Abstract
Bone epithelioid angiosarcoma (EA) is rare and characterized by large, mildly to moderately pleomorphic epithelioid cells, with abundant eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli. The tumors may arise in various locations in bone and the patients may present with unifocal or multifocal osseous disease. We present a unifocal lesion case of EA of the ilium in a 62-year-old woman. A needle biopsy of the ilium was performed and first diagnosed poorly differentiated adenocarcinoma based on CKpan and CK18 immunopositivity. The tumor was treated initially with curettage followed by chemotherapy. The final diagnosis on the surgical specimen was epithelioid angiosarcoma.
Keywords: Epithelioid angiosarcoma, bone
Introduction
Bone epithelioid angiosarcoma (EA) is one of the intraosseous epithelioid vascular tumors. It is a rare high-grade sarcoma of intraosseous vascular endothelial origin and is a rare variant of angiosarcoma [1]. It can affect any portion of the skeleton. The long tubular bones of the lower extremities are the most commonly involved [2,3]. Patients may present with unifocal or multifocal osseous disease. The tumor cells frequently express epithelial markers as well as endothelial cell markers, which may lead to a misdiagnosis of metastatic carcinoma, especially when the poorly differentiated tumor cells were happened to be encountered with the needle biopsy specimen.
Case report
A 62 years old female presented persistent pain in the low back and buttocks last month after sprain, exacerbation of sitting and walking. In addition, the right buttock pain was more serious than the left. An X-ray revealed an osteolytic lesion (Figure 1A), which showed a high-low mixed signal in both T1WI and T2WI, as well as cystic long T1, long T2 signal on MRI images in the right ilium and adjacent sacroiliac joint. On fat suppressed image, the lesion was hyperintense (Figure 1B). Computed tomography scan (CT) display, lesions were osteolytic, border is not clear, the size of 10.2 cm × 6.2 cm × 5.3 cm, extending into the adjacent soft tissue, erosion of sacroiliac joint surface (Figure 1C). B ultrasound examination has nothing to do with the internal organs, chest computer tomography in normal.
Figure 1.
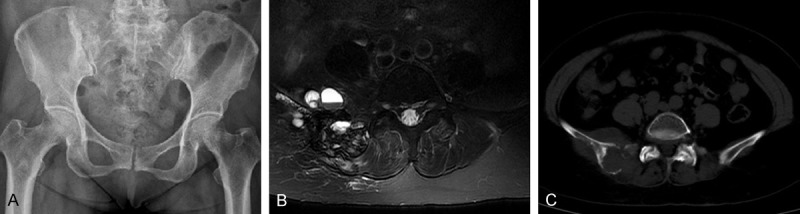
A. Radiographs showing the osteolytic lesion in the right ilium. C. Computed tomography scan revealing an osteolytic lesion with ill-defined margins and extending into the proximal soft tissue. B. Magnetic resonance image revealing a cystic, destructive soft tissue lesion of the right ilium.
A needle biopsy of the lesion was performed and first diagnosed metastatic poorly differentiated adenocarcinoma according to the epithelial nature of some tumor cells which were positive for Keratin AE1/AE3, CK18, and negative for CD34, CD31, Fli-1 was not used at that time.
The patient underwent tumor resection, postoperative chemotherapy. Grossly, operation re-section specimens were observed gray-tan tumor. The histology of the resected tumor was the same as that of the biopsy specimen. Most of the tumor was composed of patches of diffuse distribution, composition moderate pleomorphic epithelioid cells (Figure 2A) with abundant eosinophilic cytoplasm. Some tumor cells showed vesicular nuclei, prominent nucleoli (Figure 2B). There were more than mitoses per 10 high power field (HPF), and the abnormal mitosis. In some areas, spindle tumor cells to form beam fill marrow cavity (Figure 2C). Moreover, intra- and extracellular eosinophilic hyaline droplets or globules were observed (Figure 2D). The stroma varied from thin fibrovascular connective tissue to densely collagenous areas associated with multifocal chronic inflammatory cell infiltrates. Extensive hemorrhage and hemosiderin deposits, focal necrosis, and cystic changes were also distinct.
Figure 2.
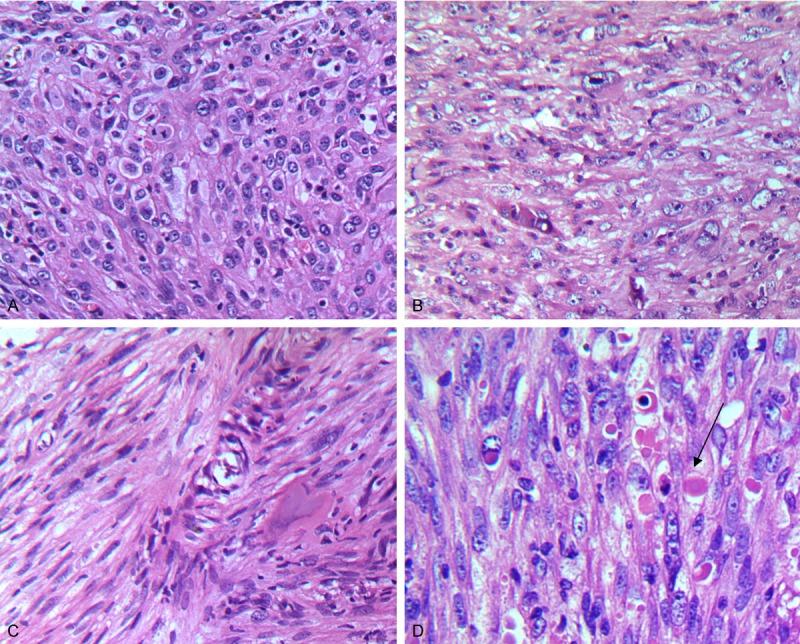
Histological features of epithelioid angiosarcoma of bone. (A) Epithelioid tumor cells arranged in sheets. (C) Foci of spindle-shaped tumor cells formed bundles. (B) Epithelioid cells with vesicular nuclei and striking nucleoli. (D) Intra- and extracellular eosinophilic hyaline droplets or globules (A: H&E ×200; B: H&E ×400; C: H&E ×200; D: H&E ×400).
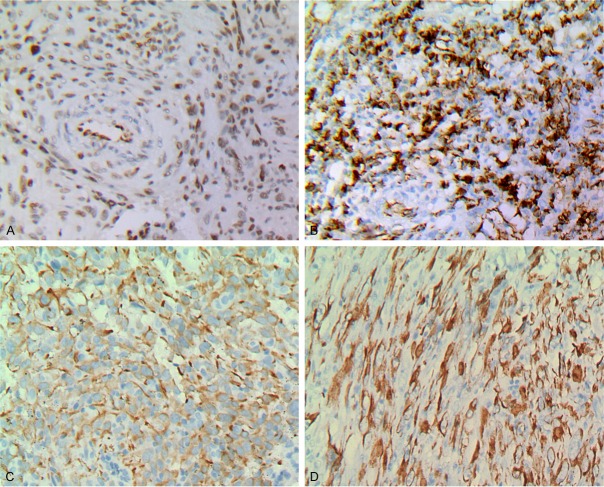
The results of immunohistochemistry excised tumors displayed, vimentin and the friend leukemia integration (FLI-1) diffuse staining (Figure 3A). Some tumor cells expressed CD31 (Figure 3B). Keratin AE1/AE3 and CK18 were focal positive (Figure 3C, 3D). However, CD34, epithelial membrane antigen (EMA), smooth muscle actin (SMA), desmin, S-100 protein and HMB45 were negative. Mitosis is high, Ki-67 is about 50%.
Figure 3.
Immunostaining: A. FLi-1 (×200); B. CD31 (×200); C. CK (×200); D. CK18 (×200).
According to the clinical features, image data, especially the histological and immunohistochemical results, the diagnosis of intraosseous epithelioid angiosarcoma was confirmed.
Discussion
Bone epithelioid angiosarcoma (EA) is a rare primary malignant bone tumor of vascular origin, the incidence rate of less than 1% [4]. More common in young and old, sixty and eighty are two small peaks [2,5]. Incidence of males is slightly higher than that of females. In addition, tumors tend to involve lower extremity long bone, such as the femur and tibia [2,6]. Pain is the most common complaint. X-ray characteristics of bone angiosarcoma are not specific, ill-defined osteolytic lesions are the prominent appearance, commonly involving the adjacent soft tissue.
Epithelioid angiosarcoma is a highly malignant tumor under the microscope often has flakes or wide distribution of cords, cells were large and round, pleomorphic epithelioid, with abundant eosinophilic cytoplasm, vesicular nuclei and significant nucleolus, and exhibit multiple and high mitotic proliferation activity. Epithelioid angiosarcoma of bone matrix, in addition to seeing the reaction of the bone, but also can have a variety of inflammatory cells, including chronic inflammatory cells, neutrophils and eosinophils [7-9]. The classic case, you can often see the blood-filled cavities and vacuole-like structures located within the cytoplasm. However, these appearances may not be easily identified if the tumor cells are poorly differentiated. In particular, when a completely epithelioid focus is encountered with scant biopsy specimen, it is easily misdiagnosed.
Most of angiosarcoma is positive immunohistochemical CD31 expression [2,9,10], so that CD31 is considered the most sensitive and specific for all types of angiosarcoma of conventional markers. CD34 is highly sensitive, is expressed in approximately 90% of the hemangioma. However, CD34 is rather non-specific [11]. FLI-1 is both a sensitive and specific marker of blood vessels, which is superior to CD31, CD34, and von Willebrand factor [12] as a marker of tumor blood vessels as the core is still in its coloring with respect to the membrane (CD31 and CD34) or cytoplasm (von Willebrand factor) coloring, FLI-1 is easier to recognize [12]. To avoid misdiagnosis, requires the use of multiple markers for diagnosis of vascular endothelial sarcoma, because there are a significant proportion of metastatic cancer can be CD34-positive (15%) and CD31-positive (38%) [13].
In the present case, there was no irregularly anastomosing vessel formation and the tumor cells were poorly differentiated. In some areas the tumor cells were positive for CD31 and in the other areas negative. When the poorly differentiated tumor cells were negative for CD31, Fli-1 was positive. When CKpan and CK18 positive happened to be encountered with the needle biopsy specimen, it is easily to make an erroneous diagnosis. Therefore, under this circumstance, the utility of the other endothelial markers, such as vwf and Factor VIII, especially Fli-1 is very helpful for the differential diagnosis. Although the significance was not clear, the presence of intra- and extracellular eosinophilic hyaline droplets or globules was another feature of this case. Maybe they can provide an evident for diagnosis of bone angiosarcoma.
The differential diagnosis includes other primary vascular tumors, especially Epithelioid hemangioendothelioma (EHE) and pseudomyogenic hemangioendothelioma (PMH) as well as metastatic carcinoma. EHE may show focal high-grade areas, with cellular atypia and a sheeted architecture, but most cells reside in small nests and trabeculae. The mitotic count is rather low (2 mitoses per 10 high-power fields), the epithelioid angiosarcoma has a greater degree of cytologic atypia and mitoses, focal areas of vessel formation, and a sheeted growth pattern comprising most of the malignancy.
Like epithelioid angiosarcoma, PMH may be composed of epithelioid cells with abundant eosinophilic cytoplasm, vesicular nuclei and prominent nucleolus although more spindle cells showing generally mild nuclear atypia and little mitotic activity was also noted. It is commonly composed of plump spindle cells. EA and PMH should be based within the cytoplasm of tumor cells without cavities, no vascular channels lined with epithelium and low proliferation index (Ki-67) to distinguish [14,15].
Treatment of angiosarcoma of bone usually includes wide resection, radiotherapy and chemotherapy.
In conclusion, bone epithelioid angiosarcoma is a rare vascular tumor. When poorly differentiated tumor cells were met with biopsy specimens, it is easy to be misdiagnosed. Therefore, H & E stained sections initial evaluation and joint use of multiple antibodies is a necessary condition for the diagnosis of epithelioid angiosarcoma.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81272420), the Scientific and Technological Development Projects in Shandong Province of China (No. 2011GSF11838), Shandong Province Natural Science Foundation (No. ZR2012HM085), and the Scientific and Technological Development Projects of Jinan City (No. 201202039).
Disclosure of conflict of interest
None.
References
- 1.Lang J, Chen L, Chen B, Chen K, Liu A, Li J, Wang J. Epithelioid angiosarcoma of the spine: a case report of a rare bone tumor. Oncol Lett. 2014;7:2170–2174. doi: 10.3892/ol.2014.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verbeke SL, Bertoni F, Bacchini P, Sciot R, Fletcher CD, Kroon HM, Hogendoorn PC, Bovee JV. Distinct histological features characterize primary angiosarcoma of bone. Histopathology. 2011;58:254–264. doi: 10.1111/j.1365-2559.2011.03750.x. [DOI] [PubMed] [Google Scholar]
- 3.Palmerini E, Maki RG, Staals EL, Alberghini M, Antonescu CR, Ferrari C, Ruggieri P, Mavrogenis A, Bertoni F, Cesari M, Paioli A, Marchesi E, Picci P, Ferrari S. Primary Angiosarcoma of Bone: A Retrospective Analysis of 60 Patients From 2 Institutions. Am J Clin Oncol. 2014;37:528–34. doi: 10.1097/COC.0b013e31827defa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshpande V, Rosenberg AE, O’Connell JX, Nielsen GP. Epithelioid angiosarcoma of the bone: a series of 10 cases. Am J Surg Pathol. 2003;27:709–716. doi: 10.1097/00000478-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Saglik Y, Yildiz Y, Atalar H, Basarir K. Primary angiosarcoma of the fibula: a case report. Acta Orthop Belg. 2007;73:799–803. [PubMed] [Google Scholar]
- 6.Mortazavi SM, Wenger D, Asadollahi S, Shariat Torbaghan S, Unni KK, Saberi S. Periosteal osteoblastoma: report of a case with a rare histopathologic presentation and review of the literature. Skeletal Radiol. 2007;36:259–264. doi: 10.1007/s00256-006-0169-2. [DOI] [PubMed] [Google Scholar]
- 7.Baliaka A, Balis G, Michalopoulou-Manolout-siou E, Papanikolaou A, Nikolaidou A. Primary angiosarcoma of bone. A case report. Hippokratia. 2013;17:180–182. [PMC free article] [PubMed] [Google Scholar]
- 8.Hasegawa T, Fujii Y, Seki K, Yang P, Hirose T, Matsuzaki K, Sano T. Epithelioid angiosarcoma of bone. Hum Pathol. 1997;28:985–989. doi: 10.1016/s0046-8177(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 9.Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135:268–272. doi: 10.5858/135.2.268. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Shen D, Sun K, Bao D, Song Q, Wang G, Chen D, Yan T, Guo W. Epithelioid angiosarcoma of bone and soft tissue: a report of seven cases with emphasis on morphologic diversity, immunohistochemical features and clinical outcome. Tumori. 2011;97:585–589. doi: 10.1177/030089161109700508. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Tao H, Ye Z, Yang D. Multicentric epithelioid angiosarcoma of bone. Orthopedics. 2012;35:e1293–1296. doi: 10.3928/01477447-20120725-39. [DOI] [PubMed] [Google Scholar]
- 12.Folpe AL, Chand EM, Goldblum JR, Weiss SW. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol. 2001;25:1061–1066. doi: 10.1097/00000478-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Gill R, O’Donnell RJ, Horvai A. Utility of immunohistochemistry for endothelial markers in distinguishing epithelioid hemangioendothelioma from carcinoma metastatic to bone. Arch Pathol Lab Med. 2009;133:967–972. doi: 10.5858/133.6.967. [DOI] [PubMed] [Google Scholar]
- 14.Hornick JL, Fletcher CD. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol. 2011;35:190–201. doi: 10.1097/PAS.0b013e3181ff0901. [DOI] [PubMed] [Google Scholar]
- 15.Kim ME, Fallon SC, Lopez ME, Hicks MJ, Brandt ML. Recurrent hemangioendothelioma in a pediatric patient: report and review of the literature. J Pediatr Surg. 2013;48:1426–1428. doi: 10.1016/j.jpedsurg.2013.03.064. [DOI] [PubMed] [Google Scholar]