Abstract
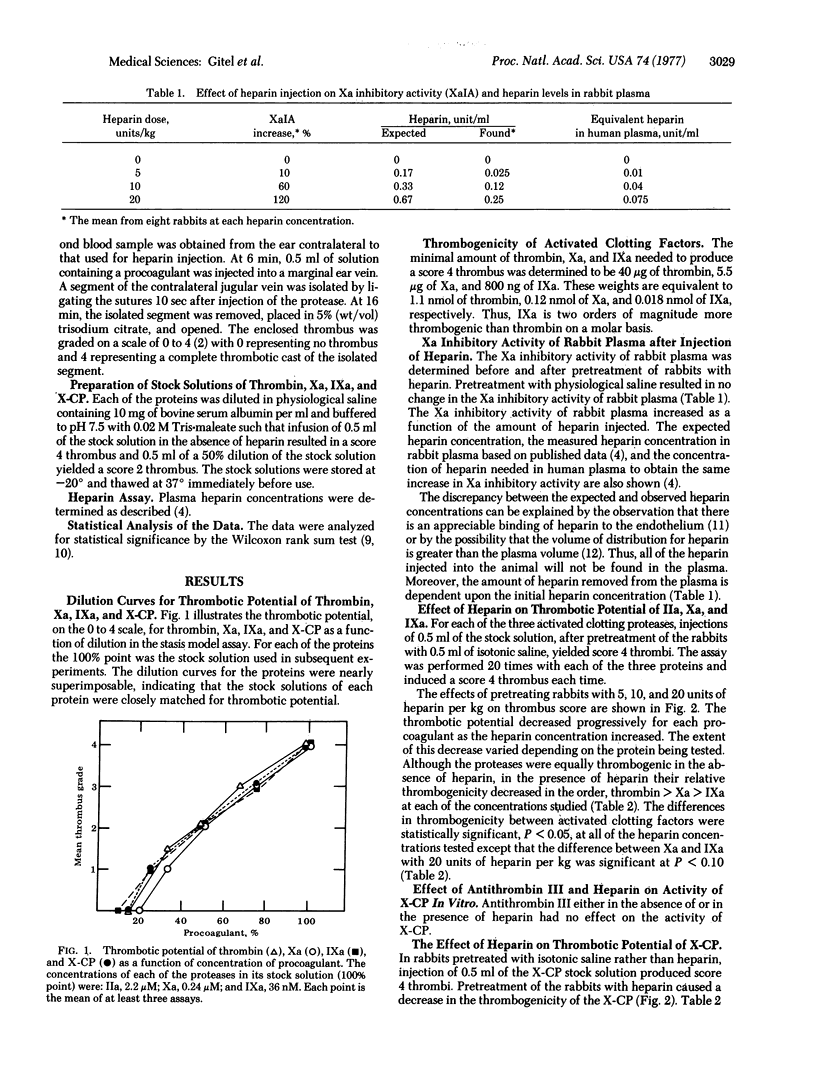
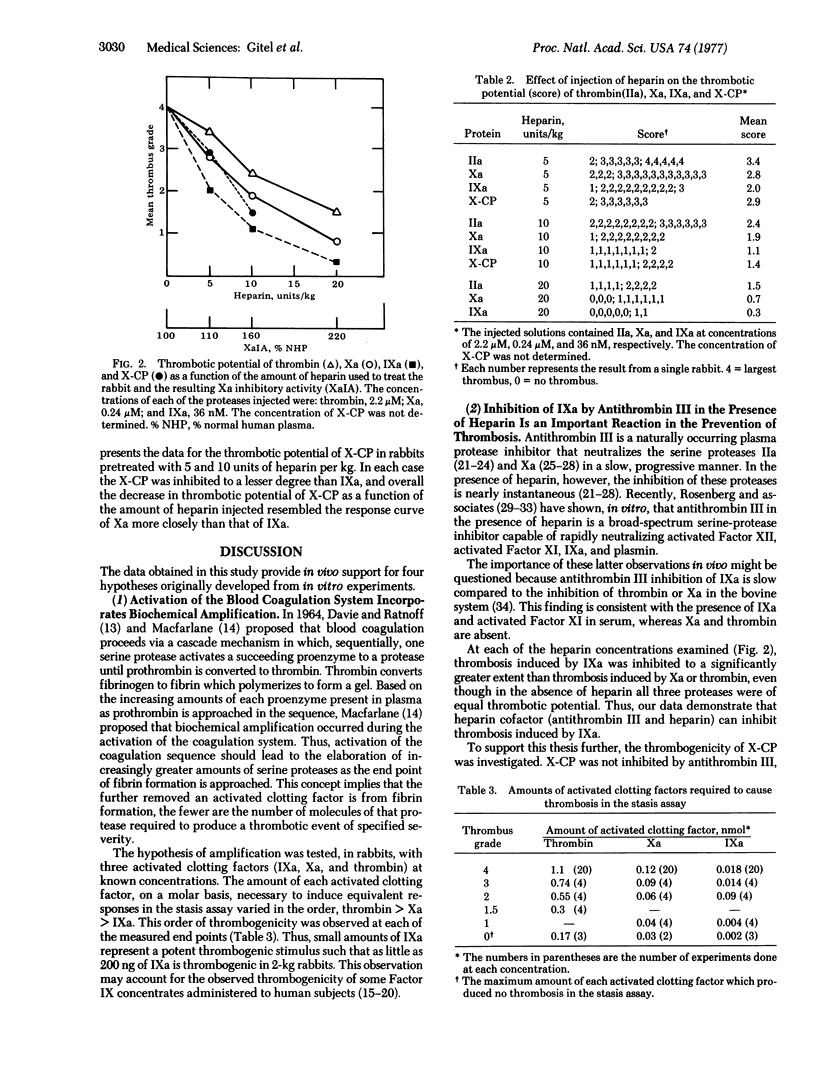
The thrombogenicity of three highly purified proteases (thrombin, activated Factor X, and activated Factor IX) was determined quantitatively in an animal model. The minimal amounts required to produce a standard score 4 thrombus were 1.1 nmol for thrombin, 0.12 nmol for activated Factor X, and 0.018 nmol for activated Factor IX. After the administration of heparin at 5, 10, and 20 units/kg in rabbits, the thrombogenicity of each of these proteases decreased progressively. The heparin-induced inhibition of thrombosis decreased in the order, activated Factor IX > activated Factor X > thrombin at each heparin concentration. These differences were statistically significant.
These in vivo data provide support for the following hypotheses originally developed from in vitro experiments: (i) activation of the blood coagulation system, which proceeds through a cascade mechanism, incorporates biochemical amplification; (ii) the inhibition of activated Factor IX by antithrombin III in the presence of heparin is an important reaction in the prevention of thrombosis; (iii) less heparin is required to inhibit thrombosis prior to thrombin generation than afterward; (iv) an increase in the reactivity of antithrombin III reflects a decreased tendency to thrombosis while a decrease in this reactivity reflects an increased tendency to thrombosis.
Keywords: coagulation, antithrombin III, animal model
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abildgaard U. Highly purified antithrombin 3 with heparin cofactor activity prepared by disc electrophoresis. Scand J Clin Lab Invest. 1968;21(1):89–91. doi: 10.3109/00365516809076981. [DOI] [PubMed] [Google Scholar]
- Biggs R., Denson K. W., Akman N., Borrett R., Hadden M. Antithrombin 3, antifactor Xa and heparin. Br J Haematol. 1970 Sep;19(3):283–305. doi: 10.1111/j.1365-2141.1970.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Blatt P. M., Lundblad R. L., Kingdon H. S., McLean G., Roberts H. R. Thrombogenic materials in prothrombin complex concentrates. Ann Intern Med. 1974 Dec;81(6):766–770. doi: 10.7326/0003-4819-81-6-766. [DOI] [PubMed] [Google Scholar]
- Butler M. J., Gordon Y. B., Irving M. H., Sola C. M., Chard T. Serum levels of fibrin (ogen) degradation fragment E antigen in the diagnosis of deep vein thrombosis after abdominal and inguinal surgery. Thromb Res. 1976 Feb;8(2):167–171. doi: 10.1016/0049-3848(76)90259-0. [DOI] [PubMed] [Google Scholar]
- DAVIE E. W., RATNOFF O. D. WATERFALL SEQUENCE FOR INTRINSIC BLOOD CLOTTING. Science. 1964 Sep 18;145(3638):1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- DE TAKATS G. Anticoagulant therapy in surgery. J Am Med Assoc. 1950 Feb 25;142(8):527–534. doi: 10.1001/jama.1950.02910260001001. [DOI] [PubMed] [Google Scholar]
- Damus P. S., Hicks M., Rosenberg R. D. Anticoagulant action of heparin. Nature. 1973 Dec 7;246(5432):355–357. doi: 10.1038/246355a0. [DOI] [PubMed] [Google Scholar]
- EGEBERG O. INHERITED ANTITHROMBIN DEFICIENCY CAUSING THROMBOPHILIA. Thromb Diath Haemorrh. 1965 Jun 15;13:516–530. [PubMed] [Google Scholar]
- Frenkel J. K. Choice of animal models for the study of disease processes in man. Introduction. Fed Proc. 1969 Jan-Feb;28(1):160–161. [PubMed] [Google Scholar]
- Hiebert L. M., Jaques L. B. The observation of heparin on endothelium after injection. Thromb Res. 1976 Feb;8(2):195–204. doi: 10.1016/0049-3848(76)90262-0. [DOI] [PubMed] [Google Scholar]
- Highsmith R. F., Rosenberg R. D. The inhibition of human plasmin by human antithrombin-heparin cofactor. J Biol Chem. 1974 Jul 25;249(14):4335–4338. [PubMed] [Google Scholar]
- Jackson C. M., Gordon J. G., Hanahan D. J. Separation of the tosyl arginine esterase activity from the factor X activating enzyme of Russell's viper venom. Biochim Biophys Acta. 1971 Nov 12;252(2):255–261. doi: 10.1016/0304-4165(71)90005-5. [DOI] [PubMed] [Google Scholar]
- KIESEWETTER R., ITTERHEIM R., GEBHARD J. Effects of vitamin B12, B6 and procaine on the conditioned escape response. Nature. 1962 Mar 24;193:1188–1188. doi: 10.1038/1931188a0. [DOI] [PubMed] [Google Scholar]
- Kasper C. K. Postoperative thromboses in hemophilia B. N Engl J Med. 1973 Jul 19;289(3):160–160. doi: 10.1056/NEJM197307192890321. [DOI] [PubMed] [Google Scholar]
- Kingdon H. S., Lundblad R. L., Veltkamp J. J., Aronson D. L. Potentially thrombogenic materials in factor IX concentrates. Thromb Diath Haemorrh. 1975 Jun 30;33(3):617–631. [PubMed] [Google Scholar]
- Kisiel W., Hermodson M. A., Davie E. W. Factor X activating enzyme from Russell's viper venom: isolation and characterization. Biochemistry. 1976 Nov 2;15(22):4901–4906. doi: 10.1021/bi00667a023. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Fujikawa K., Schmer G., Davie E. W. Inhibition of bovine factor IXa and factor Xabeta by antithrombin III. Biochemistry. 1976 Jan 27;15(2):373–377. doi: 10.1021/bi00647a021. [DOI] [PubMed] [Google Scholar]
- Letter: Prothrombin-complex concentrates and thrombosis. N Engl J Med. 1974 Feb 14;290(7):403–404. doi: 10.1056/NEJM197402142900718. [DOI] [PubMed] [Google Scholar]
- Loeliger E. A., Hensen A., Mattern M. J., Veltkamp J. J., Bruning P. F., Hemker H. C. Treatment of haemophilia B with purified Factor IX (PPSB). Folia Med Neerl. 1967;10(4):112–125. [PubMed] [Google Scholar]
- MACFARLANE R. G. AN ENZYME CASCADE IN THE BLOOD CLOTTING MECHANISM, AND ITS FUNCTION AS A BIOCHEMICAL AMPLIFIER. Nature. 1964 May 2;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- MONKHOUSE F. C., FRANCE E. S., SEEGERS W. H. Studies on the antithrombin and heparin cofactor activities of a fraction adsorbed from plasma by aluminum hydroxide. Circ Res. 1955 Jul;3(4):397–402. doi: 10.1161/01.res.3.4.397. [DOI] [PubMed] [Google Scholar]
- Marciniak E., Farley C. H., DeSimone P. A. Familial thrombosis due to antithrombin 3 deficiency. Blood. 1974 Feb;43(2):219–231. [PubMed] [Google Scholar]
- Rosenberg R. D. Actions and interactions of antithrombin and heparin. N Engl J Med. 1975 Jan 16;292(3):146–151. doi: 10.1056/NEJM197501162920307. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D. Heparin action. Circulation. 1974 Apr;49(4):603–605. doi: 10.1161/01.cir.49.4.603. [DOI] [PubMed] [Google Scholar]
- Sas G., Pepper D. S., Cash J. D. Further investigations on antithrombin III in the plasmas of patients with the abnormality of antithrombin III Budapest. Thromb Diath Haemorrh. 1975 Jun 30;33(3):564–572. [PubMed] [Google Scholar]
- Steinberg M. H., Breiling B. J. Vascular lesions in hemophilia B. N Engl J Med. 1973 Sep 13;289(11):592–592. doi: 10.1056/NEJM197309132891119. [DOI] [PubMed] [Google Scholar]
- Teien A. N., Bjoornson J. Heparin elimination in uraemic patients on Haemodialysis. Scand J Haematol. 1976 Jul;17(1):29–35. [PubMed] [Google Scholar]
- Vessey M. P., Doll R. Investigation of relation between use of oral contraceptives and thromboembolic disease. Br Med J. 1968 Apr 27;2(5599):199–205. doi: 10.1136/bmj.2.5599.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAUGH D. F., FITZGERALD M. A. Quantitative aspects of antithrombin and heparin in plasma. Am J Physiol. 1956 Mar;184(3):627–639. doi: 10.1152/ajplegacy.1956.184.3.627. [DOI] [PubMed] [Google Scholar]
- WESSLER S., REIMER S. M., SHEPS M. C. Biologic assay of a thrombosis-inducing activity in human serum. J Appl Physiol. 1959 Nov;14:943–946. doi: 10.1152/jappl.1959.14.6.943. [DOI] [PubMed] [Google Scholar]
- WESSLER S. Thrombosis in the presence of vascular stasis. Am J Med. 1962 Nov;33:648–666. doi: 10.1016/0002-9343(62)90244-9. [DOI] [PubMed] [Google Scholar]
- Wessler S., Gitel S. N., Wan L. S., Pasternack B. S. Estrogen-containing oral contraceptive agents. A basis for their thrombogenicity. JAMA. 1976 Nov 8;236(19):2179–2182. [PubMed] [Google Scholar]
- Yin E. T., Wessler S. Bovine thrombin and activated factor X. Separation and purification. J Biol Chem. 1968 Jan 10;243(1):112–117. [PubMed] [Google Scholar]
- Yin E. T., Wessler S. Heparin-accelerated inhibition of activated factor X by its natural plasma inhibitor. Biochim Biophys Acta. 1970 Feb 24;201(2):387–390. doi: 10.1016/0304-4165(70)90316-8. [DOI] [PubMed] [Google Scholar]
- Yin E. T., Wessler S., Stoll P. J. Rabbit plasma inhibitor of the activated species of blood coagulation factor X. Purification and some properties. J Biol Chem. 1971 Jun 10;246(11):3694–3702. [PubMed] [Google Scholar]



