Abstract
Pancreata from Sprague Dawley rats of different developmental stages were studied to determine the expression and cellular localization of different c-MET isoforms in the developing rat pancreas. Pancreatic mRNA and protein expression levels of c-MET at different developmental stages from embryo to adult were detected by reverse transcription-polymerase chain reaction and by western blotting. To identify the cellular localization of c-MET protein in the developing rat pancreas, double immunofluorescent staining was performed using antibodies for cell type-specific markers and for c-MET. The expression of two isoforms of c-MET (190 kDa and 170 kDa) coincided with the development of the pancreas. The 190 kDa isoform of c-MET is expressed during embryonic stages, and its expression is replaced by the expression of the 170 kDa isoform as the pancreas develops. Only the 170 kDa isoform is expressed in the adult rat pancreas. Throughout all stages of pancreatic development, c-MET is expressed by vimentin-positive cells. In contrast, c-MET staining was stronger in rat pancreata from newborn to adult stages and overlapped with insulin-positive beta-cells. The dynamic expression and localization of different c-MET isoforms in the rat pancreas during different developmental stages indicates that distinct c-MET isoform might be involved in different aspects of pancreatic development.
Keywords: c-MET, development, isoform, pancreas
Introduction
The mammal pancreas is an endodermal organ that is composed of endocrine and exocrine. Pancreatic developmental dysregulation and dysmorphogenesis, including diabetes and pancreatic cancer, can lead to a high incidence and high mortality diseases. Therefore, in-depth understanding of the molecular mechanisms regulating pancreatic development will certainly help to improve the treatment of such diseases.
c-MET tyrosine kinase, the receptor for hepatocyte growth factor (HGF), is critical for normal development and cell survival [1-4] and was initially identified because its mutant forms have oncogenic potential. The HGF/c-MET signaling pathway is involved in a variety of biological functions, such as neuroendocrine cell formation, development, proliferation, regeneration, and angiogenesis [5-8]. Recent studies have indicated that both HGF and c-MET are expressed in the pancreas; HGF is expressed in endothelial and mesenchymal cells. c-MET also localizes to pancreatic progenitor cells, islet and ductal cells [9-12]. Furthermore, HGF/c-MET signaling increases islet survival in diabetic animals. However, HGF and c-MET global knockout mice exhibit early embryonic lethality and postnatal growth retardation, thus limiting their use in elucidating the role of HGF/c-MET signaling in embryonic development [13,14]. Recent evidence indicates that the survival of beta-cells is dramatically decreased in the absence of HGF/c-MET signaling, resulting in an accelerated onset of diabetes in pancreas-specific c-MET-null mice [15].
There are two 8 kb c-met RNA species that arise from alternative splicing and have the potential to encode two c-met RTK isoforms. One isoform is a 190 kDa heterodimer consisting of the 140 kDa β and 50 kDa α subunits. The β subunit spans the membrane and contains a tyrosine kinase catalytic domain, whereas the α subunit is localized extracellularly. The second isoform is a 170 kDa protein that has been demonstrated to have a distinct biological action from the 190 kDa isoform of c-MET [16]. Both c-MET RTK isoforms are autophosphorylated in an in vitro kinase assay. To investigate the physiological roles of different c-MET isoforms during pancreas development in the rat, we detected the pancreatic expression and cellular localization of the c-MET receptor during different developmental stages. Our data indicated that the expression and localization of c-MET coincided with the development of the pancreas. Interestingly, both protein isoforms of c-MET were expressed during pancreatic development in the rat. Based on the densitometric quantification of each isoform, the highest expression level of c-MET was detected between late gestation and 2-3 weeks after birth. We also investigated the cellular localization of c-MET and confirmed the presence of c-MET in mesenchymal and beta-cells of the developing rat pancreas. To the best of our knowledge, the expression and localization of different c-MET isoforms during pancreatic development in rats is poorly understood. This information describing the temporal and spatial expression and cellular localization of different c-MET isoforms will be useful in understanding their potential role in pancreatic development.
Materials and methods
All animal procedures were performed in strict adherence to the JSTD Guide for the Care and Use of Laboratory and Animals and were approved by the Committee on the Ethics of Animal Experiments of Nanjing Medical University.
Animals and pancreatic tissue preparation
Female and male Sprague-Dawley (SD) rats were mated. Noon on the day a vaginal plug was discovered was considered embryonic day 0.5 (E0.5). E12.5, E15.5, and E18.5 rat embryos were isolated from pregnant female SD rats (Nanjing Medical University, China), and pancreata were isolated from the embryos under a dissecting microscope. Postnatal day 0 (P0), P14, P21 and adult pancreata were isolated with the unaided eye. The tissues were immediately rinsed by phosphate-buffered saline (PBS) to remove serum. The tissues were immediately snap-frozen in liquid nitrogen and stored at -80°C for RNA and protein extraction. For immunohistochemistry and double immunofluorescence, tissues were fixed in 4% paraformaldehyde overnight at 4°C.
RNA extraction and reverse transcription-polymerase chain reaction
Total RNA was extracted from five pancreata with TRIZOL reagent (Invitrogen Life Technologies, Carlsbad, California, USA). RNA quality was verified by agarose gel electrophoresis. Two micrograms of DNA-free RNA primers and reverse transcriptase was used for each PCR reaction. PCR was performed in a total volume of 25 μl; each reaction contained 25 ng of cDNA, 0.2 nmol of primer pair, and 0.3 μl of Taq DNA polymerase (QIAGEN, CA). The primer pairs were as follows: c-met, forward: 5’-CCAGTTCAGAAAACGATG-3’, reverse: 5’-TAAGTGCCCGAAGTGTAA-3’ (1050 bp); 18S rRNA, forward: 5’-ACGAACCAGAGCGAAAGC-3’, reverse: 5’-GGACATCTAAGGGCATCACAG-3’ (514 bp). The PCR conditions were as follows: 2 min at 94°C for hot start, followed by 35 cycles of 95°C for 30 s, 55.4°C for 30 s, and 72°C for 45 s, with a final extension for 5 min at 72°C. The products were separated on 1% agarose gels and detected by ethidium bromide staining. All the data were normalized to 18S rRNA levels in each sample.
Western blot analysis
Total protein was extracted from individual tissues using a protein extraction kit (Beyotime Biotechnology, China). The protein concentration was determined using the BCA protein kit (Beyotime Biotechnology, China). For each developmental stage examined, a sample containing 50 μg protein (extracted from rat pancreas) in 20 μl buffer was boiled for 3 min, separated by 8% SDS-PAGE, and then transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The nitrocellulose membranes were incubated in a solution of 5% fat-free milk in Tris-buffered saline for blocking. The membranes were then incubated with a c-MET primary antibody (sc-8108, an affinity-purified mouse monoclonal antibody, diluted 1:200; Santa Cruz, USA) for 12 h at 4°C. After being washed in TBST three times (10 min each), the membranes were incubated with a rabbit anti-mouse IgG antibody (Santa Cruz) for 1 h at room temperature. The membranes were washed again three times in TBST (10 min each), incubated with chemiluminescence reagents (ECL, Amersham Life Science, USA) for 2 min, and exposed to VA711B Blue Sensitive X-ray film. Densitometric quantification of bands was performed using Syngenetool gel analysis software (Syngene, UK).
Immunohistochemistry
The E15.5 (embryo day 15.5), E18.5, newborn and nonpregnant adult pancreas tissues were fixed with 4% paraformaldehyde in PBS for 24 h and then embedded in paraffin. Five-micron-thick sections were cut and mounted on gelatin/chrome alum-coated glass slides. After deparaffinization, the presence of c-MET and insulin was detected immunohistochemically. To expose antigenic sites of the c-MET/insulin complex, sections were heated three times to 90°C in a 600 W microwave oven and maintained at 90°C for 7 min, then allowed to cool for 1 h. Endogenous peroxidase activity was blocked by incubating in 0.5% hydrogen peroxide solution in absolute methanol for 15 min. Non-specific binding was blocked by incubating in 10% non-fat milk in phosphate-buffered saline (PBS) for 1 h at room temperature. The sections were then incubated with a monoclonal antibody (sc-8108; Santa Cruz) against c-MET or against insulin (sc-9168; Santa Cruz) at a dilution of 1:100, respectively, for 12 h at 4°C and then incubated for 1 h with horseradish peroxidase-conjugated secondary antibody (1:1000 dilution) at room temperature. The antigen-antibody complex was visualized by incubating the sections in a solution of 3’-diaminobenzidine (DAB) solution for approximately 5 min in the dark. Images were taken at × 400 magnification.
Double immunofluorescence
Five-micrometer-thick sections were cut from paraffin blocks and mounted on gelatin/chrome alum-coated glass slides. The sections were deparaffinized in xylene and rehydrated in graded ethanol and distilled water. Non-specific binding was blocked by incubating in 1% BSA for 45 min. For c-MET staining, sections were incubated with a goat anti-c-MET primary monoclonal antibody and then with a fluorescein isothiocyanate (FITC)-labeled rabbit anti-goat IgG secondary antibody (1:100, sc-2777, Santa Cruz). Sections were then further stained for either insulin or vimentin. A mouse anti-insulin monoclonal antibody (1:500, ab6995, Abcam) or a mouse anti-vimentin monoclonal antibody (1:500, CBL202, Chemicon) was used as the primary antibody, and a cy3-labeled anti-mouse IgG antibody (1:400, AP192C, Chemicon) was used as a secondary antibody. Sections were placed in Gel Mount Aqueous Mounting Medium (G0918, Sigma), covered with a cover glass, and examined under an Olympus BX51 microscope. To rule out cross-reactivity, the following controls were used: first, single staining with an alternative secondary antibody, and second, staining in the absence of the primary antibody. No staining was detected in either of the controls. Images were taken at × 400 magnification.
Experiments were repeated at least three times.
Statistical analysis
Analysis of experimental data was performed using PDQuest 7.0 software (Bio-Rad Laboratories, USA) and paired t-tests. A value of P < 0.05 was considered statistically significant. Data are presented as the mean ± standard error.
Results
Expression of c-met mRNA during rat pancreatic development
The mRNA expression of c-met throughout the development of the rat pancreas was examined by using reverse transcription-PCR (Figure 1). c-met mRNA expression was detected at E15.5, increased at postnatal stages (P0, P21) and decreased again from P21 to adult.
Figure 1.
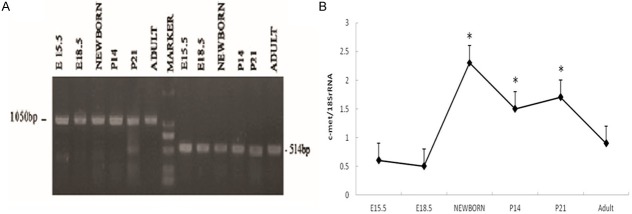
c-met mRNA expression levels in the developing rat pancreas as measured by reverse transcription-polymerase chain reaction. A: From embryonic day (E) 15.5 to adulthood, pancreatic c-met mRNA expression was greatest at newborn ages, with decreased expression in the adult (*P < 0.05 vs. E15.5, E18.5 and adult). B: c-met mRNA expression was analyzed and normalized to 18S rRNA levels. The results are indicated in percentages above the 18S rRNA value and are representative of three independent experiments.
Expression of c-MET isoforms during rat pancreatic development
To examine protein expression levels of c-MET during different stages of the developing rat pancreas, we performed western blotting on the pancreata of E15.5, E18.5, P0, P14, P21 and adult rats. The western blotting detected two isoforms of c-MET (190 kDa and 170 kDa) and showed that their expression patterns are associated with different developmental stages in the rat pancreas. The 190 kDa isoform was detected from E18.5 to P21 and was highly expressed at P21. In contrast, this isoform was not detectable in adult pancreas tissue. The 170 kDa isoform, which was detectable in the adult pancreas, was also expressed at P14 and P21 (Figure 2A). Based on the densitometric quantification of each isoform, the expression of c-MET was detected at low levels in the prenatal pancreas, dramatically increased at neonatal stages (P14, P21), and declined in the adult rat pancreas (Figure 2B). This temporal expression of the two c-MET isoforms indicates that each of them might have distinct physiological functions during the development of the rat pancreas.
Figure 2.
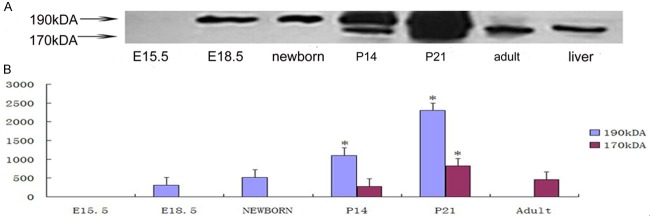
Western blot analysis of c-MET in the pancreas of E15.5, E18.5, P0, P7, P14, P21 and adult rats. A: Western blot analysis using anti-c-MET (sc-8108), an affinity-purified goat monoclonal antibody raised against a peptide mapping to the C-terminus of c-MET, revealed two bands in E18.5 and P0 samples. The molecular weight markers are indicated on the left. B: c-MET was dynamically expressed during development of the pancreas in the rat. We performed densitometric analyses for each band of each pancreatic sample using Syngenetool gel analysis software. Data are expressed as the integral optic density (IOD) of total bands and single bands for every sample of rat pancreas. Values presented are means ± standard deviation from three determinations.
Regional and temporal localization of c-MET in the rat pancreas at different developmental stages
To investigate the spatiotemporal localization of c-MET in the developing rat pancreas from fetal to postnatal stages, immunohistochemistry and double immunofluorescent staining were performed using antibodies for cell type-specific markers and for c-MET. We found that c-MET staining was modest in the developing rat pancreas from E18.5 to adult and overlapped with insulin-positive beta-cells. In addition, a few c-MET-positive cells were observed at the same developmental period and were scattered throughout the exocrine pancreas (Figures 3, 4). To further identify the cell localization pattern of c-MET, double immunofluorescent staining for c-MET and vimentin (a mesenchymal cell marker) was also conducted. Co-expression of c-MET and vimentin was detected throughout all stages of development in the rat pancreas (Figure 5). To the best of our knowledge, the expression of c-MET in the mesenchyme of the rat pancreas has not been previously reported. Collectively, the immunolocalization experiments demonstrated that c-MET was expressed not only in beta-cells but also in mesenchymal cells within the developing rat pancreas.
Figure 3.
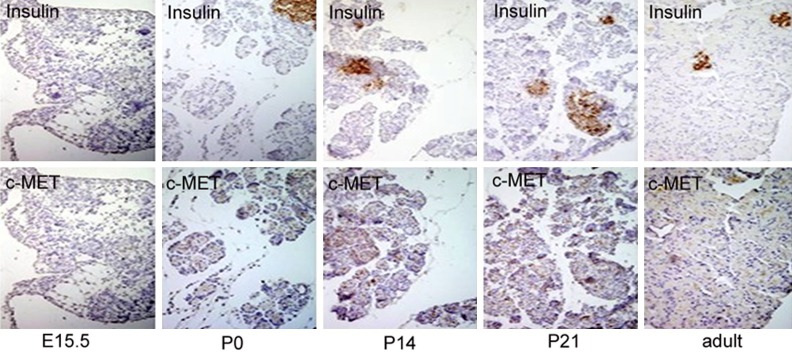
Immunohistochemical analysis of insulin and c-MET expression in the developing pancreas of E15.5, P0, P14, P21 and adult rats. Adjacent pancreatic sections from five stages were stained with antisera against insulin and c-MET. We acquired images using an OLYMPUS DP70 digital camera. Strong staining was observed for insulin for all stages except E15.5. c-MET immunolocalization experiments revealed sporadic positive staining in the developing pancreas. In P0, P14, P21 and adult rats, some pancreatic cells were stained, including non-islet cells. All magnifications were at × 400.
Figure 4.
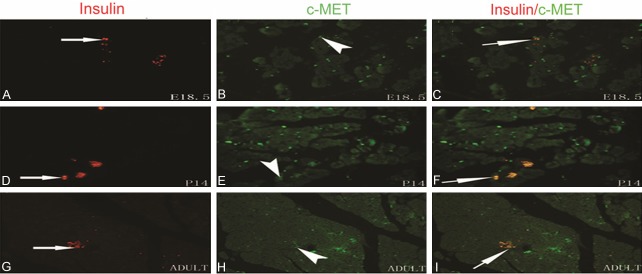
Immunofluorescence localization of c-MET and insulin in the developing pancreas in E18.5, P14, and adult rats. Immunofluorescence microscopy for c-MET (green) and insulin (red) was carried out on the same pancreas section. Modest, grouped staining for c-MET was observed in the developing pancreas from E18.5 to adult; the c-MET staining overlapped with insulin-positive beta-cells. The original magnification was × 400.
Figure 5.
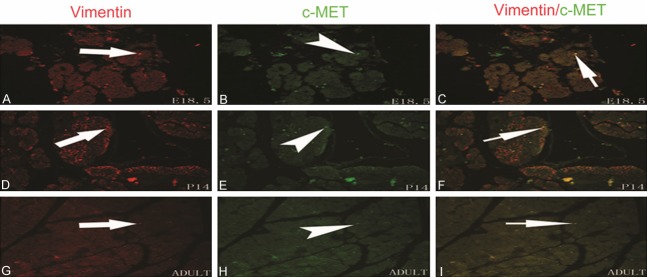
Immunofluorescence localization of c-MET and vimentin in the developing pancreas in E18.5, P14, and adult rats. Immunofluorescence microscopy for c-MET (green) and vimentin (red) was performed on the same pancreas section. Scattered but clearly stained c-MET-positive cells were detected in pancreas sections from E18.5 to adult; the c-MET staining overlapped with vimentin-positive cells. The original magnification was × 400.
Discussion
This study provides the first evidence of the expression of two protein isoforms of c-MET during pancreatic development in the rat. Each of the two isoforms is expressed at different stages of pancreatic development. The 190 kDa isoform of c-MET is expressed from E18.5 to P21 and is replaced by the 170 kDa isoform as development proceeds. Only the 170 kDa isoform is expressed in the adult pancreas. We assessed the protein expression levels of c-MET in the rat pancreas at different developmental stages. Our data indicated that c-MET protein expression levels varied temporally during the development of the rat pancreas. The expression of c-MET protein was first observed at E18.5, dramatically increased in neonatal stages (P14, P21), and decreased again in the adult rat pancreas. We also investigated the cellular localization of c-MET protein in the developing pancreas. Double labeling of c-MET and of cell type-specific markers confirmed the presence of the c-MET receptor on mesenchymal and beta-cells in the developing rat pancreas. Studies have reported that c-Met is expressed in pancreatic progenitor cells, islet, and ductal cells [9-12].
Although loss of c-Met from adult pancreas does not influence beta-cells or glucose homeostasis under basic conditions [9,11,15]. It was shown that absence of c-Met mediated cytokine induced beta-cells death and accelerated the incidence of diabetes in an animal model of low dose streptozotocin administration [15].Study has reported that HGF/c-Met signaling is critical for beta-cells adaptation during pregnancy and that its absence can lead to gestational diabetes [17]. Hepatocyte growth factor (HGF) is also a mitogen required for beta-cells replication during pregnancy [18]. The expression of HGF is up-regulated in rat islet endothelium at gestational 15 day, when the proliferation of maximal beta-cells is detected [10]. In our study, we detected that c-MET receptor localized on mesenchymal and beta-cells in the developing rat pancreas, and distinct c-MET isoform dynamically expressed in the different stages of developing rat pancreas. These results indicated that different c-MET isoform might be involved respectively in the regulation of development in the rat pancreas.
Organogenesis is a dynamic and tightly coordinated process that requires the spatial and temporal regulation of proliferation, differentiation, and morphogenesis [19-21]. The development of exocrine and endocrine pancreas tissue involves a process of differentiation that ultimately gives rise to two different enzyme-secreting cell types (acinar and duct cells) and four distinct hormone-producing cell types (α, β, δ, and PP) [22]. Descriptive studies of the developing rat pancreas have revealed that distinct phases can be delineated during embryonic stages in the rat. The mammalian pancreas originates from the foregut endoderm, commencing at approximately E9.5 (embryonic day 9.5) and proceeding to develop in an orderly fashion through three key phases: endoderm formation, pancreatic morphogenesis and the differentiation of exocrine and endocrine cells [23]. The first phase encompasses early organogenesis and is known as the “primary transition.” This phase occurs from approximately E10 to E12.5 and involves the shaping of the pancreatic diverticulum. Endocrine cells appear at this stage as individual cells that are not yet organized into islets [24,25]. This stage is followed by a “proto-differentiated state” (from E12.5 to E15.5) characterized by substantial epithelial cell proliferation, which leads to the rapid growth of the epithelium and ductule formation. During late embryonic stages, the third phase, or “secondary transitional phase” (from E15.5 to E18.5), occurs. This stage is characterized by a dramatic increase in the abundance of exocrine and insulin-positive cells (resulting from the rapid differentiation of progenitor cells), endocrine cell migration and adhesion. Primary islets take shape at this stage. However, mature islets are not fully formed until E18-E19; islets undergo further remodeling and postnatal maturation for approximately two to three weeks after birth [26]. However, the mechanisms that control the cellular differentiation and morphogenesis of the mammalian pancreas are not yet fully understood [27,28].
Several growth factors are involved in the physiological regulation of pancreatic organogenesis. HGF (signaling through its receptor c-MET) appears to be one such potential growth factor. The HGF/c-MET system stimulates the proliferation, scattering, invasion, and morphogenesis of epithelial cells [29,30]. During organogenesis, HGF/Met signaling has been involved in the formation of placenta and liver, as well as in the progress of hypaxial muscle precursors migration into limbs [4,13,14,31]. Our data demonstrate that during rat pancreatic development, c-MET mRNA and protein expression appeared around E18.5, which is during the progress ofpancreatic “secondary transitional phase”, when an increasing number of endocrine cells migrate. c-MET protein expression increased after birth and peaked at P21 (postnatal day 21); thereafter, c-MET expression declined. Interestingly, we found that the two isoforms of c-MET (190 kDa and 170 kDa) were dynamically expressed during the development of the rat pancreas. We found that the 190 kDa isoform was expressed in the fetal and neonatal pancreas, but it was not detectable in the adult pancreas. The 170 kDa isoform was expressed only after birth. This dynamic expression of c-MET isoforms during different stages of rat pancreatic development indicates that c-MET might play a role in the development of the pancreas, particularly during the postnatal remodeling stages, and that each isoform might play a distinct role during pancreatic development.
During organogenesis, epithelial cells receive essential signals from the overlying mesenchyme [32,33]. The pancreatic epithelium gives rise to exocrine and endocrine cells of the mature organ. Several experiments have shown that both exocrine and endocrine cells of the pancreas are derived from the pancreatic epithelium and that soluble factors produced by the mesenchyme are crucial for pancreatic development [34]. These mesenchyme-derived factors act on the developing epithelium to influence cell division and cellular fate decisions, even determining the proportion of endocrine and exocrine cells [35]. Recent studies revealed that mesenchymal cells derived from fetal or adult islets are capable of self-renewal and are multipotent [36]. Mesenchymal cells can be manipulated into differentiating into insulin-producing cells [37]. Moreover, studies have shown that chick foregut mesenchymal cells can differentiate into islet cells when co-cultured with pancreatic epithelium at early embryonic stages [38]. In the present work, we report evidence that c-MET is simultaneously expressed in mesenchymal and beta-cells from late embryonic to neonatal stages. Our data indicate that c-MET may play a role in the biological function of the mesenchyme during the development of the rat pancreas. However, further study is required to elucidate the different role of c-MET isoforms in this process.
In conclusion, the present study demonstrates for the first time that the two isoforms of c-MET are dynamically expressed in the rat pancreas during different developmental stages. We have revealed the spatio-temporal localization of c-MET within mesenchymal and beta-cells of the developing rat pancreas. Given that HGF/c-Met signaling is critical for beta-cells adaptation during pregnancy, the dynamic expression and localization of different c-MET isoforms in the developing rat pancreas, indicated that distinct c-MET isoform may play different role in pancreatic development, particularly at the islet remodeling stages after birth. Further investigation is needed to verify this hypothesis. These observations may highlight the HGF/c-met signal system as a potential therapeutic target for treatment of diabetes.
Acknowledgements
We would like to thank the Nanjing Medical University vivarium staff for their assistance with animal maintenance. We also thank Dr. Xiao-Ying Zang for helpful comments on a previous draft of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, Birchmeier C. The role of SF/HGF and c-Met in the development of skeletal muscle. Development. 1999;126:1621–9. doi: 10.1242/dev.126.8.1621. [DOI] [PubMed] [Google Scholar]
- 2.Giordano S, Ponzetto C, Di Renzo MF, Cooper CS, Comoglio PM. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature. 1989;339:155–6. doi: 10.1038/339155a0. [DOI] [PubMed] [Google Scholar]
- 3.Li PP, Madhavan R, Peng HB. Differential regulation of axonal growth and neuromuscular junction assembly by HGF/c-Met signaling. Dev Dyn. 2012;241:1562–74. doi: 10.1002/dvdy.23845. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 5.Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–10. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann V, Foroutan H, Sachs M, Weidner KM, Birchmeier W. Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J Cell Biol. 1995;131:1573–86. doi: 10.1083/jcb.131.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which Stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–41. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caton A, Hacker A, Naeem A, Livet J, Maina F, Bladt F, Klein R, Birchmeier C, Guthrie S. The branchial archesand HGF are growth-promoting and chemoattractant for cranial motor axons. Development. 2000;127:1751–66. doi: 10.1242/dev.127.8.1751. [DOI] [PubMed] [Google Scholar]
- 9.Dai C, Huh CG, Thorgeirsson SS, Liu Y. Beta-cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance. Am J Pathol. 2005;167:429–36. doi: 10.1016/s0002-9440(10)62987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–24. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- 11.Roccisana J, Reddy V, Vasavada RC, Gonzalez-Pertusa JA, Magnuson MA, Garcia-Ocaña A. Targeted inactivationof hepatocyte growth factor receptor c-Met in beta-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of beta-cell mass. Diabetes. 2005;54:2090–2102. doi: 10.2337/diabetes.54.7.2090. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes. 2004;53:2143–52. doi: 10.2337/diabetes.53.8.2143. [DOI] [PubMed] [Google Scholar]
- 13.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–71. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 14.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–5. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 15.Mellado-Gil J, Rosa TC, Demirci C, Gonzalez-Pertusa JA, Velazquez-Garcia S, Ernst S, Valle S, Vasavada RC, Stewart AF, Alonso LC, Garcia-Ocaña A. Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic beta-Cell death and accelerates the onset of diabetes. Diabetes. 2011;60:525–36. doi: 10.2337/db09-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues GA, Naujokas MA, Park M. Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol Cell Biol. 1991;11:2962–70. doi: 10.1128/mcb.11.6.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demirci C, Ernst S, Alvarez-Perez JC, Rosa T, Valle S, Shridhar V, Casinelli GP, Alonso LC, Vasavada RC, Garcia-Ocana A. Loss of HGF/c-Met signaling in pancreatic β-Cells leads to incomplete maternal β-Cell adaptation and gestational diabetes mellitus. Diabetes. 2012;61:1143–52. doi: 10.2337/db11-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.lvarez-Perez JC, Ernst S, Demirci C, Casinelli GP, Mellado-Gil JM, Rausell-Palamos F, Vasavada RC, Garcia-Ocaña A. Hepatocyte growth factor/c-Met signaling is required for β-cell regeneration. Diabetes. 2014;63:216–23. doi: 10.2337/db13-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crisera CA, Longaker MT, Gittes GK. Molecular approaches to understanding organogenesis. Semi Pediatr Surg. 1999;8:109–18. doi: 10.1016/s1055-8586(99)70011-9. [DOI] [PubMed] [Google Scholar]
- 20.Hammerman MR. Organogenesis of kidney and endocrine pancreas: the window opens. Organogenesis. 2007;3:59–66. doi: 10.4161/org.3.2.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo B, Strasser G, Sagolla M, Austin CD, Junttila M, Mellman I. Lkb1 regulates organogenesis and early oncogenesis along AMPK-dependent and -independent pathways. J Cell Biol. 2012;199:1117–30. doi: 10.1083/jcb.201208080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569–80. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 23.Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr Opin Genet Dev. 2002;12:540–7. doi: 10.1016/s0959-437x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 25.Mehta S, Gittes GK. Pancreatic differentiation. J Hepatobiliary Pancreat Surg. 2005;12:208–17. doi: 10.1007/s00534-005-0981-4. [DOI] [PubMed] [Google Scholar]
- 26.Habener JF, Kemp DM, Thomas MK. Transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–34. doi: 10.1210/en.2004-1576. [DOI] [PubMed] [Google Scholar]
- 27.Mfopou JK, Willems E, Leyns L, Bouwens L. Expression of regulatory genes for pancreas development during murine embryonic stem cell differentiation. Int J Dev Biol. 2005;49:915–22. doi: 10.1387/ijdb.052004jm. [DOI] [PubMed] [Google Scholar]
- 28.Min BH, Jeong SY, Kang SW, Crabo BG, Foster DN, Chun BG, Bendayan M, Park IS. Transient expression of clusterin (sulfated glycoprotein- 2) during development of rat pancreas. J Endocrinol. 1998;158:43–52. doi: 10.1677/joe.0.1580043. [DOI] [PubMed] [Google Scholar]
- 29.Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000;19:5582–9. doi: 10.1038/sj.onc.1203859. [DOI] [PubMed] [Google Scholar]
- 30.Weidner KM, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–6. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 31.Haines L, Neyt C, Gautier P, Keenan DG, Bryson-Richardson RJ, Hollway GE, Cole NJ, Currie PD. Met and Hgf signaling controls hypaxial muscle and lateral line development in the zebrafish. Development. 2004;131:4857–69. doi: 10.1242/dev.01374. [DOI] [PubMed] [Google Scholar]
- 32.Scharfmann R. Control of early development of the pancreas in rodents and humans: implications of signals from the mesenchyme. Diabetologia. 2000;43:1083–92. doi: 10.1007/s001250051498. [DOI] [PubMed] [Google Scholar]
- 33.Gradwohl G. Development of the endocrine pancreas. Diabetes Metab. 2006;32:532–3. doi: 10.1016/s1262-3636(06)72807-5. [DOI] [PubMed] [Google Scholar]
- 34.Teague WJ, Rowan-Hull AM, Jayanthi NV, Johnson PR. The competency of foregut mesenchyme in islet mesenchyme-to-epithelial transition during embryonic development. J Pediatr Surg. 2006;41:347–51. doi: 10.1016/j.jpedsurg.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Duvillié B, Attali M, Bounacer A, Ravassard P, Basmaciogullari A, Scharfmann R. The mesenchyme controls the timing of pancreatic beta-cell differentiation. Diabetes. 2006;55:582–9. doi: 10.2337/diabetes.55.03.06.db05-0839. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Liao L, Wang Q, Ma L, Ma G, Jiang X, Zhao RC. Isolation and identification of mesenchymal stem cells from human fetal pancreas. J Lab Clin Med. 2003;141:342–9. doi: 10.1016/S0022-2143(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 37.Seeberger KL, Dufour JM, Shapiro AM, Lakey JR, Rajotte RV, Korbutt GS. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab Invest. 2006;86:141–53. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- 38.Jayanthi NV, Rowan-Hull AM, Teague WJ, Johnson PR. The importance of pancreatic embryonic epithelium for mesenchyme-to-epithelial transition during islet development. Transplant Proc. 2005;37:3485–6. doi: 10.1016/j.transproceed.2005.09.026. [DOI] [PubMed] [Google Scholar]


