Abstract
Histopathological malignancy grading of human gliomas is limited by subjective interpretation of the morphological criteria. Assessment of mitotic activity is a cornerstone of grading these tumours, but mitotic figures can be hard to identify in haematoxylin-eosin stained sections. Thus, determining proliferative activity by means of Ki-67/MIB-1 immunostaining has become a useful supplement. However, this method has drawbacks, so continuous testing and evaluation are required for optimization and standardization. The aim of this study was to analyse and evaluate the Ki-67/MIB-1 proliferative indices (PIs) in a series of gliomas. We found that Ki-67/MIB-1 PIs correlated well with histological malignancy grade in all glioma subtypes, but a considerable overlap of PIs was observed between the malignancy groups. Consequently, Ki-67/MIB-1 immunostaining alone is not sufficient to adequately determine the malignancy grade. Therefore, future work is necessary to clarify the role of this immunostaining in the histopathological diagnosis of human gliomas.
Keywords: Astrocytoma, brain tumour, diagnosis, glioblastoma, immunohistochemistry, proliferation
Introduction
Histopathological classification and malignancy grading of human gliomas are based on criteria issued by the World Health Organization (WHO) [1]. However, these criteria are encumbered with subjective interpretations, giving rise to inter- and intra-observer variability [2,3]. Because proliferation is a basic process in gliomagenesis, mitotic counting constitutes a cornerstone in the grading of these tumors. Since identification and counting of mitotic figures in haematoxylin-eosin stained sections can be difficult, glioma grading is imprecise and may unfavorably impact prognosis, treatment, and follow-up.
Immunohistochemical determination of proliferative activity is a useful supplement for establishing the histopathological diagnosis of glioma. Ki-67/MIB-1 immunostaining is most commonly used and has been shown to correlate positively with tumor grade and prognosis [4-6]. Despite its widespread use, the procedure still has many uncertain and limiting factors, including problematic overlap of indices between different glioma grades and inherent problems in the immunohistochemical analysis [5-9]. Thus, publishing data on Ki-67/MIB-1 immunostaining in human gliomas is still worthwhile in order to optimize this method, with the superior goal of achieving a standardized procedure. The aim of this study was to evaluate the Ki-67/MIB-1 proliferative indices (PIs) in a series of gliomas and critically evaluate the findings and procedure.
Materials and methods
Patients
This study includes a series of gliomas in adults (over 16 years of age) who underwent operations at St. Olavs University Hospital in Trondheim, Norway, during the time period 1998-2013. Both the histopathological diagnosis (according to the WHO classification system) and determination of the Ki-67/MIB-1 PI were performed in collaboration by AJS and SHT. All patients were found by searching the electronic patient data files of the pathology department. Patients were included at primary diagnosis and all cases were diagnosed based solely on WHO classification system, and in addition the Ki-67/MIB-1 PIs were continuously registered in a spreadsheet. Diagnosis were made independent of Ki-67/MIB-1 PIs, but in cases were the PI was unusually high, a comment was made in the diagnosis. However, this did not change the WHO grade.
Immunohistochemistry
All tumor samples were fixed in buffered formalin, usually for not more than 24 hours, and then embedded in paraffin. Paraffin sections (3-μm-thick) were cut and mounted on Superfrost glass slides, deparaffinized, and dehydrated. Different antigen retrieval methods were used during the study period, including pressure cooking, microwave oven, and water bath. The Ki-67/MIB-1 antibody was supplied by Immunotech (Hamburg, Germany) and by DAKO (Glostrup, Denmark). The working dilution was 1:100 or 1:600 depending on the detection system used. The sections were incubated for 40 min at room temperature. Automatized immunohistostainers and detection systems were purveyed by DAKO (TechMate 500, Autostainer Plus, Autostainer Link 48). The staining procedures were performed according to the manufacturer’s recommendations. Positive controls were used in each staining run (“sausage block” with tonsil, appendix, pancreas, and liver). First, a standard streptavidin-biotin-peroxidase technique was used, and later the DAKO EnVision Flex+ System. Diaminobenzidine was used as the chromogene and haematoxylin as the counterstain.
Proliferation index evaluation
The immunostained sections were scanned using a 40× objective with an eye grid for the areas with the highest density of labeled tumor cells (hot spots). At least 1000 tumor cells, or alternatively three high power fields (HPF) were examined. Only immunoreactive tumor cell nuclei were counted. Necrotic areas and vascular endothelium were excluded. The Ki-67/MIB-1 PI was defined as the percentage of immunoreactive tumor cell nuclei among the total number of cells.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics version 21. The Mann-Whitney U test was applied to estimate differences in the PIs between groups of tumors. P < 0.05 was considered significant.
Results
A total of 267 glioma subtypes were examined: 186 astrocytomas, 39 oligodendrogliomas, 20 mixed gliomas, and 22 ependymal tumors. Table 1 shows all data and statistical correlations for the tumor subtypes. The Ki-67/MIB-1 PIs are graphically illustrated in Figure 1.
Table 1.
Ki-67/MIB-1 proliferative indices for glioma subtypes
| Diagnosis (abbreviations) | WHO grade | n | Median age, years (range) | Median Ki-67/MIB-1 PIa (range) | Statistical analysisb |
|---|---|---|---|---|---|
| Pilocytic astrocytoma (PILOCYT) | I | 12 | 38 (17-65) | 1.9 (0.7-8.1) | vs. diffuse astrocytoma: P = 0.004 |
| Diffuse astrocytoma (A) | II | 57 | 44 (18-78) | 5.2 (0.5-16.6) | vs. oligodendroglioma: P = 0.218 |
| vs. oligoastrocytoma: P = 0.287 | |||||
| vs. anaplastic astrocytoma and glioblastoma: P < 0.001 | |||||
| Oligo-astrocytoma (OA) | II | 13 | 42 (26-73) | 4.0 (1.0-13.3) | vs. anaplastic oligoastrocytoma: P = 0.006 |
| Oligodendroglioma (O) | II | 27 | 44 (21-72) | 4.4 (1.0-22.0) | vs. oligoastrocytoma: P = 0.798 |
| vs. anaplastic oligodendroglioma: P < 0.001 | |||||
| Anaplastic astrocytoma (AA) | III | 28 | 52 (19-82) | 13.1 (2.1-39.1) | vs. anaplastic oligoastrocytoma: P = 0.643 |
| vs. anaplastic oligodendroglioma: P = 0.122 | |||||
| vs. glioblastoma: P = 0.002 | |||||
| Anaplastic oligoastrocytoma (AOA) | III | 7 | 53 (33-71) | 12.7 (6.3-39.0) | vs. anaplastic oligodendroglioma: P = 0.006 |
| Anaplastic oligodendroglioma (AO) | III | 12 | 49 (31-78) | 21.7 (2.4-41.2) | |
| Glioblastoma (GBM) | IV | 89 | 65 (30-89) | 19.4 (2.2-80.0) | vs. anaplastic oligodendroglioma: P = 0.867 |
| vs. anaplastic oligoastrocytoma: P = 0.035 | |||||
| Sub-ependymoma (SUBEP) | I | 9 | 38 (22-62) | 1.0 (0.1-8,5) | vs. ependymoma: P = 0.126 |
| Ependymoma (EP) | II | 13 | 55 (27-74) | 2.0 (0.6-25.3) | |
| Anaplastic ependymoma | III | 0 |
PI = proliferation index;
Significance was determined using the Mann-Whitney U test.
Figure 1.
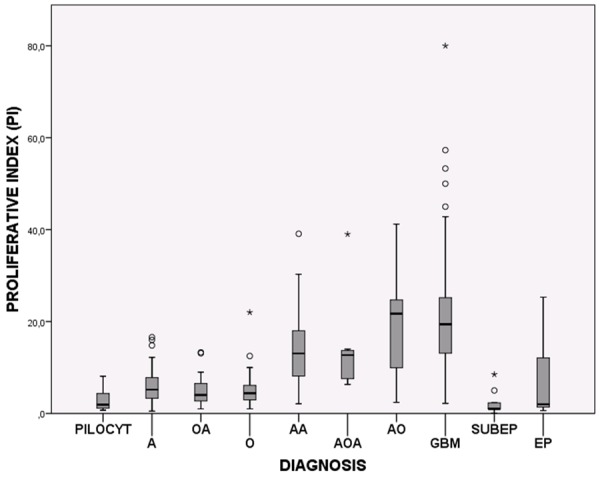
Box plots showing the distribution of Ki-67/MIB-1 PIs among the glioma subtypes. PILOCYT: Pilocytic astrocytoma, A: Diffuse astrocytoma, OA: Oligoastrocytoma, O: Oligodendroglioma, AA: Anaplastic astrocytoma, AOA: Anaplastic oligoastrocytoma, AO: Anaplastic oligodendroglioma, GBM: Glioblastoma, SUBEP: Subependymoma and EP: Ependymoma.
In general, the quality of the Ki-67/MIB-1 immunostaining was good (Figure 2). Some variation in staining intensity was observed, however, only distinctly labeled tumor cell nuclei were counted. Normal brain tissue did not show any immunoreactivity. Various distribution patterns were observed for the labeled tumor cells, both homogenous dispersion throughout the tumor tissue and hot spots, with the latter being more frequent in high grade tumors.
Figure 2.
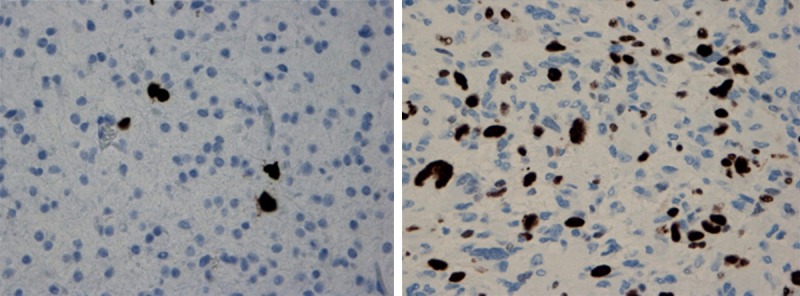
Ki-67/MIB-1 immunostaining showing low PI (~4%) in a grade II astrocytoma (left) and high PI (~30%) in a glioblastoma (right). Magnification ×400.
The Ki-67/MIB-1 PI correlated significantly with tumor grade for each glioma type. However, considerable overlap was observed between the malignancy groups (Figure 2). No significant difference was found between glioma types of the same tumor grade. Anaplastic oligodendrogliomas and anaplastic oligoastrocytomas had indices comparable to glioblastomas.
Discussion
In our material we found that the Ki-67/MIB-1 PIs correlated significantly with increasing tumor grade in all types of gliomas but an overlap occurred between the malignancy groups.
The positive correlations between Ki-67/MIB-1 PI and tumor grade in our series of gliomas are in agreement with the literature [10-14]. We found that indices were comparable between gliomas of similar malignancy grade, and indices for high-grade gliomas (grade III/IV) were significantly higher than in low-grade (grade I/II) tumors. Thus, Ki-67/MIB-1 is useful for differentiating between high and low-grade gliomas, but differentiating between grade I and grade II or grade III and grade IV is more problematic due to the overlap of values between the different tumor grades. This overlap is a main limitation of this immunostaining. For this reason, Ki-67/MIB-1 should not be used alone as a marker of tumor grade but in conjunction with histological features [15,16].
Histological grading and estimation of Ki-67/MIB-1 PI are subjected to heterogeneity-induced sampling errors, limiting their diagnostic accuracy, especially in small specimens such as stereotactical biopsies [17]. Tumor histology can appear discordant with the observed Ki-67/MIB-1 PI. In cases with histologically anaplastic glioma tissue in which mitotic figures can be difficult to find, a high index may support the high grade diagnosis. On the other hand, a low index in a cellular lesion may indicate a reactive condition (e.g., gliosis, microglial response) rather than a neoplasm [5,16]. If the index is elevated for a glioma with an otherwise benign histology, a more aggressive tumor may be indicated. Such a setting should not lead to a change in tumor grade but a remark in the biopsy report saying “with elevated Ki-67/MIB-1 PI, see comment” [5,16]. In these cases one should consider step sections as well as to correlate to radiological images and clinical history [5,16].
Ki-67/MIB-1 immunostaining to distinguish gliosis and low-grade gliomas should be interpreted with caution [5]. Normally, reactive astrocytes do not exhibit proliferative activity, but in some non-neoplastic conditions reactive astrocytes may have a proliferation rate of 1-5% [18]. In such cases, immunohistochemical analyses for mutated p53 and isocitrate dehydrogenase (IDH) proteins can be useful, though p53 immunoreactivity may occur in both settings, and there are gliomas without IDH mutation [19-21].
The procedure for Ki-67/MIB-1 immunostaining is not standardized and has various analytical and clinical elements of uncertainty [7]. Nevertheless, the method is regarded as being robust [8,9], which is also in accordance with our experience during several years with both clinical and experimental use [14,22,23]. The recommended fixative is buffered formalin, and storage time, delay in fixation and fixation time does not seem to substantially affect the staining results [8,24,25]. Loss of immunoreactivity has been described if cut sections are exposed to room air for some months [8]. A prerequisite for satisfactory immunostaining is adequate antigen retrieval [24-26]. Various antibodies against the Ki-67 antigen are commercially available, but MIB-1 is the predominant antibody [22,27]. Counting procedures vary across studies. Usually counting is performed in areas with the highest immunoreactivity (“hot spots”), and approximately 1000 cells are counted using the 40× objective. The PI is calculated as the percentage of labeled tumor cell nuclei to the total number of tumor cells [5,9]. As the expression of the Ki-67 antigen changes during the cell cycle [28], the intensity of nuclear staining will vary; principally, all types of staining should be regarded as positive [8,9]. Counting can be done manually or by digitalized image analysis systems, but manual counting has turned out to be applicable for most diagnostic purposes [5]. Defining a cut-off value is also a topic of interest due to its impact on the determination of patients classified as “high Ki-67”, which is indicative of a poorer outcome. Generally, these patients will receive more aggressive treatment. However, the definition of threshold value is not straightforward mostly due to inter-/intra-observer variability and counting procedures. Accordingly, extrapolating values from other laboratories can be deceptive; thus, Ki-67/MIB-1 immunostaining should be interpreted in the context of one’s own practice [5]. Each pathology department should regularly adjust its Ki-67/MIB-1 PIs by tumor grade and survival and develop its own in-house policy. Such a work-up will constitute an important part of a department’s quality assurance and accreditation programs [29]. For astrocytomas, a cut-off of approximately 10% has appeared clinically feasible [6,16]. However, the predictive value of Ki-67/MIB-1 is ambiguous [7,30].
This study also has limitations inherent to the Ki-67/MIB-1 immunohistochemistry, including definition of immunoreactive tumor cell nuclei, sampling error and counting procedures. In addition, no statistical analysis of intra- or inter-observer variability was done. The statistics may also be influenced by the fact that not all glioma cases during the study period were immunostained.
Overall, Ki-67/MIB-1 immunostaining is a useful supplement to the histopathological diagnosis of human gliomas. However, the procedure cannot be used alone, but should be used in combination with established histopathological features of malignancy. The analytical and clinical performance of Ki-67/MIB-1 immunostaining in glioma diagnosis is not sufficiently determined. This limits its clinical utility and underlines the need for further research and standardization of procedures between laboratories [7]. To improve the diagnostics for human gliomas, a battery of proliferation markers might be considered [23]. Progress has been made in the recent years towards introducing molecular genetics in glioma diagnosis [31]. This has the potential to move us towards a more personalized medicine in the care of glioma patients.
Acknowledgements
We are grateful to Prof. Johannes Attems, Dr. David Scheie, Prof. Ivar Skjåk Nordrum, Prof. Christina Vogt and Dr. Erlend Hassel for their critical reading of the manuscript. Anne Jarstein Skjulsvik, MD, was funded by St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway. Jørgen Negård Mørk, MD, and Morten Overrein Torp, MD, were funded by the Norwegian State Educational Loan Fund, Norway. Sverre Helge Torp, Prof, PhD, was funded by Norwegian University of Science and Technology (NTNU), Trondheim, Norway.
Disclosure of conflict of interest
None.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79:1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol. 2010;120:297–304. doi: 10.1007/s00401-010-0725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takei H, Powell SZ. Novel immunohistochemical markers in the diagnosis of nonglial tumors of nervous system. Adv Anat Pathol. 2010;17:150–153. doi: 10.1097/PAP.0b013e3181cfb7ae. [DOI] [PubMed] [Google Scholar]
- 5.Prayson RA. The utility of MIB-1/Ki-67 immunostaining in the evaluation of central nervous system neoplasms. Adv Anat Pathol. 2005;12:144–148. doi: 10.1097/01.pap.0000163957.21409.52. [DOI] [PubMed] [Google Scholar]
- 6.Johannessen AL, Torp SH. The clinical value of Ki-67/MIB-1 labeling index in human astrocytomas. Pathol Oncol Res. 2006;12:143–147. doi: 10.1007/BF02893360. [DOI] [PubMed] [Google Scholar]
- 7.Berghoff AS, Stefanits H, Woehrer A, Heinzl H, Preusser M, Hainfellner JA. Clinical neuropathology practice guide 3-2013: levels of evidence and clinical utility of prognostic and predictive candidate brain tumor biomarkers. Clin Neuropathol. 2013;32:148–158. doi: 10.5414/NP300646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, McShane L, Paik S, Penault-Llorca F, Prudkin L, Regan M, Salter J, Sotiriou C, Smith IE, Viale G, Zujewski JA, Hayes DF. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol. 2013;66:512–516. doi: 10.1136/jclinpath-2012-201085. [DOI] [PubMed] [Google Scholar]
- 10.Schiffer D, Cavalla P, Migheli A, Chio A, Giordana MT, Marino S, Attanasio A. Apoptosis and cell proliferation in human neuroepithelial tumors. Neurosci Lett. 1995;195:81–84. doi: 10.1016/0304-3940(95)11784-t. [DOI] [PubMed] [Google Scholar]
- 11.Karamitopoulou E, Perentes E, Diamantis I, Maraziotis T. Ki-67 immunoreactivity in human central nervous system tumors: a study with MIB 1 monoclonal antibody on archival material. Acta Neuropathol. 1994;87:47–54. doi: 10.1007/BF00386253. [DOI] [PubMed] [Google Scholar]
- 12.Coons SW, Johnson PC, Pearl DK. The prognostic significance of Ki-67 labeling indices for oligodendrogliomas. Neurosurgery. 1997;41:878–84. doi: 10.1097/00006123-199710000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, Oka H, Kawano N, Tanaka S, Utsuki S, Fujii K. Prognostic value of Ki-67 (MIB-1) and p53 in ependymomas. Brain Tumor Pathol. 2001;18:151–154. doi: 10.1007/BF02479429. [DOI] [PubMed] [Google Scholar]
- 14.Torp SH. Diagnostic and prognostic role of Ki67 immunostaining in human astrocytomas using four different antibodies. Clin Neuropathol. 2002;21:252–257. [PubMed] [Google Scholar]
- 15.Smith C, Ironside JW. Diagnosis and pathogenesis of gliomas. Current Diagnostic Pathology. 2007;13:180–192. [Google Scholar]
- 16.Trembath D, Miller CR, Perry A. Gray zones in brain tumor classification: evolving concepts. Adv Anat Pathol. 2008;15:287–297. doi: 10.1097/PAP.0b013e3181836a03. [DOI] [PubMed] [Google Scholar]
- 17.Coons SW, Johnson PC. Regional heterogeneity in the proliferative activity of human gliomas as measured by the Ki-67 labeling index. J Neuropathol Exp Neurol. 1993;52:609–618. doi: 10.1097/00005072-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Colodner KJ, Montana RA, Anthony DC, Folkerth RD, De Girolami U, Feany MB. Proliferative potential of human astrocytes. J Neuropathol Exp Neurol. 2005;64:163–169. doi: 10.1093/jnen/64.2.163. [DOI] [PubMed] [Google Scholar]
- 19.Kurtkaya-Yapicier O, Scheithauer BW, Hebrink D, James CD. p53 in nonneoplastic central nervous system lesions: an immunohistochemical and genetic sequencing study. Neurosurgery. 2002;51:1246–1254. doi: 10.1097/00006123-200211000-00021. discussion 1254-1245. [DOI] [PubMed] [Google Scholar]
- 20.Capper D, Sahm F, Hartmann C, Meyermann R, von Deimling A, Schittenhelm J. Application of mutant IDH1 antibody to differentiate diffuse glioma from nonneoplastic central nervous system lesions and therapy-induced changes. Am J Surg Pathol. 2010;34:1199–1204. doi: 10.1097/PAS.0b013e3181e7740d. [DOI] [PubMed] [Google Scholar]
- 21.Kloosterhof NK, Bralten LB, Dubbink HJ, French PJ, van den Bent MJ. Isocitrate dehydrogenase-1 mutations: a fundamentally new understanding of diffuse glioma? Lancet Oncol. 2011;12:83–91. doi: 10.1016/S1470-2045(10)70053-X. [DOI] [PubMed] [Google Scholar]
- 22.Torp SH. Proliferative activity in human glioblastomas: evaluation of different Ki67 equivalent antibodies. Mol Pathol. 1997;50:198–200. doi: 10.1136/mp.50.4.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habberstad AH, Gulati S, Torp SH. Evaluation of the proliferation markers Ki-67/MIB-1, mitosin, survivin, pHH3, and DNA topoisomerase IIalpha in human anaplastic astrocytomas--an immunohistochemical study. Diagn Pathol. 2011;6:43. doi: 10.1186/1746-1596-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992;168:357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- 25.Wester K, Wahlund E, Sundstrom C, Ranefall P, Bengtsson E, Russell PJ, Ow KT, Malmstrom PU, Busch C. Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol. 2000;8:61–70. [PubMed] [Google Scholar]
- 26.Taylor CR, Shi SR, Chaiwun B, Young L, Imam SA, Cote RJ. Strategies for improving the immunohistochemical staining of various intranuclear prognostic markers in formalin-paraffin sections: androgen receptor, estrogen receptor, progesterone receptor, p53 protein, proliferating cell nuclear antigen, and Ki-67 antigen revealed by antigen retrieval techniques. Hum Pathol. 1994;25:263–270. doi: 10.1016/0046-8177(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 27.Zabaglo L, Salter J, Anderson H, Quinn E, Hills M, Detre S, A’Hern R, Dowsett M. Comparative validation of the SP6 antibody to Ki67 in breast cancer. J Clin Pathol. 2010;63:800–804. doi: 10.1136/jcp.2010.077578. [DOI] [PubMed] [Google Scholar]
- 28.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Luporsi E, Andre F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B, Arnould L, Gompel A, Egele C, Poulet B, Clough KB, Crouet H, Fourquet A, Lefranc JP, Mathelin C, Rouyer N, Serin D, Spielmann M, Haugh M, Chenard MP, Brain E, de Cremoux P, Bellocq JP. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012;132:895–915. doi: 10.1007/s10549-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moskowitz SI, Jin T, Prayson RA. Role of MIB1 in predicting survival in patients with glioblastomas. J Neurooncol. 2006;76:193–200. doi: 10.1007/s11060-005-5262-1. [DOI] [PubMed] [Google Scholar]
- 31.Theeler BJ, Yung WK, Fuller GN, De Groot JF. Moving toward molecular classification of diffuse gliomas in adults. Neurology. 2012;79:1917–1926. doi: 10.1212/WNL.0b013e318271f7cb. [DOI] [PMC free article] [PubMed] [Google Scholar]


