume. The date of death was obtained using the social security index database [25]. Time to death was the time from surgery to death. Patients whose deaths were unconfirmed were censored at the time of their last clinic visit.
Fig. 1.
Examples of patients with bGBMs that cross the corpus callosum. a, b A 46-year old patient presented with headaches as well as confusion and memory loss, and had evidence of a bGBM that crosses the body of the corpus callosum and underwent surgical debulking. c, d A patient who is 58 years old and presented with headaches and seizures and had evidence of a bGBM that crossed the genus of the corpus callosum and underwent surgical debulking
General treatment strategy
The choice of surgical resection versus needle biopsy was typically based on the discretion of the surgeon. Motor and somatosensory evoked potentials and surgical navigation were typically used for tumors near motor and/or somatosensory cortex. The use of other surgical adjuncts (i.e. cortical and subcortical mapping, ultrasound, and functional imaging) was based on surgeon preference. In the majority of cases, the primary goal was to debulk the eccentric portion of the bGBM and not to resect the tumor involving the corpus callosum. In general, a unilateral craniotomy was done with the primary intent of debulking based on the side of maximal tumor bulk and/or mass effect. For lesions with symmetric bilateral involvement, the non-dominant side was typically chosen for the approach. Tumor was debulked on the contralateral side using a unilateral approach if the tumor was deemed accessible by the operating surgeon. The use and choice of adjuvant radiation and/or chemotherapy was determined by a multi-disciplinary team, including the surgeon, radiation oncologist, oncologist, and the patients themselves.
Statistical analysis
Summary data were presented as mean ± standard deviation and median [interquartile range (IQR)] for parametric and non-parametric data, respectively. The Student’s t test and Fisher exact test were used to make inter-group comparison for continuous and categorical data, respectively. A multivariate proportional hazards regression analysis was used to identify whether a butterfly location was independently associated with worse outcomes for patients with GBM after controlling for factors previously known to be associated with survival. Matched pair analyses were also used to compare survival for patients with and without bGBM, as well as bGBM patients who underwent biopsy and debulking surgical resection. Stepwise multivariate proportional hazards regression analyses were used to identify independent associations with survival. For surgical resection, percent resections were dichotomized in 5 % intervals, and separate stepwise multivariate proportional hazards regression analyses were done to find the percent resection most significantly associated with decreased hazards of death. Values with p < 0.05 in these analyses were considered statistically significant. Overall survival was plotted using the Kaplan–Meier method, and Log-rank analysis was used to compare Kaplan–Meier plots (GraphPad Prism 5, La Jolla, CA, USA). JMP9 (SAS, Cary, NC, USA) was used unless otherwise specified.
Results
Pre, peri, and postoperative patient characteristics of butterfly and non-butterfly patients
The pre, peri, and postoperative characteristics of the 336 patients with newly diagnosed bGBM and non-bGBM are summarized in Table 2. 48 (14 %) patients presented with a bGBM. The average age of all (bGBM and non-bGBM) patients was 60.5 ± 13.9 years, and 205 (61 %) were male. The median [IQR] KPS prior to surgery was 80 [70–90], and 96 (29 %) presented with seizures, 127 (38 %) presented with headaches, 170 (51 %) with motor deficits, 95 (28 %) with language deficits, 48 (14 %) with vision deficits, and 119 (35 %) with confusion/memory loss. The median [IQR] pre and postoperative contrast-enhancing tumor volume was 30.1 [14.1–56.4] and 3.0 [0.3–12.3] cm3, respectively. This equated to a mean ± - SEM percent resection of 71.4 ± 2.0 %.
Table 2.
Pre, peri, and postoperative characteristics of all patients undergoing surgery of a newly diagnosed glioblastoma (GBM) from January 1997 to July 2012
| Characteristics | bGBM biopsy number (%) N = 19 |
bGBM resection number (%) N = 29 |
Non-bGBM surgery number (%) N = 288 |
All GBM number (%) N = 336 |
bGBM vs. non-bGBM p value |
|---|---|---|---|---|---|
| Study population (n = 336) | |||||
| Agea | 54.2 ± 17.9 | 61.7 ± 12.8 | 60.7 ± 13.7 | 60.5 ± 13.9 | 0.35 |
| Male | 11 (58 %) | 14 (48 %) | 180 (63 %) | 205 (61 %) | 0.54 |
| KPSc | 80 (80–90) | 80 (70–80) | 80 (70–90) | 80 (70–90) | 0.65 |
| Pre-operative symptoms | |||||
| Seizures | 1 (5 %) | 3 (10 %) | 92 (32 %) | 96 (29 %) | 0.0005 |
| Headaches | 12 (63 %) | 8 (28 %) | 107 (37 %) | 127 (38 %) | 0.63 |
| Nausea/vomiting | 4 (21 %) | 2 (7 %) | 28 (10 %) | 34 (10 %) | 0.60 |
| Motor deficit | 6 (32 %) | 14 (48 %) | 150 (33 %) | 170 (51 %) | 0.21 |
| Language deficit | 1 (5 %) | 7 (24 %) | 87 (30 %) | 95 (28 %) | 0.05 |
| Visual deficit | 3 (16 %) | 6 (21 %) | 39 (14 %) | 48 (14 %) | 0.37 |
| Confusion/memory loss | 13 (68 %) | 19 (66 %) | 87 (30 %) | 119 (35 %) | 0.0001 |
| Radiographics | |||||
| Tumor vol (cm3)c | 39.2 (24.0–59.9) | 45.1 (28.5–75.3) | 28.8 (13.0–54.2) | 30.1 (14.1–56.4) | 0.007 |
| Surgical variables | |||||
| Needle biopsy | 19 (100 %) | 0 (0 %) | 29 (10 %) | 48 (14 %) | 0.0001 |
| Postop tumor vol (cm3)c | 39.2 (24.0–59.9) | 15.5 (5.8–25.0) | 2.3 (0.0–7.9) | 3.0 (0.3–12.3) | 0.0001 |
| Percent resection (%)b | 0 ± 0 % | 61.4 ± 4.9 % | 77.9 ± 1.9 % | 71.4 ± 2.0 % | 0.0001 |
| Perioperative variables | |||||
| Motor deficit | 3 (16 %) | 3 (10 %) | 25 (9 %) | 31 (9 %) | 0.442 |
| Language deficit | 1 (5 %) | 1 (3 %) | 11 (4 %) | 13 (4 %) | 0.99 |
| Vision deficit | 0 (0 %) | 1 (3 %) | 14 (5 %) | 15 (4 %) | 0.71 |
| Hospital stay (days)c | 7 (4–11) | 8 (5–9) | 4 (3–7) | 4 (3–8) | 0.21 |
| Adjuvant therapy | |||||
| Temozolomide | 13 (68 %) | 16 (55 %) | 198 (69 %) | 227 (68 %) | 0.99 |
| Radiation therapy | 13 (68 %) | 19 (66 %) | 209 (73 %) | 241 (72 %) | 0.39 |
| Survival | |||||
| Died at last follow-up | 15 (79 %) | 26 (90 %) | 228 (79 %) | 269 (80 %) | 0.43 |
| Median survival (months) | 4.2 | 6.4 | 12.5 | 12.1 | 0.0001 |
| 6-month survival rate (%, N) | 7 (39 %) | 15 (52 %) | 199 (75 %) | 219 (71 %) | |
| 12-month survival rate (%, N) | 5 (32 %) | 5 (16 %) | 141 (55 %) | 150 (51 %) | |
| 18-month survival rate (%, N) | 2 (9 %) | 1 (0 %) | 68 (31 %) | 69 (27 %) |
Groups are separated by those who underwent biopsy of a bGBM (n = 19), surgical resection of a bGBM (n = 29), surgery for a non-bGBM (n = 288), and all GBM (n = 336)
KPS Karnofsky performance score
Bold values indicate p < 0.05
Mean ± standard deviation;
mean ± standard error of the mean;
median (interquartile range)
Following surgery, 31 (9 %), 13 (4 %), and 15 (4 %) incurred a new motor, language, and vision deficit, respectively. At last follow-up, 227 (68 %) underwent temozolomide chemotherapy and 241 (72 %) underwent radiation therapy. 224 (67 %) underwent temozolomide/radiation therapy according to the Stupp protocol [26]. Among those patients who received temozolomide/radiation therapy, the mean ± standard error of the mean (SEM) of adjuvant temozolomide cycles was 1.1 ± 0.6. Of the patients who did not undergo temozolomide/radiation chemotherapy, 48 (14 %) underwent other types of chemotherapy, 27 (8 %) were determined to not be candidates for chemotherapy (i.e. thrombocytopenia, poor functional status, etc.), and 34 (10 %) were lost to follow-up and may have had their adjuvant therapy at another hospital and their records were not available for review. 269 (80 %) patients died at last follow-up, where the median survival was 12.1 months. The 6, 12, and 18-month survival rates were 71, 51, and 27 %, respectively. The median [IQR] follow-up time for surviving patients was 10.5 [1.0–17.3] months.
Differences between patients with and without butterfly GBM
The differences between patients undergoing surgery for a bGBM and non-bGBM are summarized in Table 2. Preoperatively, patients undergoing surgery for a bGBM less frequently presented with seizures (p = 0.0005) and language deficits (p = 0.05), and more frequently confusion and/or memory loss (p = 0.0001). Moreover, patients with bGBM typically had larger tumors (p = 0.007). Among patients with bGBM, 48.5 ± 4.1 % of the volume was on the left and 45.9 ± 4.7 % on the right, and 4 (8 %) involved the motor cortex. There were no differences between the cohorts in regards to age, gender, KPS, and other presenting symptoms. Perioperatively, patients with bGBM more frequently underwent needle biopsy (p = 0.0001), had larger postoperative volumes (p = 0.0001), and underwent less percent resection (p = 0.0001). There were no statistical differences between patients with and without a bGBM in regards to development of iatrogenic deficits, length of hospital stay, and use of adjuvant therapies.
Association between a butterfly glioblastoma and survival
In multivariate analysis, after controlling for factors previously shown to be associated with survival (age [1, 4, 27, 28], KPS [1, 4, 27, 28], extent of resection [23], temozolomide [26, 29], and radiation [30]), a bGBM was independently associated with poorer survival [HR (95 % CI) 1.848 (1.250–2.685), p < 0.003] (Table 3). The median survival for patients who underwent surgery of a bGBM was significantly shorter than non-bGBM patients (5.1 vs. 12.5 months, p < 0.0001) (Fig. 2a). Moreover, in order to account for differences in confusion/memory loss between patients with and without bGBM, a butterfly location remained significantly associated with poorer survival [HR (95 % CI) 1.704 (1.140–2.504), p = 0.005). This also remained true after controlling for other preoperative differences between patients with and without bGBM including seizures and language deficits [HR (95 % CI) 1.554 (1.064–2.844), p = 0.01).
Table 3.
Independent associations with survival
| Variables | Hazards ratio (95 % CI) | p value |
|---|---|---|
| Butterfly tumor location and survival for all GBM | ||
| Butterfly tumor location | 1.848 (1.250–2.685) | 0.003 |
| Factors controlled for in multivariate analysis | ||
| Increasing age | 1.026 (1.018–1.037) | <0.0001 |
| Increasing KPS | 0.984 (0.973–0.996) | 0.008 |
| Extent of resection | 0.993 (0.989–0.997) | 0.002 |
| Radiation therapy | 0.879 (0.477–1.545) | 0.67 |
| Temozolomide chemotherapy | 0.486 (0.281–0.895 | 0.02 |
| Factors associated with survival among only butterfly GBM patients | ||
| Increasing percent resection | 0.987 (0.977–0.997) | 0.01 |
| >65 % resection | 0.397 (0.179–0.821) | 0.01 |
| Postoperative radiation | 0.431 (0.225–0.812) | 0.009 |
| Temozolomide chemotherapy | 0.413 (0.212–0.784) | 0.007 |
Independent association of a butterfly tumor location with survival for adult patients who underwent surgery of a newly diagnosed intracranial glioblastoma (GBM) after controlling for perioperative variables previously shown to be associated with survival (age, Karnofsky performance score, extent of resection, temozolomide chemotherapy, and radiation therapy). Factors independently associated with prolonged survival for patients who underwent surgical resection of a butterfly glioblastoma (GBM). >65 % resection had the greatest decrease in hazards ratio among percent resection
Bold values indicate p < 0.05
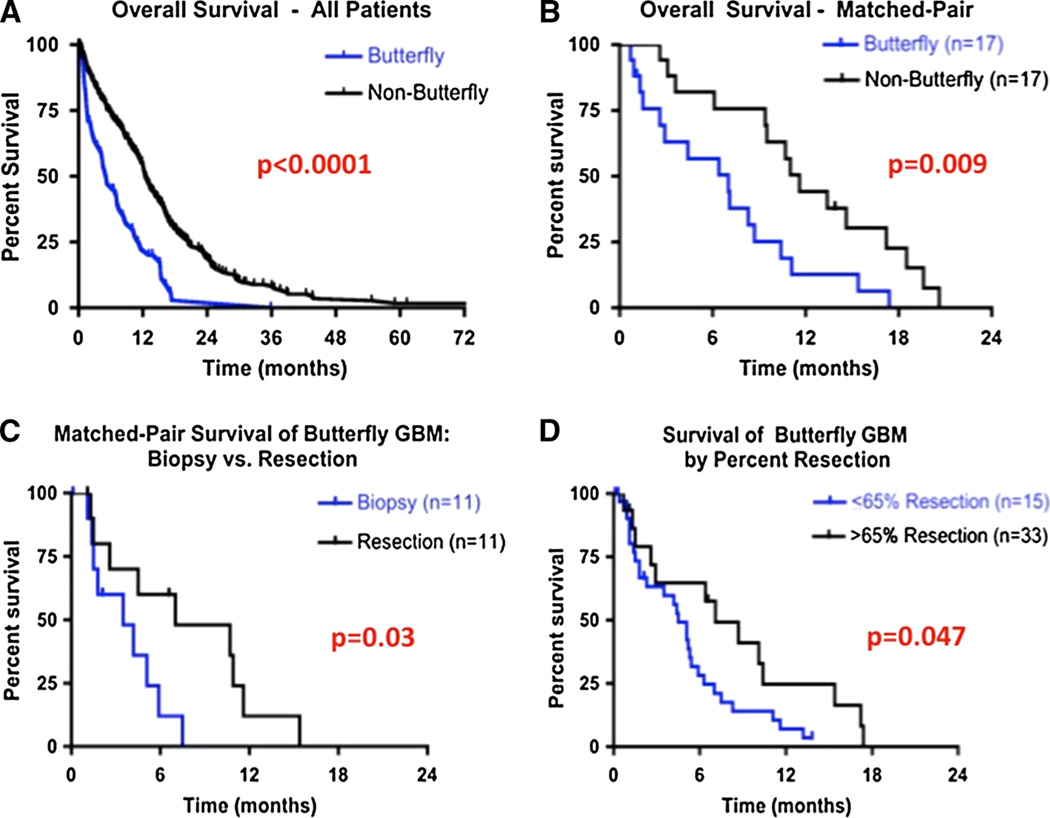
Fig. 2.
Overall survival curves for patients with glioblastoma (GBM). a, b Overall survival for patients who underwent surgery of a newly diagnosed butterfly (bGBM) and non-butterfly glioblastoma (nonbGBM). a Overall survival of all GBM patients. The median survival for patients who underwent surgery of a bGBM was significantly shorter than patients who underwent surgery of a non-bGBM (5.1 vs. 12.5 months, p < 0.0001). The 6-and 12-month survival rates for patients with bGBM were 47 and 22 % as compared to 75 and 55 % for patients with non-bGBM, respectively. b Overall survival of bGBM patients matched with non-bGBM. In order to control for potential pre and perioperative differences, groups were matched for age (±5 years), KPS (±10 points), preoperative tumor size (±5 cm3), eloquent cortex involvement (yes/no), extent of resection (±5 %), temozolomide (yes/no), and radiation (yes/no). The median survival for patients with bGBM (n = 17) was significantly shorter than matched patients with non-bGBM (n = 17) (7.0 vs. 11.6 months, p = 0.009). c, d Overall survival for bGBM patients by increasing surgical resection. c Overall survival of bGBM patients who underwent debulking surgery matched to patients who underwent biopsy. Groups were matched for age (±5 years), KPS (±10 points), preoperative tumor size (±5 cm3), percent tumor on contralateral side (±5 %), motor cortex involvement (yes/no), temozolomide chemotherapy (yes/no), and radiation therapy (yes/no). The median survival for patients who underwent surgical resection (n = 11) was significantly longer than for patients who underwent biopsy of a bGBM (n = 11) (7.0 vs. 3.5 months, p = 0.03). The 6- and 12-month survival rates for patients who underwent surgical resection of a bGBM were 60 and 12 % as compared to 12 and 0 % for matched patients who underwent needle biopsy, respectively. d Survival of bGBM patients who underwent >65 % resection as compared to patients who underwent ≤65 % resection. The median survival for patients who underwent >65 % percent resection (n = 15) was significantly longer than patients who underwent ≤65 % percent resection (n = 33) (7.1 vs. 4.5 months, p = 0.047). The 6- and 12-month survival rates for patients who underwent >65 % resection were 64.6 and 24.6 % as compared to 28.1 and 7.0 % who underwent ≤65 % resection, respectively
In order to further account for potential pre and perioperative differences that could contribute to differences in outcomes, matched pair analyses were also conducted. Groups were matched for age (±5 years), KPS (±10), preoperative tumor size (±5 cm3), eloquent cortex involvement (yes/no), extent of resection (±5 %), temozolomide chemotherapy (yes/no), and radiation therapy (yes/no). In this matched-pair analysis, the median survival for patients with bGBM (n = 17) was significantly shorter than matched patients with non-bGBM (n = 17) (7.0 vs. 11.6 months, p = 0.009) (Fig. 2b). The 6- and 12-month survival rates for patients who underwent biopsy of a bGBM were 12 % (n = 2) and 0 % (n = 1), respectively, while the 6- and 12-month survival rates for patients who underwent resection of a bGBM were 60 % (n = 7) and 12 % (n = 2), respectively. In sub-group analysis, there were no significant differences in the number of adjuvant temozolomide cycles between patients with and without bGBM in this matched-pair analysis (0.4 ± 0.3 vs. 0.7 ± 0.5, p = 0.45).
Impact of surgical resection versus biopsy for patients with butterfly GBM
Among patients with bGBM, 29 (60 %) underwent debulking surgical resection and 19 (40 %) underwent needle biopsy. In order to understand the effect of surgery for patients with bGBM, a matched pair analysis was conducted to assess potential differences in surgical selection and survival between patients who underwent needle biopsy and surgical resection.
Groups were matched for age (±5 years), KPS (±10), preoperative tumor volume (±5 cm3), percent tumor on contralateral side (±5 %), motor cortex involvement (yes/no), temozolomide (yes/no), and radiation (yes/no). The median survival for patients who underwent surgical resection (n = 11) was significantly longer than for patients who underwent biopsy (n = 11) of a bGBM (7.0 vs. 3.5 months, p = 0.03) (Fig. 2c). In sub-group analyses for these matched pairs, there was no significant difference in the number of adjuvant temozolomide cycles between patients who underwent needle and surgical resection of a bGBM (1.0 ± 0.6 vs. 1.5 ± 0.3, p = 0.48).
Factors independently associated with improved outcomes for patients with butterfly GBM
In univariate analysis, the factors associated with prolonged survival for patients who underwent surgery of a bGBM were: increasing percent of resection, radiation, and temozolomide. In multivariate analysis, increasing percent resection [HR (95 % CI) 0.987 (0.977–0.997), p = 0.01], radiation [HR (95 % CI) 0.431 (0.225–0.812), p = 0.009], and temozolomide [HR (95 % CI) 0.413 (0.212–0. 784), p = 0.007] remained significantly associated with prolonged survival (Table 3).
Among percent resection, resection > 65 % had the most significantly decreased hazards of death [HR (95 % CI) 0.397 (0.179–0.821), p < 0.01]. In Log-Rank analysis, patients who underwent >65 % percent resection (n = 15) had significantly longer median survival than patients who underwent ≤65 % percent resection (n = 33) (7.1 vs. 4.5 months, p = 0.047) (Fig. 2d).
Discussion
In this study, 48 (14 %) patients with newly diagnosed GBM presented with a bGBM. Among patients who underwent surgery of a newly diagnosed GBM at our institution, patients with bGBM typically had larger tumors, more frequently underwent needle biopsy, and had less extensive resection. In multivariate and matched-pair analyses, a bGBM was independently associated with poorer survival, with a median survival 60 % less than matched patients with non-bGBM. In regards to identifying the optimal treatment for patients with bGBM, patients who underwent debulking surgical resection had a twofold improved median survival as compared to matched patients who underwent needle biopsy (7.0 vs. 3.5 months). Patients with bGBM who underwent increasing percent resection, postoperative radiation, and temozolomide chemotherapy had the highest likelihood of prolonged survival.
Butterfly GBM may represent a distinct clinical subset of GBM. These tumors involve the bilateral hemispheres by invading the corpus callosum [31]. While the majority of outcome studies on GBM show that younger age, higher preoperative functional status, increased resection, radiation, and temozolomide are associated with improved outcomes [1, 2, 4–6, 27, 28, 32–39], these findings may not be applicable to bGBM. bGBM can be larger, more difficult to resect, and associated with more morbidity than GBM located elsewhere [7, 10, 12, 15, 40]. As a result, the majority of bGBM patients in previous studies have undergone needle biopsy rather than surgical resection and been withheld adjuvant therapies [7, 9, 10, 12, 15, 18, 41]. This withholding of aggressive therapies for bGBM patients may be because these tumors are assumed to have poorer prognoses than GBM in other locations, and aggressive treatment may be considered futile. This assumption may be erroneous because it is primarily based on anecdotal data [10–17].
Previous studies on bGBM are limited [7, 8, 10–17, 19, 20]. Studies on bGBM have combined this subset of patients into the same cohort of patients with non-bGBM even though they only account for 3–15 % of patients [1, 27, 38, 42, 43]. The majority of studies limited to only patients with bGBM are small case series [10–17] aimed to report rare presenting symptoms including catatonia [11], muscle atrophy [13], neuropsychological deficits [14], and leptomeningeal metastases [16]. To date, there have been few previous non-case report-based studies limited to bGBM patients (Table 1) [7, 9, 18, 20, 41]. Stelzer et al. in 1997 evaluated 105 patients, where 32 patients had contrast-enhancing tumor in the corpus callosum [18]. Younger patients (age < 50) that were high-functioning (KPS > 60) had worse outcomes if they had corpus callosum involvement [18]. However, in this study, volumetric measurements were not made, extent of contralateral disease was not evaluated, adjuvant chemotherapy were not typically given, and the study had mixed histology [18]. Dziurzynski et al. evaluated the outcomes of 23 patients with bGBM, where 12 patients underwent needle biopsy and 11 underwent resection [7]. Only 8 of these patients underwent temozolomide and radiation therapy, and only 11 underwent radiation therapy [7]. Because of the small patient numbers, they were unable to make statistically significant conclusions about the efficacy of aggressive treatments [7]. Therefore, it remains unclear if aggressive treatment is warranted for this small subset of GBM patients with presumed poor prognosis. As a result, the treatment algorithm remains unclear and most patients undergo biopsy to confirm diagnosis, and are not frequently offered radiation and chemotherapy [7, 10–17].
The present study provides several potential important observations about patients with bGBM. Patients with bGBM have a significantly poorer survival than patients with non-bGBM. Despite this poor prognosis among patients with GBM, patients with bGBM can benefit from aggressive treatment. In matched-pair analysis, patients with bGBM who underwent surgical resection had improved survival as compared to patients who underwent needle biopsy. Moreover, in multivariate analysis, increasing resection, temozolomide chemotherapy, and radiation were each independently associated with prolonged survival. This study ultimately shows that despite their poor prognosis, aggressive therapies can improve outcomes for patients with bGBM. Moreover, there was no increase in perioperative deficits for patients who underwent surgery of a bGBM as compared to a non-bGBM.
Strength and limitations
We believe this study provides valuable information for patients with bGBM. First, this is the first study to demonstrate this subset of GBM is clinically distinct than non-bGBM. This has been an assumption by many, but has not been rigorously evaluated. Second, this study shows that debulking surgical resection may result in improved outcomes for patients with bGBM as compared to needle biopsy. Previous studies have been limited by patient numbers to make this evaluation [7, 10–17]. Third, this study shows that increasing percent resection, temozolomide chemotherapy, and radiation therapy are each independently associated with prolonged survival for bGBM patients. Previous studies have yet to make multivariate analyses to evaluate the effectiveness of these therapies in prolonging survival.
This study, however, has some limitations. One limitation is that the tumors in this study did not routinely undergo assessment of molecular markers and genotypes including O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation [44] and isocitrate dehydrogenase 1 (IDH1) mutations [45], as well as molecular sub-classifications including proneural, classical, and mesenchymal [46], which were not routinely done at our institution. These molecular markers and sub-classifications may also be associated with survival, but were not analyzed in this study. Furthermore, this study found that patients who underwent >65 % percent resection had the greatest reduction in the risk of death, which is different than the 98 and 78 % thresholds found in previous studies on all GBM [4, 6]. These previous studies were much higher powered to find the minimum percent resection thresholds because they involved all patients with GBM, not just bGBM [4, 6]. This study was just designed to evaluate the percent resection associated with the greatest reduction in the risk of death among patients with bGBM. Additionally, not all the patients in this study underwent temozolomide and radiation therapy. Some of these patients were poor candidates for adjuvant therapy, while others were lost-to-follow-up and had their adjuvant care at another hospital making it difficult to determine if they received adjuvant therapy. The relevance of this study’s findings may be altered if all patients received uniform treatments. This study is also based on retrospective information, which may create inherent biases associated with patient and treatment selection. It would also benefit from evaluation of other outcome measures in addition to survival including quality of life and neuropsychological assessments, which were not available in our retrospective patient population. However, we tried to create a uniform patient population by utilizing strict inclusion criteria by only including newly diagnosed GBM as well as controlling for potential confounding variables by performing matched-pair and multivariate analyses. We believe our findings offer useful insights for patients with bGBM. Nonetheless, prospective studies, most likely multi-institutional in design, that incorporate other outcome measures including quality of life and neuropsychological assessments are needed.
Conclusions
This present study shows that while patients with bGBM have poorer prognoses compared to non-bGBM, these patients can also benefit from aggressive treatments including debulking surgery, increasing percent resection, temozolomide chemotherapy, and radiation therapy.
Acknowledgments
K.L.C. is funded by a NIH T32 training grant. A.Q.H. is supported by NIH Grant 5R01NS070024.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
Ethical standards This study complies with all the current laws.
Contributor Information
Kaisorn L. Chaichana, Email: kaisorn@jhmi.edu, Department of Neurosurgery, Neuro-Oncology Outcomes Laboratory, The Johns Hopkins Hospital, Johns Hopkins University, 1800 Orleans Street, Zayed 6007B, Baltimore, MD 21202, USA.
Ignacio Jusue-Torres, Department of Neurosurgery, Neuro-Oncology Outcomes Laboratory, The Johns Hopkins Hospital, Johns Hopkins University, 1800 Orleans Street, Zayed 6007B, Baltimore, MD 21202, USA.
Ana Maria Lemos, Department of Neurosurgery, Neuro-Oncology Outcomes Laboratory, The Johns Hopkins Hospital, Johns Hopkins University, 1800 Orleans Street, Zayed 6007B, Baltimore, MD 21202, USA.
Aaron Gokaslan, Department of Neurosurgery, Neuro-Oncology Outcomes Laboratory, The Johns Hopkins Hospital, Johns Hopkins University, 1800 Orleans Street, Zayed 6007B, Baltimore, MD 21202, USA.
Eibar Ernesto Cabrera-Aldana, Department of Neurosurgery, Neuro-Oncology Outcomes Laboratory, The Johns Hopkins Hospital, Johns Hopkins University, 1800 Orleans Street, Zayed 6007B, Baltimore, MD 21202, USA.
Ahmed Ashary, Department of Neurosurgery, Neuro-Oncology Outcomes Laboratory, The Johns Hopkins Hospital, Johns Hopkins University, 1800 Orleans Street, Zayed 6007B, Baltimore, MD 21202, USA.
Alessandro Olivi, Department of Neurosurgery, Neuro-Oncology Outcomes Laboratory, The Johns Hopkins Hospital, Johns Hopkins University, 1800 Orleans Street, Zayed 6007B, Baltimore, MD 21202, USA.
Alfredo Quinones-Hinojosa, Department of Neurosurgery, The Johns Hopkins Hospital, Johns Hopkins University, Cancer Research Building II, 1550 Orleans Street, Room 247, Baltimore, MD 21231, USA, aquinon2@jhmi.edu.
References
- 1.Chaichana K, Parker S, Olivi A, Quinones-Hinojosa A. A proposed classification system that projects outcomes based on preoperative variables for adult patients with glioblastoma multiforme. J Neurosurg. 2010;112:997–1004. doi: 10.3171/2009.9.JNS09805. [DOI] [PubMed] [Google Scholar]
- 2.Chaichana KL, Chaichana KK, Olivi A, Weingart JD, Bennett R, Brem H, Quinones-Hinojosa A. Surgical outcomes for older patients with glioblastoma multiforme: preoperative factors associated with decreased survival. Clinical article. J Neurosurg. 2011;114:587–594. doi: 10.3171/2010.8.JNS1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaichana KL, Halthore AN, Parker SL, Olivi A, Weingart JD, Brem H, Quinones-Hinojosa A. Factors involved in maintaining prolonged functional independence following supratentorial glioblastoma resection. Clinical article. J Neurosurg. 2011;114:604–612. doi: 10.3171/2010.4.JNS091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 5.Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, Lillehei KO, Bernstein M, Brem H, Sloan A, Berger MS, Chang S. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 6.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 7.Dziurzynski K, Blas-Boria D, Suki D, Cahill DP, Prabhu SS, Puduvalli V, Levine N. Butterfly glioblastomas: a retrospective review and qualitative assessment of outcomes. J Neurooncol. 2012;109:555–563. doi: 10.1007/s11060-012-0926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsa AT, Wachhorst S, Lamborn KR, Prados MD, McDermott MW, Berger MS, Chang SM. Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. J Neurosurg. 2005;102:622–628. doi: 10.3171/jns.2005.102.4.0622. [DOI] [PubMed] [Google Scholar]
- 9.Balana C, Capellades J, Teixidor P, Roussos I, Ballester R, Cuello M, Arellano A, Florensa R, Rosell R. Clinical course of high-grade glioma patients with a “biopsy-only” surgical approach: a need for individualised treatment. Clin Transl Oncol. 2007;9:797–803. doi: 10.1007/s12094-007-0142-0. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal A. Butterfly glioma of the corpus callosum. J Cancer Res Ther. 2009;5:43–45. doi: 10.4103/0973-1482.48769. [DOI] [PubMed] [Google Scholar]
- 11.Arora M, Praharaj SK. Butterfly glioma of corpus callosum presenting as catatonia. World J Biol Psychiatry. 2007;8:54–55. doi: 10.1080/15622970600960116. [DOI] [PubMed] [Google Scholar]
- 12.Galldiks N, Schroeter M, Fink GR, Kracht LW. Interesting image. PET imaging of a butterfly glioblastoma. Clin Nucl Med. 2010;35:49–50. doi: 10.1097/RLU.0b013e3181c361e8. [DOI] [PubMed] [Google Scholar]
- 13.Hammersen S, Brock M, Cervos-Navarro J. Adult neuronal ceroid lipofuscinosis with clinical findings consistent with a butterfly glioma. Case report. J Neurosurg. 1998;88:314–318. doi: 10.3171/jns.1998.88.2.0314. [DOI] [PubMed] [Google Scholar]
- 14.Osawa A, Maeshima S, Kubo K, Itakura T. Neuropsychological deficits associated with a tumour in the posterior corpus callosum: a report of two cases. Brain Inj. 2006;20:673–676. doi: 10.1080/02699050600676958. [DOI] [PubMed] [Google Scholar]
- 15.Roche S, Godward S, Middleton A, Lane RJ. Bifrontal glioma presenting as a gross movement disorder. Mov Disord. 1993;8:120–122. doi: 10.1002/mds.870080124. [DOI] [PubMed] [Google Scholar]
- 16.Witoonpanich P, Bamrungrak K, Jinawath A, Wongwaisayawan S, Phudhichareonrat S, Witoonpanich R. Glioblastoma multiforme at the corpus callosum with spinal leptomeningeal metastasis. Clin Neurol Neurosurg. 2011;113:407–410. doi: 10.1016/j.clineuro.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Zakrzewska M, Szybka M, Zakrzewski K, Biernat W, Kordek R, Rieske P, Golanska E, Zawlik I, Piaskowski S, Liberski PP. Diverse molecular pattern in a bihemispheric glioblastoma (butterfly glioma) in a 16-year-old boy. Cancer Genet Cytogenet. 2007;177:125–130. doi: 10.1016/j.cancergencyto.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Stelzer KJ, Sauvé KI, Spence AM, Griffin TW, Berger MS. Corpus callosum involvement as a prognostic factor for patients with high-grade astrocytoma. Int J Radiat Oncol Biol Phys. 1997;38:27–30. doi: 10.1016/s0360-3016(96)00632-3. [DOI] [PubMed] [Google Scholar]
- 19.Matsukado Y, Maccarty CS, Kernohan JW. The growth of glioblastoma multiforme (astrocytomas, grades 3 and 4) in neurosurgical practice. J Neurosurg. 1961;18:636–644. doi: 10.3171/jns.1961.18.5.0636. [DOI] [PubMed] [Google Scholar]
- 20.Devaux BC, O’Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg. 1993;78:767–775. doi: 10.3171/jns.1993.78.5.0767. [DOI] [PubMed] [Google Scholar]
- 21.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl) 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. discussion 226–229. [DOI] [PubMed] [Google Scholar]
- 23.Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, Raza SM, Pascual-Gallego M, Ibrahim A, Hernandez-Hermann M, Gomez L, Ye X, Weingart JD, Olivi A, Blakeley J, Gallia GL, Lim M, Brem H, Quinones-Hinojosa A. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16:113–122. doi: 10.1093/neuonc/not137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaichana KL, Cabrera-Aldana E, Jusue-Torres I, Wijesekera O, Olivi A, Rahman M, Quinones-Hinojosa A. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg. 2014 doi: 10.1016/j.wneu.2014.01.019. (in press) [DOI] [PubMed] [Google Scholar]
- 25.Rootsweb: Social Security Index Database [Google Scholar]
- 26.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 27.Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89:219–224. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 28.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6:227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2008:CD007415. doi: 10.1002/14651858.CD007415. [DOI] [PubMed] [Google Scholar]
- 30.Genc M, Zorlu AF, Atahan IL. Accelerated hyperfractionated radiotherapy in supratentorial malignant astrocytomas. Radiother Oncol. 2000;56:233–238. doi: 10.1016/s0167-8140(00)00198-5. [DOI] [PubMed] [Google Scholar]
- 31.Kallenberg K, Goldmann T, Menke J, Strik H, Bock HC, Stockhammer F, Buhk JH, Frahm J, Dechent P, Knauth M. Glioma infiltration of the corpus callosum: early signs detected by DTI. J Neurooncol. 2013;112:217–222. doi: 10.1007/s11060-013-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaichana KL, Garzon-Muvdi T, Parker S, Weingart JD, Olivi A, Bennett R, Brem H, Quinones-Hinojosa A. Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann Surg Oncol. 2011;18:239–245. doi: 10.1245/s10434-010-1242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaichana KL, Parker SL, Mukherjee D, Cheng JS, Gokaslan ZL, McGirt MJ. Assessment of the extent of surgical resection as a predictor of survival in patients with primary osseous spinal neoplasms. Clin Neurosurg. 2011;58:117–121. doi: 10.1227/neu.0b013e318226fff7. [DOI] [PubMed] [Google Scholar]
- 34.Chaichana KL, Parker SL, Olivi A, Quinones-Hinojosa A. Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. Clinical article. J Neurosurg. 2009;111:282–292. doi: 10.3171/2009.2.JNS081132. [DOI] [PubMed] [Google Scholar]
- 35.Chaichana KL, Zadnik P, Weingart JD, Olivi A, Gallia GL, Blakeley J, Lim M, Brem H, Quinones-Hinojosa A. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg. 2012;118(4):812–820. doi: 10.3171/2012.9.JNS1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaichana KL, Zaidi H, Pendleton C, McGirt MJ, Grossman R, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H. The efficacy of carmustine wafers for older patients with glioblastoma multiforme: prolonging survival. Neurol Res. 2011;33:759–764. doi: 10.1179/1743132811Y.0000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52:371–379. doi: 10.1016/s0090-3019(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 38.McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, Quinones-Hinojosa AR. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 39.McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–469. doi: 10.1227/01.NEU.0000349763.42238.E9. discussion 469–470. [DOI] [PubMed] [Google Scholar]
- 40.Chang SM, Parney IF, McDermott M, Barker FG, 2nd, Schmidt MH, Huang W, Laws ER, Jr, Lillehei KO, Bernstein M, Brem H, Sloan AE, Berger M. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98:1175–1181. doi: 10.3171/jns.2003.98.6.1175. [DOI] [PubMed] [Google Scholar]
- 41.Ramakrishna R, Barber J, Kennedy G, Rizvi A, Goodkin R, Winn RH, Ojemann GA, Berger MS, Spence AM, Rostomily RC. Imaging features of invasion and preoperative and postoperative tumor burden in previously untreated glioblastoma: correlation with survival. Surg Neurol Int. 2010;1 doi: 10.4103/2152-7806.68337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, Wildrick DM. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–1055. doi: 10.1097/00006123-199805000-00054. discussion 1055–1056. [DOI] [PubMed] [Google Scholar]
- 43.Ferroli P, Schiariti M, Finocchiaro G, Salmaggi A, Castiglione M, Acerbi F, Tringali G, Farinotti M, Broggi M, Roberto C, Maccagnano E, Broggi G. Operability of glioblastomas: “sins of action” versus “sins of non-action”. Neurol Sci. 2013;34(12):2107–2116. doi: 10.1007/s10072-013-1345-5. [DOI] [PubMed] [Google Scholar]
- 44.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 45.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerber NK, Goenka A, Turcan S, Reyngold M, Makarov V, Kannan K, Beal K, Omuro A, Yamada Y, Gutin P, Brennan CW, Huse JT, Chan TA. Transcriptional diversity of long-term glioblastoma survivors. Neuro Oncol. 2014;16(9):1186–1195. doi: 10.1093/neuonc/nou043. [DOI] [PMC free article] [PubMed] [Google Scholar]