Abstract
Background and aim: Chitosan, is a natural polymer, plays an important role in prevention of tendon adhesion in tendon healing process. However, the molecular mechanisms underlying the prevention effect is unclear. Here we investigated the effects of chitosan on Achilles tendon injury rats and fibroblasts. Methods: Eight weeks after surgery, gliding excursion and the content of collagen fibers in Achilles tendon injury rats were determined to evaluate the chitosan effect on tendon healing. Fibroblasts isolated from scar tissue of repaired tendon were treated with different concentration of chitosan, and then cell inhibition, apoptosis and cell cycle were measured using MTT and Flow Cytometry respectively. The expression of microRNAs (miRNAs) was quantified by real-time PCR and protein expression of TGF-β1, Smad3 and P21 were quantified by western blotting. MiR-29b inhibitor was transfected in cells to evaluate the mechanism underlying the effects of chitosan on tendon fibroblasts. Results: The gliding excursion of repaired tendon was increased and the content of collagen fibers was decreased by chitosan in rats. Chitosan inhibited the fibroblasts growth and arrested cells in G1 phase. Chitosan also elevated the expression of miR-29b and P21 while reduced the levels of TGFβ1 and Smad3 in both repaired tendon and fibroblasts. In addition, miR-29b inhibitor revered the effects of chitosan on fibroblasts. Conclusions: The current study demonstrated that chitosan improving the condition of tendon healing after surgery, which is reduced by the high expression of miR-29b and its down-regulation of TGF-β1/Smad3 level and inhibition of fibroblasts growth.
Keywords: Tendon injury, chitosan, TGF-β1, miR-29b, fibroblasts
Introduction
In human body, Achilles tendon is the strongest as well as thickest tendon. Achilles tendon injury is a common but serious sports-related injury, the incidence of which is gradually improving with the popularity of mass sports [1]. Tendon rupture is not only an unbearable pain to the patient, but a possibility to be permanent disability. Surgery is a main strategy to treat tendon rupture in clinic.
However, tendon healing is a complex process involving the endogenous and exogenous mechanism [2]. In endogenous healing, the cells of tendon sheath proliferate and secret collagen. In exogenous process, hyperplasia of fibroblasts in tendon sheath stretches and damages cut tendon ends, which is necessary in the process of healing but also results in tendon adhesion [3]. Adhesion is regarded as the major problem of wound healing after surgery to plague clinicians.
Chitosan, a linear polymer of D-glucosamine, is well known to prevent the adhesion after tendon surgery [4-6]. The chitosan products are widely used in wound healing due to its biocompatibility, biodegradability, non-toxicity and adsorption properties [7]. It was reported that the inhibition of fibroblasts growth [8] and collagen synthesis are involved in the tendon adhesion by chitosan. Nevertheless, the mechanism underlying the effect of chitosan on improving the function of postoperative tendon is still unclear.
Transforming growth factor-beta (TGF-β) is a type of cytokine, and the role in pro-fibrosis is widely studied [9]. It was also shown that TGF-β appears to promote the tendon fibroblast proliferation and secretion of collagen [10], which is the core in adhesion formation after tendon surgery. Treated with TGF-β1 inhibitor has been reported to improve postoperative range of motion in zone-II flexor tendons in vivo study [11]. Smad proteins, transform TGF-β signals from the cell membrane to the nucleus, which act as a critical role in TGF-β regulation [12]. The recent study showed that knockout of Smad3 gene decreases scarring in flexor digitorum longus tendon repair model [13].
In addition, microRNAs (miRNAs) are involved in regulation of gene expression in various physiological processes via binding to the 3’untranslated regions of target genes. Significant changes occur in key miRNAs during wound healing [14]. It is also well known that miRNAs take part in the inhibition of fibroblasts by regulation of TGF-β1 pathway [15-17]. Therefore, we hypothesized that miRNAs may play a role in the effects of chitosan on tendon healing via regulation of TGF-β1/Smad3 pathway. The rat Achilles tendon injured model was established to test this hypothesis in present study.
Materials and methods
Experimental model
Six weeks old male Sprague Dawley rats were used in this experiment. All experiments with animals were approved by animal committee for ethics of The First Affiliated Hospital of Medical School of Zhejiang University. Rats were anaesthetized using halothane (50 mg/kg weight). A longitudinal incision was made from the base of the middle digit to the heel of the hind paw under tourniquet control. The flexor tendon lying below the exposed muscle was divided and the wound sutured. 50 mg of chitosan was administered into the wound site of eight animals following surgical procedure (Group 1). A further eight animals received 50 mg normal saline into the wound (Group 2).
Rats were sacrificed at eight weeks after operation, the gliding excursion of tendon was determined and the collagen fiber content in adhesions was calculated by formula as follow: total content of collagen fibers = content of hydroxyproline/12.5.
Repaired tendon tissue was isolated to make homogenate and the expression of miR-155, miR-29b, miR-21, miR-133b, let-7 and protein expression of TGF-β1, P21, p-Smad3 and Smad3 were detected.
Fibroblasts extraction and culture
Fibroblasts were extracted from scar tissue of repaired tendon sites and incubated in DMEM containing 10% fetal bovine serum, 1% penicillin, 1% streptomycin and 200 U/ml collagenase IV (Invitrogen). The suspension was filtered after digestion to get Fibroblast cells and then cultured with DMEM at 37°C in humidified atmosphere of 5% CO2. MTT was used to measure the cell number of fibroblasts.
Cell cycle analysis
Cell cycle analysis of fibroblast cells was performed via flow cytometry using a FACSCalibur (Becton Dickinson). Briefly, cells were harvested, and then fixed and permeabilized in 100% ice-cold methanol. PI staining was performed by incubation with propidium iodide (50 µg/ml) plus RNase A (125 µg/ml) for 45 min at room temperature. Flow cytometric analysis was performed.
Apoptosis analysis
Apoptosis was determined by staining cells with PI (BD Biosciences, 556463) and Annexin V-FITC (BD Biosciences, 556419) according to the manufacturer’s protocol, followed by flow cytometry analysis. In brief, cells were harvested as described above and then trypsinized. Samples containing 1 × 105 cells were washed with cold PBS and resuspended in 100 μl binding buffer. Then, 2 μl Annexin V-FITC and 5 μl PI were added to the cells and incubated for 15 min at room temperature darkly. An additional 400 μl binding buffer was added to the reaction prior to analysis.
Western blotting
Cells were harvested and prepared to do western blotting. Proteins were loaded onto the sodium dodecyl sulfate-polyacrylamide gels for electrophoresis and transferred to PVDF membranes, which were then blocked with 5% BSA prior to incubation with the indicated primary antibodies and the secondary antibodies (Cell signaling, USA). Immunoreactivity was determined using enhanced chemiluminescence (Millipore, USA) and observed using autoradiography (Protein Simple, USA). β-actin was served as a control of the amount of protein loading.
Quantitative real-time PCR
MiRNAs from cells were extracted using the TRIzol reagent (Invitrogen, USA) and 2 µg of RNA was used to reverse transcription-PCR with ImProm-II™ (Promega, USA) following the manufacturer’s instructions. The miRNAs levels were quantified by real-time PCR using TransStartTM SYBR Green qPCR Supermix (TransGen Biotech, China), and with U6 small nuclear RNA served as an internal normalized reference.
Luciferase reporter assay
The PCR-amplified CDS fragments of TGF-β1 exon were cloned into pCDNA3.1/ZEO(+)-luc vector to generate TGF-β1 CDS reporter constructs, and then vectors with miR-29b binding sites were cotransfected into fibroblasts together with pCDNA3 plasmid by Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. After 24 h, relative luciferase activities were measured using the Dual-Luciferase Reporter Assay (Promega, USA).
Downregulation of miR-29b and p-Smad3
Fibroblasts were transiently transfected with miR-29b inhibitor using the Lipofectamine 2000 reagent (Invitrogen, USA) according to the manufacturer’s instructions. The miR-29b inhibitor was synthesized by RiboBio Co., Ltd. (Guangzhou, China). In brief, cells were harvested and transfected with miR-29b inhibitor and then incubated in fresh RPMI-1640 with 10% FBS for 24 h. Transfection efficiency was monitored by fluorescent images. p-Smad3 was suppressed by 3 μmol/lSIS3 hydrochloride (Absin, Shanghai).
Statistical analysis
All data were presented as means ± SD. Each experiment in vitro was performed at least in triplicate. Variance (ANOVA) or Student’s t-test by SPSS 17.0 software was performed to statistical analysis. P value < 0.05 was regarded as statistically significant.
Results
The effects of chitosan on repaired tendon tissue in rats
The model of Achilles tendon injury in rats was established. As showed in Figure 1A, the gliding excursion was significantly increased by chitosan treatment at eight weeks after surgery. The content of collagen fibers in tendon tissue was decreased in chitosan group (Figure 1B). The expression of miRNAs including miR-155, miR-29b, miR-21, miR-133b and let7 were detected in repaired tendon tissue, and only miR-29b by chitosan interference was observed significantly higher than that in control group (Figure 1C). As showed in Figure 1D, the protein expression of TGF-β1 and p-Smad3 were decreased and P21 was increased in group treated with chitosan.
Figure 1.
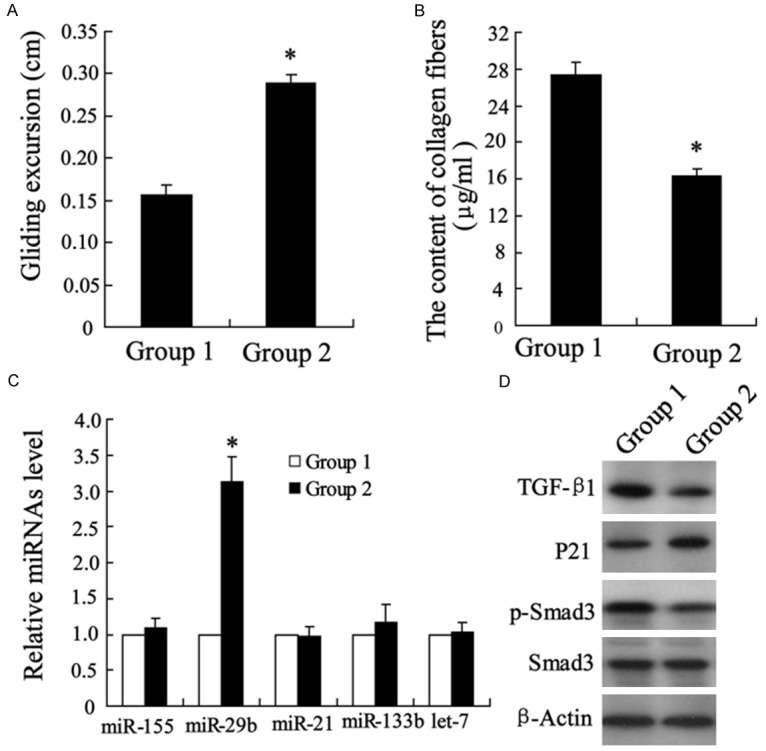
The effects of chitosan on repaired tendon tissue in rats. The Achilles tendon injury model of rats was established. Eight weeks after surgery, repaired tendon tissues were isolated from rats. Gliding excursion (A) and the content of collagen fibers (B) were measured. MicroRNAs expression was detected by quantitative real-time PCR (C). TGF-β1, P21, p-Smad3 and Smad3 proteins were detected by Western blotting (D). Achilles tendon injured rats with 50 mg normal saline or chitosan were regarded as Group 1 or Group 2 respectively. *P < 0.05 by Student’s t-test, indicates a significant difference from the Group 1.
The effects of chitosan on fibroblasts survival, apoptosis and cell cycle
To identify the mechanisms underlying the effects of chitosan on the healing of Achilles tendon injury, fibroblasts were isolated from the scar tissue of repaired tendon. Treated the fibroblasts with different concentrations (0 μg/ml, 5 μg/ml, 10 μg/ml and 20 μg/ml) of chitosan resulted in an inhibition of cell growth in a dose-dependent manner (Figure 2A), but had no effect on apoptosis rate (Figure 2B). In addition, the chitosan dose-dependently increased the cells number in G1 phase and decreased the number in S phase (Figure 2C, 2D). The low concentration of chitosan (5 μg/ml) had no significant changes in cell growth, apoptosis rate and cell cycle.
Figure 2.
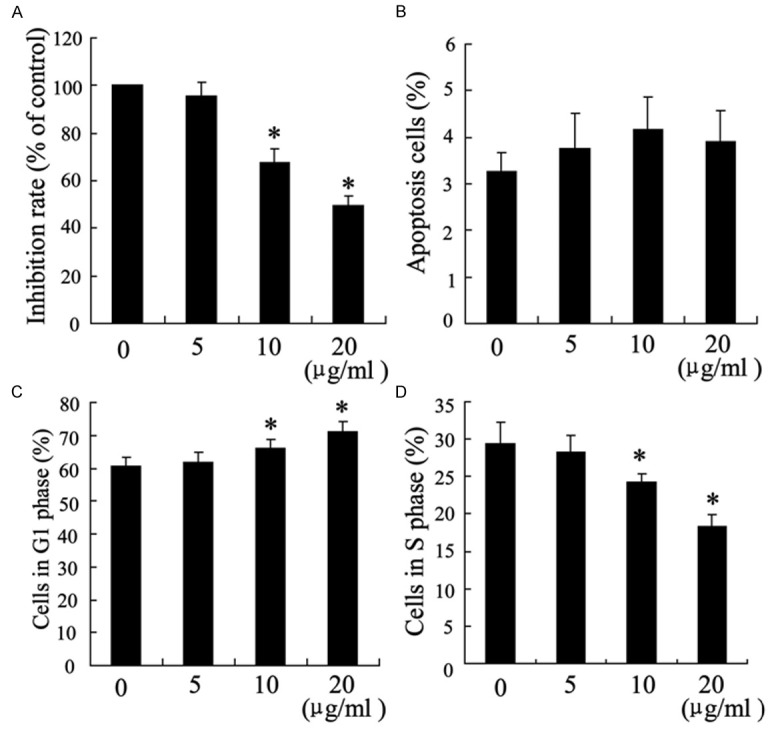
The effects of chitosan on fibroblasts survival, apoptosis and cell cycle. Fibroblasts were treated with chitosan (0 μg/ml, 5 μg/ml, 10 μg/ml and 20 μg/ml) for 24 hours. 104 cells per ml were used to determine cell survival rate by MTT assays (A). Apoptosis (B) and cell cycle (C, D) were evaluated with Flow Cytometry. *P < 0.05 by Student’s t-test, indicates a significant difference from the group without chitosan treatment. All experiments were repeated three times.
The effects of chitosan on miR-29b, TGF-β1, p-Smad3 and P21 expression in fibroblasts
At 24 after treatment with chitosan (10 μg/ml and 20 μg/ml), miR-29b was expressed at a higher level in fibroblasts in a dose-dependent manner (Figure 3A), and the expression of TGF-β1 and p-Smad3 were lower than that in control group (Figure 3B). Similar to the results of vivo study, P21 was also higher expressed in chitosan treatment group (Figure 3B).
Figure 3.
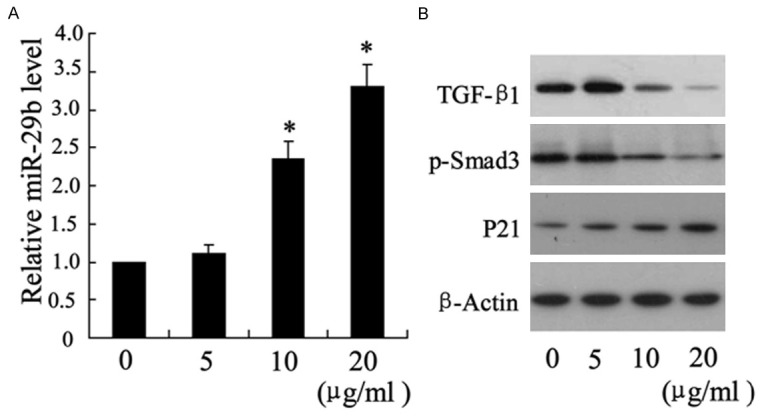
The effects of chitosan on miR-29b, TGF-β1, p-Smad3 and P21 expression in fibroblasts. Fibroblasts were treated with chitosan (0 μg/ml, 5 μg/ml, 10 μg/ml and 20 μg/ml) for 24 hours. The expression of miR-29b was detected by real-time PCR (A), TGF-β1, p-Smad3 and P21 were measured by western blotting (B). *P < 0.05 by Student’s t-test, indicates a significant difference from the group without chitosan treatment. All experiments were repeated three times.
The effects of chitosan on binding of miR-29b to CDS of TGF-β1
To investigate the effect of chitosan on binding of miR-29b to TGF-β1, we conducted luciferase reporters with miR-29b binging sits on the coding sequence (CDS) of TGF-β1. As shown in Figure 4, the chitosan treatment increased the level of relative luciferase activity in a dose-dependent manner, which implied the enhancement in binding of miR-29b and TGF CDS.
Figure 4.
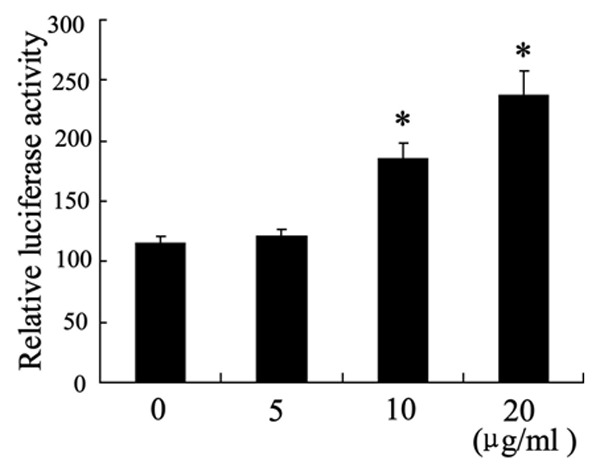
The effects of chitosan on binding of miR-29b to CDS of TGF-β1. Fibroblasts were treated with chitosan (0 μg/ml, 5 μg/ml, 10 μg/ml and 20 μg/ml) for 24 hours. The relative luciferase activity was determined by CDS reporter assays. *P < 0.05 by Student’s t-test, indicates a significant difference from the group without chitosan treatment.
The effect of miR-29b inhibitor on TGF-β1/Smad3 and P21 expression in fibroblasts
The expression of miR-29b was down-regulated by miR-29b inhibitor (Figure 5A). The TGF-β1/p-Smad3 and P21 were detected to evaluate the role of miR-29b. As shown in Figure 5B, 5C, the expression of TGF-β1/p-Smad3 were reduced and P21 was elevated by chitosan. MiR-29b inhibitor reversed the effect of chitosan on TGF-β1/p-Smad3 and P21 expression (Figure 5B, 5C). In addition, SIS3 hydrochloride, which is a specific p-Smad3 inhibitor, reversed the effect of miR-29b on P21 expression (Figure 5C).
Figure 5.
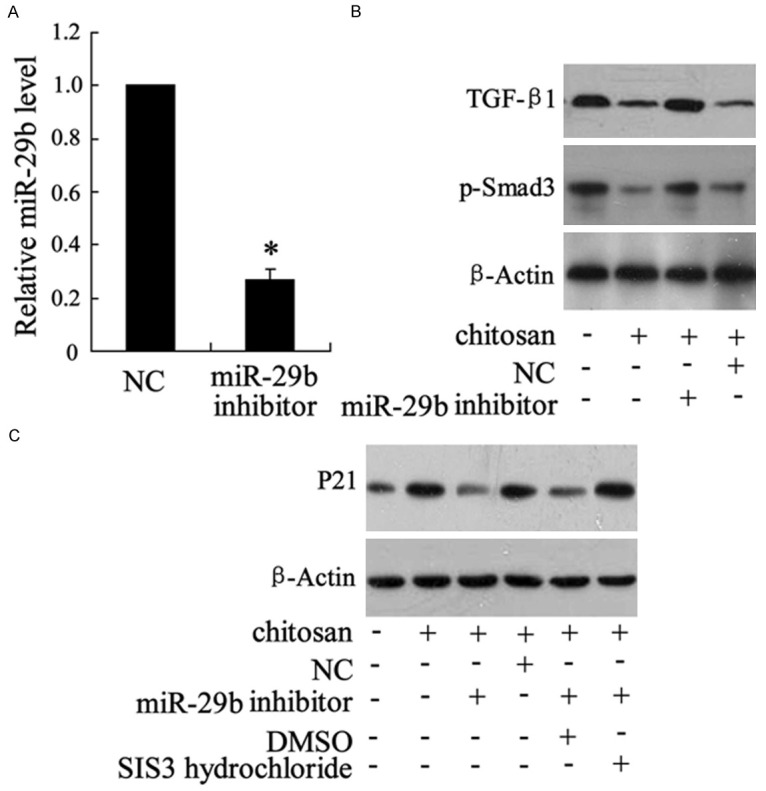
The effect of miR-29b inhibitor on TGF-β1/Smad3 and P21 expression in fibroblasts. Fibroblasts were transfected with miR-29b inhibitor. 50 μg/ml chitosan was used in this experiment. The expression of miR-29b was detected by real-time PCR (A), TGF-β1 and p-Smad3 were measured by western blotting (B), P21 was also determined by western blotting (C). *P < 0.05 by Student’s t-test, indicates a significant difference from the NC group.
The effect of miR-29b inhibitor on cell growth and cell cycle in fibroblasts
Chitosan significantly reduced the survival of tendon fibroblasts and cell number in S phase but elevated the cells number in G1 phase (Figure 6). The effect of chitosan on cell survival and cell cycle were reversed by miR-29b inhibitor (Figure 6).
Figure 6.
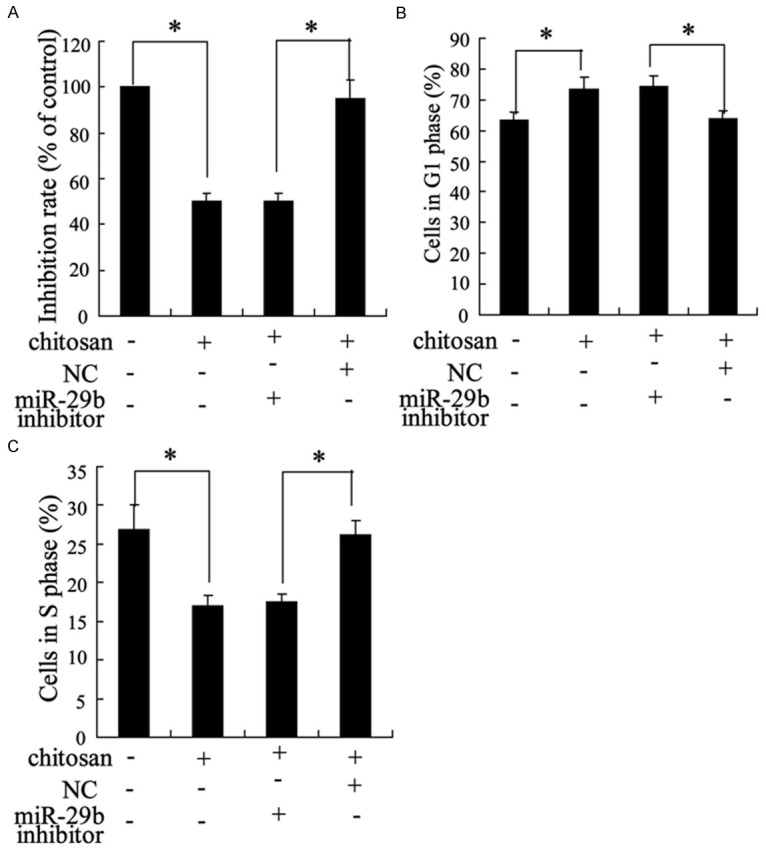
The effect of miR-29b inhibitor on cell growth and cell cycle in fibroblasts. Fibroblasts were treated with chitosan and (or) miR-29b inhibitor. MTT assay was used to detect the cell survival rate (A) and Flow Cytometry was applied to determine the cell cycle (B, C). *P < 0.05 by Student’s t-test, indicates a significant difference between two groups.
Discussion
Tendon adhesion accompanied by Achilles tendon healing after surgery is a puzzled problem to orthopeadic doctors. This study was conducted to investigate the molecular mechanisms underlying the positive effects of chitosan on postoperative Achilles tendon healing. The major finding of this study is that treatment with chitosan markedly increased the expression of miR-29b in both rat repaired tendon tissue and fibroblasts isolated from it, which contributed to the inhibition of fibroblasts survival and make cell cycle arrest in G1 phase via regulating TGF-β1/p-Smad3 pathway and P21 expression.
Chitosan, is a natural polymer from deacetylation of chitin (poly-N-acetylglucosamine), has been widely applied to topical dressing in wound healing due to its stypticity, antimicrobial and nontoxic, biocompatible and biodegradable properties [18-20]. Xia et al. demonstrated that chitosan inhibits tendon sheath cells proliferation and collagen production [21]. We also found that chitosan decreases the content of collagen fibers in repaired tendon tissue and inhibits fibroblasts survival, which could be one of the reasons for the increase of tendon gliding. In addition, the expression of P21, which is the gene encodes a potent cyclin-dependent kinase inhibitor and functions as a regulator of cell cycle progression at G1 [22], was up-regulated by chitosan treatment in fibroblasts. It was confirmed by cell cycle experiments that tendon fibroblasts was arrested in G1 phase by chitosan.
To further clarify the molecule mechanisms of chitosan involved in the inhibition of collagen production and fibroblasts growth, we investigated the effects of chitosan on the expression of miRNAs. Quantitative real-time PCR was employed to determine the expression of miR-155, miR-29b, miR-21 and miR-133b and let-7 in repaired tendon tissue, which are proved having a close relationship with fibrosis or tendon fibroblast proliferation [23,24]. The present data showed that only the level of miR-29b was increased by chitosan treatment in tendon tissue. The up-regulation of miR-29b was also observed in fibroblasts. These results imply that miR-29b is involved in the protective effects of chitosan in tendon injury.
Down-regulation of miR-29b has been shown to promote cardiac fibrosis and systemic sclerosis via the TGF-β1/Smad3 pathway [15,25]. TGF-β1 is a key mediator of tissue fibrosis in pathological status and phosphorylation of Smad family plays an important role in transmitting TGF-β1 from cell surface receptor to the nucleus [26]. In addition, It was reported that the TGF-β1/Smad3 pathway was involved in the promoting both tendon fibroblast proliferation and collagen synthesis [13,27], the underlying mechanism of which is related to the regulation of cell cycle by TGF-β1/Smad3 [28]. The present study showed that the levels of TGF-β1 and p-Smad3 were decreased while the expression of miR-29b was increased by chitosan in rat repaired tendon tissue and fibroblasts. It was also observed that chitosan enhanced the binding of miR-29b to CDS of TGF-β1 in a dose-dependent manner in fibroblasts. A further study showed that miR-29b inhibitor attenuated the effects of chitosan on the expression of TGF-1β, p-Smad3 and P21, and decreased the inhibition of fibroblasts induced by chitosan. SIS3 hydrochloride, is a specific inhibitor of Smad3 phosphorylation, up-regulated the expression of P21 protein. These results suggest that regulation of TGF-β1/Smad3 via miR-29b is involved in the inhibition of fibroblasts proliferation by chitosan in process of tendon healing after surgery.
In conclusion, our study revealed that chitosan increases the gliding excursion of Achilles tendon injuried rats after surgery and inhibits the tendon fibroblasts growth in vitro experiments. Our results also suggest that the positive effects of chitosan on tendon healing are contributing to the high expression of miR-29b and its down-regulation of TGF-β1/Smad3 and up-regulation of fibroblasts number in G1 phase. The present findings offer a theoretical basis for better application of chitosan to tendon healing.
Disclosure of conflict of interest
None.
References
- 1.Maquirriain J. Achilles tendon rupture: avoiding tendon lengthening during surgical repair and rehabilitation. Yale J Biol Med. 2011;84:289–300. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen CH, Wu YF, Tang JB. Molecular Biology of Tendon Healing. Tendon Surgery of the Hand. 2012:44. [Google Scholar]
- 3.Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care. 2013;22:407–8. 10–12. doi: 10.12968/jowc.2013.22.8.407. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13:382–5. [PubMed] [Google Scholar]
- 5.Dai T, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther. 2011;9:857–79. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Altiok D, Altiok E, Tihminlioglu F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J Mater Sci Mater Med. 2010;21:2227–36. doi: 10.1007/s10856-010-4065-x. [DOI] [PubMed] [Google Scholar]
- 8.Jing C, Wan-shun L, Bao-qin H. Study on mechanism of preventing tendon adhesion by CTSb compound membrane. Journal of Functional Materials (China) 2011 [Google Scholar]
- 9.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. J Hand Surg Am. 2002;27:615–20. doi: 10.1053/jhsu.2002.34004. [DOI] [PubMed] [Google Scholar]
- 11.Bates SJ, Morrow E, Zhang AY, Pham H, Longaker MT, Chang J. Mannose-6-phosphate, an inhibitor of transforming growth factor-beta, improves range of motion after flexor tendon repair. J Bone Joint Surg Am. 2006;88:2465–72. doi: 10.2106/JBJS.E.00143. [DOI] [PubMed] [Google Scholar]
- 12.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 13.Katzel EB, Wolenski M, Loiselle AE, Basile P, Flick LM, Langstein HN, Hilton MJ, Awad HA, Hammert WC, O’Keefe RJ. Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J Orthop Res. 2011;29:684–93. doi: 10.1002/jor.21235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson PM, Chuang TD, Sriram S, Pi L, Luo XP, Petersen BE, Schultz GS. MicroRNA signature in wound healing following excimer laser ablation: role of miR-133b on TGF-beta1, CTGF, SMA, and COL1A1 expression levels in rabbit corneal fibroblasts. Invest Ophthalmol Vis Sci. 2013;54:6944–51. doi: 10.1167/iovs.13-12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM, Lan HY. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-beta/Smad3 signaling. Mol Ther. 2014;22:974–85. doi: 10.1038/mt.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu H, Luo H, Li Y, Zhou Y, Jiang Y, Chai J, Xiao X, You Y, Zuo X. MicroRNA-21 in scleroderma fibrosis and its function in TGF-beta-regulated fibrosis-related genes expression. J Clin Immunol. 2013;33:1100–9. doi: 10.1007/s10875-013-9896-z. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Ricardo S. Role of microRNA machinery in kidney fibrosis. Clin Exp Pharmacol Physiol. 2014;41:543–50. doi: 10.1111/1440-1681.12249. [DOI] [PubMed] [Google Scholar]
- 18.Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4:1457–65. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 19.Ong SY, Wu J, Moochhala SM, Tan MH, Lu J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29:4323–32. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 20.Kojima K, Okamoto Y, Kojima K, Miyatake K, Fujise H, Shigemasa Y, Minami S. Effects of chitin and chitosan on collagen synthesis in wound healing. J Vet Med Sci. 2004;66:1595–8. doi: 10.1292/jvms.66.1595. [DOI] [PubMed] [Google Scholar]
- 21.Xia CS, Hong GX, Dou RR, Yang XY. Effects of chitosan on cell proliferation and collagen production of tendon sheath fibroblasts, epitenon tenocytes, and endotenon tenocytes. Chin J Traumatol. 2005;8:369–74. [PubMed] [Google Scholar]
- 22.Ouellet S, Vigneault F, Lessard M, Leclerc S, Drouin R, Guerin SL. Transcriptional regulation of the cyclin-dependent kinase inhibitor 1A (p21) gene by NFI in proliferating human cells. Nucleic Acids Res. 2006;34:6472–87. doi: 10.1093/nar/gkl861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendias CL, Gumucio JP, Lynch EB. Mechanical loading and TGF-beta change the expression of multiple miRNAs in tendon fibroblasts. J Appl Physiol. 2012;113:56–62. doi: 10.1152/japplphysiol.00301.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin MM, Lee EJ, Buckenberger JA, Schmittgen TD, Elton TS. MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts. J Biol Chem. 2006;281:18277–84. doi: 10.1074/jbc.M601496200. [DOI] [PubMed] [Google Scholar]
- 25.Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–43. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 26.Ihn H. The role of TGF-beta signaling in the pathogenesis of fibrosis in scleroderma. Arch Immunol Ther Exp (Warsz) 2002;50:325–31. [PubMed] [Google Scholar]
- 27.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–98. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 28.Pardali K, Kowanetz M, Heldin CH, Moustakas A. Smad pathway-specific transcriptional regulation of the cell cycle inhibitor p21(WAF1/Cip1) J Cell Physiol. 2005;204:260–72. doi: 10.1002/jcp.20304. [DOI] [PubMed] [Google Scholar]


