Abstract
Circulating miRNAs are promising biomarkers for predicting the aggressiveness of hepatocellular carcinoma (HCC). We aimed to identify differentially expressed miRNAs in the serum of HCC patients with different Barcelona Clinic Liver Cancer (BCLC) stage, and to investigate the potential of serum miRNAs as biomarkers for patient outcomes. In the discovery stage, TaqMan Low-Density Array was used to test the difference in levels of serum miRNAs between 20 patients with portal vein tumor thrombosis (PVTT) and 20 patients without PVTT. The detected serum miRNAs then were validated in 182 patients. Fifteen serum miRNAs showed more than two-fold higher expression in patients with PVTT, and miR-128-2 was found to be significantly up-regulated and was selected for further validation. In the validation stage, patients were divided into two groups with low or high serum miR-128-2 using the median expression level of all 182 cases as the cut-off point. Kaplan-Meier analysis revealed that patients with low level of serum miR-128-2 had favorable trends of survival (log rank = 13.031, p < 0.001). The median survivals for patients with a low and high level of serum miR-128-2 were 625 (95% CI, 527–722) days and 426 (95% CI, 362–491) days, respectively. MiR-128-2 was also an independent factor of overall survival (p = 0.001, HR 2.793, 95%CI 1.550, 5.033). Serum levels of the ubiquitously expressed miR-128-2 showed no significant correlation with parameters of liver damage or liver function. In addition, expressions of miR-128-2 in HCC tissues were up-regulated in comparison with adjacent non-tumor tissues. In conclusion, serum level of miR-128-2 serves as a noninvasive biomarker for the overall survival of patients with hepatocellular carcinoma.
Introduction
Hepatocellular carcinoma (HCC) accounts for 85–90% of all primary liver cancers, is an extremely poor prognostic cancer [1, 2]. About 80% of patients present with locally advanced or metastatic disease [3]. It is mainly due to the highly vascular nature of HCC tumors, which show the propensity to spread and invade into neighboring or distant tissues [4]. In addition, underlying chronic liver disease increases the complexity and heterogeneity of HCC pathology [5]. The treatment of HCC is usually performed according to the recommendation of the Barcelona Clinic Liver Cancer (BCLC) staging system, which not only takes the tumor associated parameters into consideration, but also includes liver function and performance status. It has also been confirmed as a prognostic relevance for the overall survival in HCC patients [6, 7].
MicroRNAs (miRNAs), about 22 nucleotides long, are non-coding RNAs that negatively regulate gene expression at the post-transcriptional level [8]. Because a single miRNA can regulate hundreds of downstream genes with different biologic entities, the information gained from miRNA profiling may provide more accurate classification of cancer subtypes than the use of expression profiles of protein-coding genes [9]. In liver cancer, numerous studies have reported the association of miRNA expression profiles with the onset and progression of tumor. MiRNAs such as miR-17-5p, miR-21, miR-181b, miR-143, miR-221 and miR-224, which are associated with cell proliferation, apoptosis inhibition, and migration promotion, are found to be upregulated in HCC tissues [10–15]. Given the difficult access of tumor tissues from advanced HCC patients, circulating miRNAs are preferable noninvasive biomarkers for diagnosis and prognosis assessment. Recent findings demonstrated that human serum and plasma contain a large amount of stable miRNAs, and that the expression profile of these miRNAs holds great promise as a novel non-invasive biomarker [16]. The profile of circulating microRNAs has been explored in a variety of studies aiming to improve the early detection of HCC and to predict the response to therapy [17–19].
Venous metastasis, with tumor thrombi in the portal vein and the inferior vena cava, is a major hallmark of metastatic HCC [20]. Chen SQ et al reported that 40%–90.2% of advanced HCC patients had portal vein tumor thrombosis (PVTT) [21]. Even in patients with HCC tumor smaller than 2cm, 40.5% of them had microscopic venous invasion [22]. The presence of PVTT represents limited benefit of various treatment and poor survival outcome in HCC patients. Till now, no biomarkers related to PVTT have been reported. In this study, we hypothesize that there is a serum miRNA profile that can be used as a fingerprint for patients with different status of PVTT and survival outcomes. To address this hypothesis, we screened serum miRNAs by using TaqMan Low-Density Array (TLDA) in patients with PVTT or not, followed by an extensively validated study in a cohort of 182 patients.
Materials and Methods
Study subjects
HCC patients who were treated between January 2012 and July 2013 in Integrative Department, Fudan University Shanghai Cancer Center, were retrospectively enrolled into the present study. Inclusion criteria were as follows: histologically confirmed HCC or clinical diagnosis based on dynamic imaging and an underlying chronic liver disease; a good performance status (ECOG level <2); favorable liver functions of Child-Pugh class A or B. Patients with a history of another malignant within the last five years were excluded. Five milliliters of venous blood was collected from each participant at his/her first admission to the hospital. The whole blood was centrifuged at 4°C, 3000 r.p.m. for 10 min, which was followed by an additional centrifugation at 12,000 r.p.m. for 15min to completely remove all remaining cells. The serum samples were portioned in aliquots and stored at -80°C until analysis. This study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center, Shanghai, China, and written informed consent was obtained from each participant, in accordance with the institutional guidelines of our hospital.
RNA isolation
Total RNA was extracted from 500ul of serum using a miR-PARIS kit (AM1556) according to the manufacturer’s instructions for enrichment procedure for small RNAs. To allow for normalization of sample-to-sample variation in RNA isolation, synthetic Caenorhabditis elegans miRNAcel-miR-54(purchased as a custom RNA oligo nucleotide from Qiagen) was added (50pmol/l in a 5-ul total volume) to each denatured sample.
MiRNA profiling using the TaqMan Low-Density Array
MiRNA profiling assays of two samples (100ul serum sample from each patient and then mixed together) were performed using the TLDA (Applied Biosystems, CA, USA). Each sample was analyzed with an A & B card for duplicate detection of a total of 754 miRNAs together with endogenous and negative controls. In order to increase the sensitivity of the TLDA, a pre-amplification was performed after the Megaplex reverse transcription (RT) reactions. All procedures were carried out according to the protocols recommended by the manufacturer. qRT-PCR was carried out on an Applied Biosystems 7900HT thermocycler using the recommended cycling conditions. The array data were analyzed using ∆∆CT method, DataAssist Software (Applied Biosystems).
Quantitative real-time reverse-transcription (RT)-PCR assays
We used TaqMan miRNA probes (Applied Biosystems) to perform qRT–PCR assays according to the manufacturer’s instructions. In brief, 2 ul aliquot of enriched small RNAs from serum samples were reverse transcribed using the Taq-Man MicroRNA Reverse Transcription Kit (Applied Biosystems, San Diego, CA). Then 2 ul of the cDNA solution was used as template for the PCR stage. PCR reaction was performed using 10 ml TaqMan Universal Master Mix (2×) (Applied Biosystems, USA), 1 ul gene-specific probe, and nuclease-free H2O in a final volume of 20 ul. No-template controls for both RT step and PCR step were included to ensure target specific amplification. All reactions were run in duplicate. The CT values of the different samples were compared using the ∆∆CT method [23]. The relative expression levels of target miRNAs were normalized by cel-miR-54.
Clinical chemistry
Standard parameters of liver function and alpha-fetoprotein (AFP) levels were measured at the central laboratory of the Fudan University Shanghai Cancer Center.
Statistical methods
Differences in patient characteristics between two groups were evaluated by the Chi-squared test, the student’s t-test or the nonparametric Wilcoxon-Mann-Whitney test according to the variable type. We defined the follow-up duration from the date of diagnosis to the last follow up. The overall survival (OS) was calculated from the date of a definitive diagnosis to death or to the date of the last follow up. Kaplan-Meier method was used to compare the OS between patients in different groups. Only covariates significantly associated with outcomes at univariate analysis (two-sided P value<0.10) are shown and included in the multivariate model. Results were reported as hazard ratios (HR) with 95% confidence intervals (CI). Potential covariates were adjusted in multivariate model by Forward LR method.
The correlation coefficients between the expression level of miRNA and laboratory parameters were calculated by using the Spearman correlation. P values < 0.05 were considered to be significant. Data were analyzed using the SPSS version 20 (IBM, Chicago, IL).
Results
Detection of differentially expressed serum miRNAs in HCC patients with PVTT compared with no PVTT
TLDA was performed to identify candidate miRNAs associated with PVTT and survival in HCC patients. miRNA profiling in serum samples from 20 HCC patients with PVTT was compared with profiling in serum samples from 20 HCC patients without PVTT. In PVTT group, tumor thrombi involving the main portal vein trunk in six patients, and tumor thrombi involving right/left portal vein in another fourteen patients. Clinical variables were similar between the two groups, with the exception of the serum alpha-fetoprotein, BCLC stage and the median overall survival (Table 1). Of the 754 miRNAs incorporated in the array, 216 and 212 miRNAs were detected in serum of HCC patients with PVTT and without PVTT, respectively. Overall, 15 miRNAs showed more than 2-fold higher expression in PVTT group than no-PVTT group, and miR-128-2 was found to be significantly upregulated. Furthermore, miR-128-2 has never been reported in HCC, and its expression level in serum is detachable. Thus, it was selected for further validation (Table 2).
Table 1. Patients’ characteristics.
| Parameter | Cohort 1(n = 40) | Cohort 2(n = 182) | ||
|---|---|---|---|---|
| PVTT(n = 20) | No-PVTT(n = 20) | P value | ||
| Gender, male/female | 18/2 | 17/3 | 0.904 | 155/27 |
| Age, years, mean±SD | 50.4±7.5 | 53.9±9.3 | 0.24 | 53.1±10.5 |
| Hepatitis B, n (%) | 17(85) | 18(90) | 0.697 | 159 (87) |
| Child-pugh stage (A/B/C) | 18/2/0 | 19/1/0 | 0.737 | 163/19/0 |
| BCLC (B/C) | 0/20 | 20/0 | 0.000 | 98/84 |
| Treatment | ||||
| Local therapy, n (%) | 20(100) | 20(100) | 1 | 177(97.3) |
| Sorafenib, n (%) | 1(5) | 0(0) | 0.317 | 33(18.1) |
| Laboratory results | ||||
| TBIL (umol/L), mean±SD | 17.7±9.46 | 15.7±6.1 | 0.856 | 18.3±12 |
| DBIL(umol/L), mean±SD | 8.1±6.1 | 5.5±3.9 | 0.083 | 7.0±7.2 |
| ALT (IU/L), mean±SD | 72.6±103.2 | 39.8±22.5 | 0.338 | 47.5±50.4 |
| AST (IU/L), mean±SD | 65.5±38.3 | 50.2±31.4 | 0.062 | 61.1±64.2 |
| γ-GGT (IU/L), mean±SD | 167.9±75 | 149.9±188.6 | 0.053 | 174.5±198.0 |
| LDH (IU/L), mean±SD | 268.3±117.8 | 207.3±97.3 | 0.086 | 240.4±146.6 |
| ALP (IU/L), mean±SD | 180.5±157.4 | 125.6±76.5 | 0.104 | 146.3±113.6 |
| ALB (g/L), mean±SD | 38.1±4 | 39.8±4.9 | 0.057 | 39.2±4.6 |
| AFP (ng/ml), mean±SD | 3011±1230 | 1089±1556 | 0.000 | 1415±1615 |
| Median OS (95% CI), days | 289.5(242.2, 446.9) | 541.5(395.2, 829.3) | 0.013 | 525.6(466.2,585) |
Table 2. Serum miRNAs overexpressed in HCC patients with PVTT in comparison with no PVTT.
| MiRNA | CT value for PVTT | CT value for no-PVTT | Fold-change |
|---|---|---|---|
| hsa-miR-128-2 | 28.9625 | 35.244 | 60.8355 |
| hsa-miR-545 | 29.9643 | 32.9926 | 6.3806 |
| hsa-miR-138 | 32.9851 | 36.0033 | 6.3363 |
| hsa-miR-511 | 30.978 | 33.9827 | 6.2772 |
| hsa-miR-193b | 25.9747 | 28.9215 | 6.0303 |
| hsa-miR-224 | 27.9724 | 30.0886 | 3.3906 |
| hsa-miR-1267 | 28.0004 | 30.1077 | 3.37 |
| hsa-miR-125b | 27.9226 | 29.9798 | 3.2547 |
| hsa-miR-148b | 28.8768 | 30.1027 | 3.2133 |
| hsa-miR-375 | 25.9421 | 27.9755 | 3.2016 |
| hsa-miR-642 | 31.9555 | 33.9612 | 3.1408 |
| hsa-miR-374b | 31.9823 | 33.9681 | 3.0977 |
| hsa-miR-885-5p | 22.9879 | 24.9635 | 3.076 |
| hsa-miR-381 | 32.9906 | 34.9648 | 3.0729 |
| hsa-miR-625 | 31.0133 | 32.9356 | 2.9644 |
We then performed individual qRT–PCR detection on another 182 samples to quantify the serum expression of miR-128-2 in HCC patients. Most of the patients in this cohort were men (85%), were long-term carriers of hepatitis B virus (HBV) (87%). All of the patients presented with locally advanced or metastatic HCC, and preserved liver function of Child A/B. 31.3% (57/182) of patients had PVTT (Table 1). In 107 patients the diagnosis of HCC was confirmed by histopathological examination of biopsy material, whereas in another 75 patients HCC was clinically diagnosed. The mean duration of follow-up was 656±393 days.
Serum miRNA-128-2 is a prognostic marker for HCC patients
Patients were divided into two groups with low or high serum miR-128-2 using the median expression level of all 182 cases as the cut-off point. Kaplan-Meier analysis revealed that patients with low level of serum miR-128-2 had favorable trends of OS (log rank = 13.031, p < 0.001; Fig. 1A). The median survivals for patients with a low level of serum miR-128-2 and that with a high level of serum miR-128-2 were 625 (95% CI, 527–722) days and 426 (95% CI, 362–491) days, respectively. BCLC stage is a strong significant factor for HCC prognosis. We then grouped patients according to the BCLC stage and the status of PVTT, and compared the survival curves according to miR-128-2 levels in each subgroup of patients. The significant differences were all evident, which indicated that low level of serum miR-128-2 presented better survival (S1 and S2 Figs.).
Fig 1. Kaplane Meier curves for different subsets of patients.
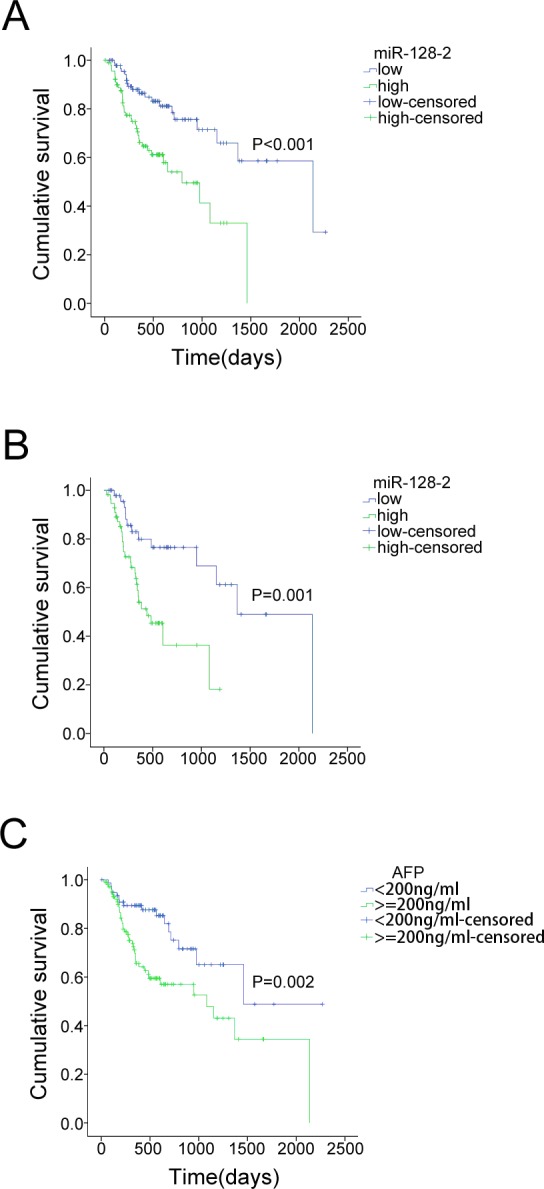
A. Kaplane Meier curve for overall survival in a cohort of 182 HCC patients grouped by the serum level of miR-128-2. The serum miR-128-2 level was analyzed by TaqMan real-time qPCR, and the median miR-128-2 expression level was chosen as the cut-off point for separating the miR-128-2 low-level cases (n = 91) from the miR-128-2 high-level cases (n = 91). Low level of serum miR-128-2 was associated with better survival. B. Low level of serum miR-128-2 was still related with better survival in the subset of patients with serum level of AFP higher than 200ng/ml. C. Patients with serum level of AFP lower than 200ng/ml present better survival in comparison with patients with AFP level higher than 200ng/ml.
To examine whether the serum miR-128-2 level was associated with specific stages of the disease, patients were classified according to the BCLC stage. No significant difference was found on the expression level of miR-128-2 between patients with stage B and stage C (p = 0.274). Similarly, the miR-128-2 level in patients with PVTT was not significantly different from HCC patients without PVTT (p = 0.733), which suggested that miR-128-2 was independent from BCLC stage. In addition, it might not associate with the presence of PVTT.
Serum AFP is a biomarker conventionally used in the diagnosis of HCC and the prediction of treatment response and survival. Following the AASLD Guidelines, an AFP level >200 ng/ml is favorable for the diagnosis of HCC when liver lesions are >2cm in size [24]. Thus, we examined the effect of miR-128-2 in two subsets of patients, using 200ng/ml of the serum AFP level as the cut-off point. In group one, which patients with serum AFP level lower than 200ng/ml, Kaplan-Meier Analysis revealed that patients with low or high level of miR-128-2 showed no significant difference in OS (log rank = 1.991, p = 0.158). Contrarily, in group two, which patients with serum AFP level higher than 200ng/ml, lower miR-128-2 presented with better survival (log rank = 10.156, p = 0.001; Fig. 1B), which indicated that the predictive effect of miR-128-2 might be dependent on serum AFP level. Further analysis found a weak positive correlation between serum miR-128-2 level and AFP levels (r = 0.198, p = 0.009, Table 3).
Table 3. Correlation of serum miR-128-2 levels and laboratory parameters.
| Variables | miR-128-2 | |
|---|---|---|
| Rank correlation coefficient (r) | P-value | |
| TBIL (umol/L) | -0.031 | 0.688 |
| D-TBIL (umol/L) | 0.013 | 0.865 |
| ALT(IU/L) | 0.144 | 0.059 |
| AST(IU/L) | 0.035 | 0.641 |
| γ-GGT (IU/L) | 0.093 | 0.224 |
| LDH(IU/L) | 0.125 | 0.101 |
| ALP(IU/L) | 0.049 | 0.519 |
| ALB (g/L) | 0.014 | 0.859 |
| AFP(ng/ml) | 0.198 | 0.009 |
| PT | -0.46 | 0.550 |
| INR | -0.040 | 0.601 |
TBIL, total bilirubin
D-TBIL, direct bilirubin
ALT, alanine aminotransferase
AST, aspartate aminotransferase
γ-GGT, γ-glutamyl transferase
LDH, lactic dehydrogenase
ALP, alkaline phosphatase
ALB, albumin
PT, prothrombintime activity percentage
INR, international normalized ratio.
Because serum AFP level affects prognosis (log rank = 9.214, p = 0.002; Fig. 1C) and to exclude confounding effects, we then performed a Cox proportional hazards regression analysis. A multivariate analysis confirmed that the serum miR-128-2 level was an independent prognostic factor for OS (p = 0.001, HR 2.793, 95%CI 1.550, 5.033). In addition, multivariate analysis further confirmed that low BCLC stage and local therapy were independent factors for better survival of HCC patients (Table 4).
Table 4. Univariate and multivariate Cox regression analyses of parameters associated with overall survival of all HCC patients.
| Parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR(95% CI) | p-value | HR(95% CI) | p-value | |
| Gender (male vs. female) | 1.659(0.66, 4.172) | 0.282 | ||
| HBV (no vs. yes) | 0.767(0.375, 1.567) | 0.466 | ||
| Cirrhosis (no vs. yes) | 1.268(0.506, 3.181) | 0.613 | ||
| AFP (<200 vs. >200ng/ml) | 2.397(1.339, 4.289) | 0.003 | 1.757(0.940, 3.284) | 0.077 |
| Child-pugh (A vs. B) | 2.04(1.041, 3.996) | 0.038 | 1.500(0.758, 2.969) | 0.244 |
| PVTT (no vs. yes) | 3.145(1.85, 5.345) | 0.000 | 1.475(0.776, 2.802) | 0.236 |
| BCLC (B vs. C) | 4.083(2.228, 7.482) | 0.000 | 2.481(1.166, 5.280) | 0.018 |
| Local therapy | 0.235(0.084, 0.654) | 0.006 | 0.227(0.075, 0.687) | 0.009 |
| Sorafenib | 1.423(0.791, 2.562) | 0.239 | ||
| Serum miR-128-2 (low vs. high) | 2.791(1.577, 4.941) | 0.000 | 2.793(1.550, 5.033) | 0.001 |
Serum level of miR-128-2 didn’t correlate with inflammatory activity in the liver and its function
To investigate if the prognostic significance of serum miR-128-2 correlated with inflammatory activity in the liver and its function, we assessed parameters of total bilirubin (TBIL), direct bilirubin (D-BIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (γ-GGT), lactic dehydrogenase (LDH), alkaline phosphatase (ALP), albumin (ALB), prothrombintime activity percentage (PT), and international normalized ratio (INR) (Table 3). Serum levels of the ubiquitously expressed miR-128-2 showed no significant correlation with parameters of liver damage or liver function.
Expression of miR-128-2 up-regulated in HCC tissues
To identify the miR-128-2 expression level in HCC tissues, we analyzed the miRNA expression in a large number of human HCC samples from the GEO, a public functional genomics data repository supporting MIAME- compliant data submissions. The miRNA expression profiles of 181 tumors and the paired non-tumor liver were obtained from the study GSE36376 [25]. All data were quartile normalized. The baseline characteristics of patients were shown on S1 Table. The analysis revealed that miR-128-2was significantly overexpressed when compared with adjacent non-tumor tissue (Fig. 2A). In addition, patients were grouped in subjects with low or high tissue miR-128-2 using the median expression level of the 181 cases as the cut-off point and Kaplan-Meier analysis was done. The OS of patients with low or high tissue miR-128-2 wasn’t significantly different (P = 0.704). We then analyzed the relation between miR-128-2 expression and survival in the subgroup of patients with BCLC B or C stage. Finally, 77 cases were included in the analysis, and the median expression level of the 77 cases was used as the cut-off point. The results showed that patients with lower miR-128-2 were associated with better survival outcome (log rank = 5.594, p = 0.018; Fig. 2B). Taken together, miR-128-2 was upregualted in HCC tissues. Higher expression level of miR-128-2 is associated with a poor prognosis in patients with advanced HCC.
Fig 2. miR-128-2 expression was up-regulated in tumor tissues and correlates with shorter survival in patients with advanced HCC.
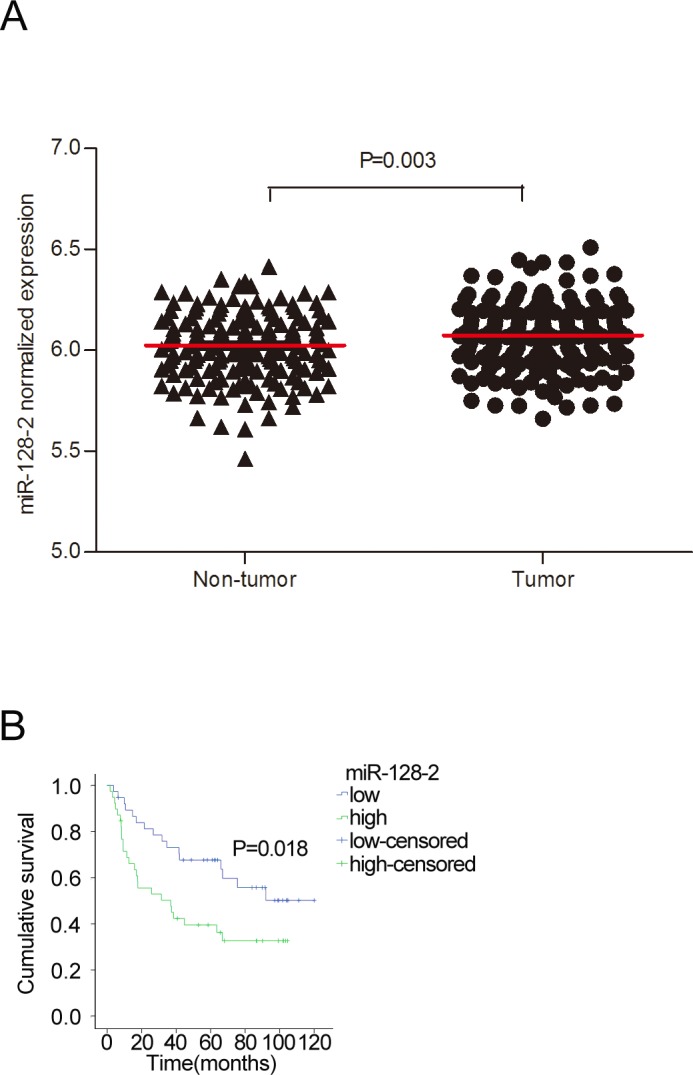
A. A comparison of miR-128-2 expression levels in HCC tissues and adjacent non-cancer tissues. Statistical significance was calculated. B. A Kaplane Meier survival analysis of the HCC patients with BCLC B/C stage grouped according to the expression level of miR-128-2 in the tumor tissue. The median miR-128-2 expression level of the 77 cases was chosen as the cut-off point for separating the miR-128-2 low-expression tumors (n = 39) from miR-128-2 high-expression tumors (n = 38). Low level of miR-128-2 in HCC tissue was associated with better survival.
Discussion
The aim of this study was to identify differentially expressed miRNAs in the serum of HCC patients with different BCLC stage, and investigate the potential of serum miRNA as a biomarker for venous metastasis and survival. We firstly conducted an array in two subsets of HCC patients. One subset of HCC patients was diagnosed at BCLC B stage, with a relatively longer survival, and the other subset was diagnosed at BCLC C stage (with PVTT but not extra liver metastasis), with a shorter survival. Thus, the differentially expressed miRNAs were prospected to be associated with tumor progression and survival outcome. The systematic screening found that miR-128-2 was the most significantly up-regulated miRNA in the serum of patients with BCLC C stage. In further validation, we confirmed that serum miR-128-2 level could be a new prognostic parameter in HCC patients that is independent from BCLC stage.
MiR-128 is encoded by two distinct genes, miR-128-1 and miR-128-2, which are processed into an identical mature sequence [26]. MiR-128 is frequently reported as a tumor suppresser in the malignance of nervous system, for example neuroblastoma, glioblastoma and medulloblastoma [27–29]. In contrast to these, Volinia S et al conducted a large-scale miRnome analysis on 540 samples including lung, breast, stomach, prostate, colon, and pancreatic tumors. They identified that elevated miR-128b was shared in colon, lung, and pancreas cancer [30]. The role of miR-128 in liver cancer has not yet been reported. We used the public data from GEO to analyze the expression level of miR-128 in HCC tissues. The results revealed that miR-128-2 was significantly overexpressed in HCC tissues in comparison with the adjacent non-tumor tissues, which indicated that miR-128 was related with the pathogenesis of HCC. However, it is still unknown whether the dysregulation of miR-128 is a cause or effect of the cancer.
The presence of PVTT is strongly correlated with a poor prognosis for HCC patients [31, 32]. Aberrantly expressed miRNAs contribute to the formation of PVTT has ever been reported. Liu S et al. comparatively analyzed the miRNA and mRNA expression profile of PVTT and the corresponding parenchyma tumor tissue. They found that miR-135a showed the greatest increase in PVTT tissue. Further in vitro and in vivo studies demonstrated that overexpression of miR-135a favored invasive and metastatic behavior [33]. PVTT cases in the literature are usually from developing countries. Yang P et al. uncovered a causative link between HBV infection and development of PVTT. Mechanistically, elevated TGF-b activity, resulting from the persistent presence of HBV in the liver tissue, suppresses the expression of microRNA-34a, leading to enhanced production of chemokine CCL22, which recruits regulatory T cells to facilitate immune escape [34]. Circulating miRNA profile in HCC patients with PVTT has not yet been reported. Initially, our study aimed to indentify certain serum miRNAs, which differentially express in patients with PVTT in comparison with no-PVTT. Then further validate whether it can be a biomarker for PVTT development and survival. Serum miR-128-2 showed the greatest increase in the screening. However, it failed to show association with the presence of PVTT in further validation. Biomarkers reflected the aggressiveness of tumor are likely to provide more accurate information for the prognosis of HCC patients. Since its stability in circulation and the easy access of blood samples, circulating miRNAs hold great promise. Circulating miRNA expression levels are not always consistent with corresponding tissue. For example, miR-122, a liver specific miRNA, has been reported as downregulated in rodent and human HCC tissues when compared with adjacent non-tumor tissues [35]. However, an elevation of serum level of miR-122 was evident in HCC patients [36, 37]. It indicates that circulating miRNAs may not always directly associate with changes occurring in tumor tissues but may also reflect indirect effects. In present study, we found a consistent expression level of miR-128-2 in serum and HCC tissues.
In conclusion, we identified serum miR-128-2 as an independent prognostic parameter for OS in HCC patients.
Supporting Information
Patients with high level of serum miR-128-2 had poor survival in subset of patients with BCLC stage B and subset of patients with BCLC stage C.
(TIF)
Patients with high level of serum miR-128-2 had poor survival in the subset of patients with PVTT and subset of patients without PVTT.
(TIF)
(DOC)
Acknowledgments
We gratefully thank all of the patients who contributed to this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no funding or support to report.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global Cancer Statistics. CA Cancer J Clin 61:69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2. El-Serag HB (2011) Hepatocellular carcinoma. New Engl J Med 365:1118–1127. 10.1056/NEJMra1001683 [DOI] [PubMed] [Google Scholar]
- 3. Bruix J, Sherman M, American Association for the Study of Liver Diseases (2011) Management of hepatocellular carcinoma: An update. Hepatology 53:1020–1022. 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang ZY (2001) Hepatocellular carcinoma—Cause, treatment and metastasis. World J Gastroenterol 7:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, et al. (2011) Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology 54:463–471. 10.1002/hep.24397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huitzil-Melendez FD, Capanu M, O'Reilly EM, Duffy A, Gansukh B, et al. (2010) Advanced hepatocellular carcinoma: which staging systems best predict prognosis. J Clin Oncol 28: 2889–2895. 10.1200/JCO.2009.25.9895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peck-Radosavljevic M (2012) Back to basics: Staging and prognosis in HCC for medical oncologist. J Hepatol 56:488–489. 10.1016/j.jhep.2011.06.023 [DOI] [PubMed] [Google Scholar]
- 8. Bartel DP (2004) MicrRNAs: Genomics, biogensis, mechanism and function. Cell 116:281–297. [DOI] [PubMed] [Google Scholar]
- 9. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, et al. (2005) MicroRNA expression profiles classify human cancers. Nature 435: 834–838. [DOI] [PubMed] [Google Scholar]
- 10. Yang F, Yin Y, Wang F, Wang Y, Zhang L, et al. (2010) miR-17–5p Promotes migration of human hepatocellular carcinoma cells through the p38 mitogen-activated protein kinase-heat shock protein 27 pathway. Hepatology 51:1614–1623. 10.1002/hep.23566 [DOI] [PubMed] [Google Scholar]
- 11. Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, et al. (2007) MicroRNA- 21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cance. Gastroenterology 133:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang B, Hsu SH, Majumder S, Kutay H, Huang W, et al. (2010) TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene 29:1787–1797. 10.1038/onc.2009.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang X, Liu S, Hu T, Liu S, He Y, et al. (2009) Up-regulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology 50:490–499. 10.1002/hep.23008 [DOI] [PubMed] [Google Scholar]
- 14. Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, et al. (2008) MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene 27:5651–5661. 10.1038/onc.2008.178 [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, Lee AT, Ma JZ, Wang J, Ren J, et al. (2008) Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J Biol Chem 283: 13205–15. 10.1074/jbc.M707629200 [DOI] [PubMed] [Google Scholar]
- 16. Chen X, Ba Y, Ma L, Cai X, Yin Y, et al. (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18: 997–1006. 10.1038/cr.2008.282 [DOI] [PubMed] [Google Scholar]
- 17. Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, et al. (2010) Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBVpositive hepatocarcinoma. Cancer Res 70:9798–9807. 10.1158/0008-5472.CAN-10-1001 [DOI] [PubMed] [Google Scholar]
- 18. Zhou J, Yu L, Gao X, Hu J, Wang J, et al. (2011) Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol Off J Am Soc Clin Oncol 29:4781–4788. [DOI] [PubMed] [Google Scholar]
- 19. Ji J, Shi J, Budhu A, Yu Z, Forgues M, et al. (2009) MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med 361:1437–47. 10.1056/NEJMoa0901282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuki K, Hirohashi S, Sakamoto M, Kanai T, Shimosato Y (1990) Growth and spread of hepatocellular carcinoma, a review of 240 consecutive autopsy cases. Cancer 66:2174–2179. [DOI] [PubMed] [Google Scholar]
- 21. Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, et al. (2007) Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology 54:499–502. [PubMed] [Google Scholar]
- 22. Chau GY, Lui WY, King KL, Wu CW (2005) Evaluation of effect of hemihepatic vascular occlusion and the Pringle maneuver during hepatic resection for patients with hepatocellular carcinoma and impaired liver function. World J Surg 29:1374–83. [DOI] [PubMed] [Google Scholar]
- 23. Giovannetti E, Funel N, Peters GJ, Del Chiaro M, Erozenci LA, et al. (2010) MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res 70:4528–4538. 10.1158/0008-5472.CAN-09-4467 [DOI] [PubMed] [Google Scholar]
- 24. Lok AS, Lai CL (1989) alpha-Fetoprotein monitoring in Chinese patients with chronic hepatitis B virus infection: role in the early detection of hepatocellular carcinoma. Hepatology 9:110–115. [DOI] [PubMed] [Google Scholar]
- 25. Lim HY, Sohn I, Deng S, Lee J, Jung SH, et al. (2013) Prediction of disease-free survival in hepatocellular carcinoma by gene expression profiling. Ann Surg Oncol 20:3747–3753. 10.1245/s10434-013-3070-y [DOI] [PubMed] [Google Scholar]
- 26. Adlakha YK, Saini N (2011) MicroRNA-128 downregulates Bax and induces apoptosis in human embryonic kidney cells. Cell Mol Life Sci 68:1415–1428. 10.1007/s00018-010-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evangelisti C, Florian MC, Massimi I, Dominici C, Giannini G, et al. (2009) MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB J 23:4276–4287. 10.1096/fj.09-134965 [DOI] [PubMed] [Google Scholar]
- 28. Ciafrè SA, Galardi S, Mangiola A, Ferracin M, Liu CG, et al. (2005) Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun 334: 1351–1358. [DOI] [PubMed] [Google Scholar]
- 29. Venkataraman S, Alimova I, Fan R, Harris P, Foreman N, et al. (2010) MicroRNA 128a increases intracellular ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer cell growth by promoting senescence. PLoS One 5:e10748 10.1371/journal.pone.0010748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, et al. (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 103: 2257–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin DX, Zhang QY, Li X, Ye QW, Lin F, et al. (2011) An aggressive approach leads to improved survival in hepatocellular carcinoma patients with portal vein tumor thrombus. J Cancer Res Clin Oncol 137:139–149. 10.1007/s00432-010-0868-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chok KS, Cheung TT, Chan SC, Poon RT, Fan ST, et al. (2010) Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol 17:2073–2080. 10.1245/s10434-010-0940-4 [DOI] [PubMed] [Google Scholar]
- 33. Liu S, Guo W, Shi J, Li N, Yu X, et al. (2012) MicroRNA-135a contributes to the development of portal vein tumor thrombus by promoting metastasis in hepatocellular carcinoma. J Hepatol 56:389–396. 10.1016/j.jhep.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 34. Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, et al. (2012) TGF-b-miR-34a- CCL22 Signaling-Induced Treg Cell Recruitment Promotes Venous Metastases of HBV-Positive Hepatocellular Carcinoma. Cancer Cell 22:291–303. 10.1016/j.ccr.2012.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, et al. (2006) Downregulation of miR-122 in the rodent and human hepatocellular carcinoma. J Cell Biochem 99:671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qi P, Cheng SQ, Wang H, Li N, Chen YF, et al. (2011) Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS One 6:e28486 10.1371/journal.pone.0028486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu J, Wu C, Che X, Wang L, Yu D, et al. (2011) Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog 50: 136–42. 10.1002/mc.20712 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patients with high level of serum miR-128-2 had poor survival in subset of patients with BCLC stage B and subset of patients with BCLC stage C.
(TIF)
Patients with high level of serum miR-128-2 had poor survival in the subset of patients with PVTT and subset of patients without PVTT.
(TIF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


