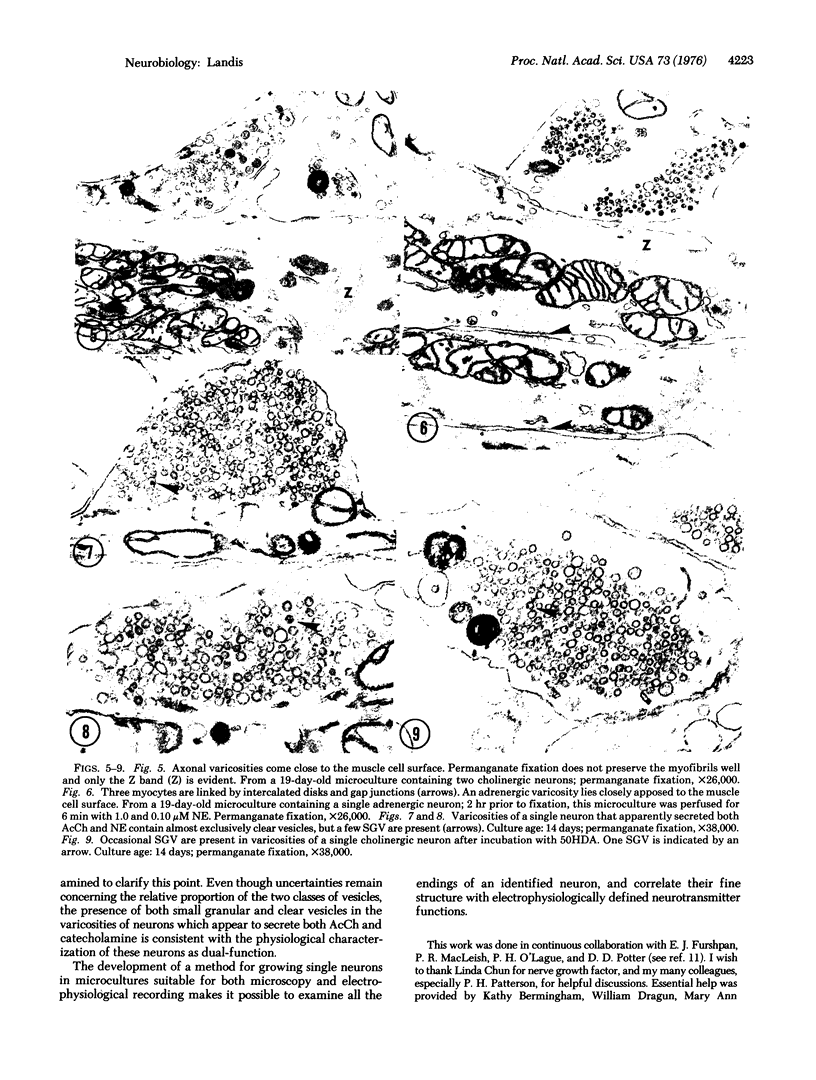
Abstract
Microcultures containing single sympathetic principal neurons and small numbers of dissociated heart myocytes were prepared from newborn rats. After the transmitter properties of the neuron were studied by electrophysiological experiments, the microculture was examined with the electron microscope. Single neurons of either putative cholinergic or putative adrenergic character made morphological synapses on themselves (autapses), although only cholinargic autapses were detected electrophysiologically. Numerous axonal varicosities were present adjacent to the myocytes but no synaptic specializations were evident. After permanganate fixation to localize endogenous norepinephrine, the endings of neurons which appeared to secrete catecholamines contained many small granular vesicles, while the endings of neurons which appeared to secrete acetylcholine contained none. The endings of neurons which apparently secreted both catecholamines and acetylcholine contained only occasional small granular vesicles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton H., Bunge R. P. A comparison of the uptake and release of [3H]norepinephrine in rat autonomic and sensory ganglia in tissue culture. Brain Res. 1975 Oct 24;97(1):157–162. doi: 10.1016/0006-8993(75)90924-5. [DOI] [PubMed] [Google Scholar]
- Eränkö L. Ultrastructure of the developing sympathetic nerve cell and the storage of catecholamines. Brain Res. 1972 Nov 13;46:159–175. doi: 10.1016/0006-8993(72)90013-3. [DOI] [PubMed] [Google Scholar]
- Eränkö O. Light and electron microscopic histochemical evidence of granular and non-granular storage of catecholamines in the sympathetic ganglion of the rat. Histochem J. 1972 May;4(3):213–224. doi: 10.1007/BF01890993. [DOI] [PubMed] [Google Scholar]
- FORSSMANN W. G. STUDIEN UEBER DEN FEINBAU DES GANGLION CERVICALE SUPERIUS DER RATTE. I. NORMALE STRUKTUR. Acta Anat (Basel) 1964;59:106–140. [PubMed] [Google Scholar]
- Furshpan E. J., MacLeish P. R., O'Lague P. H., Potter D. D. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo M. A. Electron microscopy of sympathetic tissues. Pharmacol Rev. 1966 Mar;18(1):387–399. [PubMed] [Google Scholar]
- Hökfelt T. Distribution of noradrenaline storing particles in peripheral adrenergic neurons as revealed by electron microscopy. Acta Physiol Scand. 1969 Aug;76(4):427–440. doi: 10.1111/j.1748-1716.1969.tb04488.x. [DOI] [PubMed] [Google Scholar]
- Hökfelt T. In vitro studies on central and peripheral monoamine neurons at the ultrastructural level. Z Zellforsch Mikrosk Anat. 1968;91(1):1–74. doi: 10.1007/BF00336984. [DOI] [PubMed] [Google Scholar]
- Johnson M., Ross D., Meyers M., Rees R., Bunge R., Wakshull E., Burton H. Synaptic vesicle cytochemistry changes when cultured sympathetic neurones develop cholinergic interactions. Nature. 1976 Jul 22;262(5566):308–310. doi: 10.1038/262308a0. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Patterson P. H. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973 Nov;59(2 Pt 1):329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida S., Sears M. Fine structural innervation of the dilator muscle of the iris of the albino guinea pig studied with permanganate fixation. Exp Eye Res. 1969 Jul;8(3):292–296. doi: 10.1016/s0014-4835(69)80041-2. [DOI] [PubMed] [Google Scholar]
- O'Lague P. H., MacLeish P. R., Nurse C. A., Claude P., Furshpan E. J., Potter D. D. Physiological and morphological studies on developing sympathetic neurons in dissociated cell culture. Cold Spring Harb Symp Quant Biol. 1976;40:399–407. doi: 10.1101/sqb.1976.040.01.038. [DOI] [PubMed] [Google Scholar]
- O'Lague P. H., Obata K., Claude P., Furshpan E. J., Potter D. D. Evidence for cholinergic synapses between dissociated rat sympathetic neurons in cell culture. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3602–3606. doi: 10.1073/pnas.71.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The influence of non-neuronal cells on catecholamine and acetylcholine synthesis and accumulation in cultures of dissociated sympathetic neurons. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3607–3610. doi: 10.1073/pnas.71.9.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Reichardt L. F., Chun L. L. Biochemical studies on the development of primary sympathetic neurons in cell culture. Cold Spring Harb Symp Quant Biol. 1976;40:389–397. doi: 10.1101/sqb.1976.040.01.037. [DOI] [PubMed] [Google Scholar]
- RICHARDSON K. C. THE FINE STRUCTURE OF THE ALBINO RABBIT IRIS WITH SPECIAL REFERENCE TO THE IDENTIFICATION OF ADRENERGIC AND CHOLINERGIC NERVES AND NERVE ENDINGS IN ITS INTRINSIC MUSCLES. Am J Anat. 1964 Mar;114:173–205. doi: 10.1002/aja.1001140202. [DOI] [PubMed] [Google Scholar]
- Rees R., Bunge R. P. Morphological and cytochemical studies of synapses formed in culture between isolated rat superior cervical ganglion neurons. J Comp Neurol. 1974 Sep 1;157(1):1–11. doi: 10.1002/cne.901570102. [DOI] [PubMed] [Google Scholar]
- Richardson K. C. Electron microscopic identification of autonomic nerve endings. Nature. 1966 May 14;210(5037):756–756. doi: 10.1038/210756a0. [DOI] [PubMed] [Google Scholar]
- Thaemert J. C. Fine structure of neuromuscular relationships in mouse heart. Anat Rec. 1969 Apr;163(4):575–585. doi: 10.1002/ar.1091630409. [DOI] [PubMed] [Google Scholar]
- Tranzer J. P., Thoenen H. Electronmicroscopic localization of 5-hydroxydopamine (3,4,5-trihydroxy-phenyl-ethylamine), a new 'false' sympathetic transmitter. Experientia. 1967 Sep 15;23(9):743–745. doi: 10.1007/BF02154151. [DOI] [PubMed] [Google Scholar]
- Van Orden L. S., 3rd, Schaefer J. M., Antonaccio M. J., Smith C. B. Fine structural identification of a reserpine-resistant norepinephrine store in adrenergic nerve terminals in guinea-pig atria. J Pharmacol Exp Ther. 1974 Mar;188(3):668–675. [PubMed] [Google Scholar]
- Van Orden L. S., 3rd, Schaefer J. M., Burke J. P., Lodoen F. V. Differentiation of norepinephrine storage compartments in peripheral adrenergic nerves. J Pharmacol Exp Ther. 1970 Sep;174(3):357–368. [PubMed] [Google Scholar]
- Van der Loos H., Glaser E. M. Autapses in neocortex cerebri: synapses between a pyramidal cell's axon and its own dendrites. Brain Res. 1972 Dec 24;48:355–360. doi: 10.1016/0006-8993(72)90189-8. [DOI] [PubMed] [Google Scholar]





