Abstract
Researches have shown that the onset of diabetes is closely associated with oxidative stress and the chronic exposure leads to the development of complications such as diabetic cardiomyopathy. One of the central adaptive responses against the oxidative stresses is the activation of the nuclear transcriptional factor, NF-E2-related factor 2 (Nrf2), which then activates more than 20 different antioxidative enzymes. Kelch-like ECH associated protein 1 (Keap1) targets and binds to Nrf2 for proteosomal degradation. The aim of the present study was to investigate the status of Nrf2 mediated antioxidant system in myocardial biopsies of non-diabetic (NDM) and type-2 diabetic (DM-T2) cardiomyopathy patients. The western blot analysis of antioxidant proteins, real-time PCR analysis of Nrf2/Keap1 gene and bisulphate DNA sequencing analysis to study the methylation status of the CpG islands of Keap1 promoter DNA were performed. The immunoblot analysis showed the decreased level of antioxidant proteins other than Keap1 in the diabetic cardiopathy patients. Similarly, mRNA levels of Keap1 showed 5-fold increase in diabetic patients. Further analysis on promoter region of Keap1 gene revealed 80% demethylation in diabetic patients. Altogether, our results indicated that demethylation of the CpG islands in the Keap1 promoter will activate the expression of Keap1 protein, which then increases the targeting of Nrf2 for proteosomal degradation. Decreased Nrf2 activity represses the transcription of many antioxidant enzyme genes and alters the redox-balance up on diabetes. Thus, our study clearly demonstrates the failure of Nrf2 mediated antioxidant system revealed in biopsies of diabetic cardiomyopathy.
Keywords: Nrf2, antioxidant system, CpG islands, bisulphate sequencing
Introduction
Oxidative stress plays an important role in the development and progression of chronic diabetic complications by increasing the generation of reactive oxygen species (ROS) [1-3]. Possible sources of oxidative stress in diabetes include auto-oxidation of glucose or shifts in redox balances from shunting glucose to the polyol pathway, which decreases the overall reducing capability of the cell [2]. Diabetes also causes mitochondrial superoxide overproduction in endothelial cells of both large and small blood vessels. The balance between the rate of free radical generation and elimination is very important in the development of diabetic complications. This increased superoxide production causes the activation of several signal pathways involved in the pathogenesis of chronic complications such as diabetic cardiomyopathy, diabetic retinopathy and diabetic nephropathy. Diabetic cardiomyopathy is a pathological condition, where myocyte hypertrophy and focal fibrosis of cardiac tissue was observed. The affected individuals manifest both systolic and diastolic dysfunction and the risk of heart failure in diabetics is significantly higher than the normal population [4]. Although several therapeutic targets are under practice, the development and progression of cardiomyopathy in diabetic patients was still unpreventable.
The molecular mechanisms of pathological change in diabetes, and its role in multi-organ damage via oxidative stress, have progressed but the picture is still not clear. Therefore, to develop an effective drug research to prevent or delay these lethal complications for diabetic patients is urgently required. As an initial step, therapy of diabetic complications focusing on the antioxidant-mediated prevention has been attractive, but hitherto, there was no potential antioxidant found implemented efficiently in clinics [5,6]. The NFE2-related factor 2 (Nrf2), a master of transcription factor for cellular detoxification responses and redox status [7,8]. Under normal physiological conditions Nrf2 located in the cytoplasm and combines with kelch-like ECH-associated protein 1 (Keap1), a negative regulator [9]. Keap1 could mediate a rapid ubiquitination and subsequent degradation of Nrf2 by the proteasome [9]. Upon exposure of cells to oxidative stress or electrophilic compounds, Nrf2 is free from Keap1 and translocates into the nucleus to bind to antioxidant-responsive elements (AREs) in the genes encoding antioxidant enzymes such as NADPH quinone oxidoreductase (NQO1), glutathione S-transferase, heme oxygenase-1 (HO1), and γ-glutamylcysteine synthetase, increasing their expression to play a role of detoxification, antioxidation, and anti-inflammation [9,10].
Recently, several studies have indicated preventive effect of Nrf2 on high glucose- (HG-) induced oxidative damage in the cultured cells and potentially on the diabetic complications in animal models [11]. Although there are reports/reviews available on the general features of Nrf2 in the oxidative stress and damage related to diabetes [12,13], the present study was to demonstrate the failure of nrf2-mediated antioxidant system in the development of diabetic complications in relation to epigenetic changes in promoter DNA demethylation of Keap1 gene.
Materials and methods
Patient selection and cardiac sample collection
The ventricular cardiac biopsy samples were derived from patients affected by post-myocardial infarction (MI) cardiomyopathy undergoing surgical coronary revascularization as described previously [14]. Patients with DM-T2 (n=20) and without diabetes (NDM, n=20) were included in the study and did not differ significantly in any clinical parameter other than the presence of DM-T2 (Table 1). All diabetic patients were treated with oral hypoglycemic agents and had an acceptable glycemic control (HbA1c < 8%), and for 72 hours after surgery they received insulin therapy. From each patient, 6 biopsy samples were harvested: 3 from the infarct border area (peri-infarct zone) and 3 from the non-ischemic, remote myocardium (remote zone). Bioptic specimens were taken after informed consent disclosing future use for research. The Ethical Committee of the Liao Cheng People’s Hospital had authorized our study. Samples were immediately snap-frozen in liquid nitrogen and stored at -80°C for RNA or protein extraction.
Table 1.
Characteristics of the patients enrolled in the study
| Parameters | Diabetic patients (n=20) | Non-diabetic patients >(n=20) |
|---|---|---|
| Sex (M/F) | 8/12 | 8/12 |
| Age (years) | 65-70 | 65-70 |
| Duration of diabetes, (years) | 8-10 years | - |
| BMI, kg/m2 | 28.9±0.9 | 27.2±0.7 |
| HbA1c, % | 7.2±0.1 | 5.3±0.1* |
| FBG, mg/dL | 151.3±9.5 | 81.5±3.9* |
| LDL cholesterol, mg/dL | 132.3±7.1 | 128±5.1 |
| HDL cholesterol, mg/dL | 43.3±1.6 | 48.2±1.8* |
| TG, mg/dL | 167.5±10.3 | 126±8.5* |
| SBP, mm Hg | 133.9±3.7 | 127.3±3.2 |
| DBP, mm Hg | 76.3±2.1 | 74.1±2.4 |
BMI, indicates body mass index; FBG, fasting blood glucose; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; SDP, systolic blood pressure; DBP, diastolic blood pressure; CHD, coronary heart disease.
P < 0.01 vs. diabetic patients.
Western blot analysis
The biopsies samples were washed in ice-cold phosphate buffered saline (PBS) and were homogenated/lysed in RIPA buffer (Cell Signaling Technology). The lysates were centrifuged, and the protein content of the supernatant was determined by the Bradford method [15]. The soluble proteins, 10-20 µg, were loaded and separated by 10% SDS-PAGE and blotted onto a polyvinylidene fluoride membrane. Then, the membranes were blocked with 5% nonfat dry milk powder solution for 1 h at room temperature before an overnight incubation with primary antibodies superoxide dismutase (SOD), glutathione reductase (GR), glutathione-s-transferase (GST), NQO1, HO1, Nrf2 and Keap1 (Santa Cruz Biotech.) at 4°C. After rinsing the membranes, they were incubated with secondary antibody for 1 h at room temperature and the bands were made more visible by enhanced chemiluminescence. The intensity of each band was normalized to that of β-actin and quantified using the Image J analysis.
Real-time PCR analysis
Total RNA was extracted from the biopsy samples with Quick-RNA MicroPrep solution (Zymo Research) and followed the protocol with the kit. Then the purified total RNA was reverse-transcribed by iScript Reverse Transcription Supermix for real-time PCR (Bio-Rad) and followed the protocol with the kit. The reverse transcribed RNA was analyzed by real-time PCR using the SsoFast EvaGreen supermix (Bio-Rad). Roche’s ProbeFinder has designed the optimal real-time PCR assay for Nrf2 and Keap1 genes. The primer sequence for Nrf2 forward: 5’-ACACGGTCCACAGCTCATC-3’, reverse: 5’-TGCCTCCAAAGTATGTCAATCA-3’ with product size 96 bp; Keap1 forward: 5’-GGGTCCCCTACAGCCAAG-3’, reverse: 5’-TGGGGTTCCAGAAGATAAGC-3’ with product size 66 bp and β-actin forward: 5’-CCAACCGCGAGAAGATGA-3’, reverse: 5’-CCAGAGGCGTACAGGGATAG-3’ with product size 97 bp. Each reaction was carried out in triplicate and a standard curve was prepared using a serial dilution of a reference sample. The relative copy numbers were obtained from the standard curve and were normalized to the values obtained for β-actin as internal control.
Bisulfite conversion and DNA sequencing
The genomic DNA isolated from biopsy samples were subjected to bisulfite conversion by EZ DNA Methylation-Direct™ kit (Zymo Research Corporation, Orange, CA). The bisulfite converted DNA was then used for bisulfite genomic DNA sequencing (BGS). The bisulfite-modified DNAs were amplified by bisulfite sequencing PCR using Platinum PCR SuperMix High Fidelity (Invitrogen, Carlsbad, CA) with primers specific to human Keap1 promoter region (-430 to -110) with 330 bp size (forward: 5’-TTAGTTATTTAGGAGGTTGT-3’, reverse: 5’-AACCCCCCTTCTCA- CTA-3’). The primers were designed using the Methyl Primer Express Software from Applied Biosystems Inc. The PCR products were purified by gel extraction using the Zymoclean™ Gel DNA recovery kit (Zymo Research Corporation, Orange, CA), and sequenced to determine the status of CpG methylation. Then the sequenced data of each sample was analyzed for DNA methylation in the Keap1 promoter by BISMA software (http://biochem.jacobs-university.de/BDPC/BISMA/) using default filtering threshold settings [16].
Result
Western blot analysis of antioxidant proteins
The levels of antioxidants protein such as SOD, GR, GST, NQO1, HO1, Nrf2 and Keap1 were analyzed in biopsy samples from diabetic and non-diabetic cardiomyopathy patients using western blot analysis. The SOD, GR, GST, NQO1, HO1 and Nrf2 levels of non-diabetic and diabetic samples were compared between the remote zone and peri-infarct. Naturally, Keap1 shows contrary results from these proteins (Figure 1). Both the remote zone and peri-infarct samples from diabetic patients showed decreased protein levels of antioxidant system than that of non-diabetic patients. However, significant decreased levels were observed in peri-infarct than that of remote zone samples, which suggest that diabetic induced defect in antioxidant system of heart muscles. A drastic difference in Keap1 proteins becomes our point of interest and proceeds for further investigations.
Figure 1.
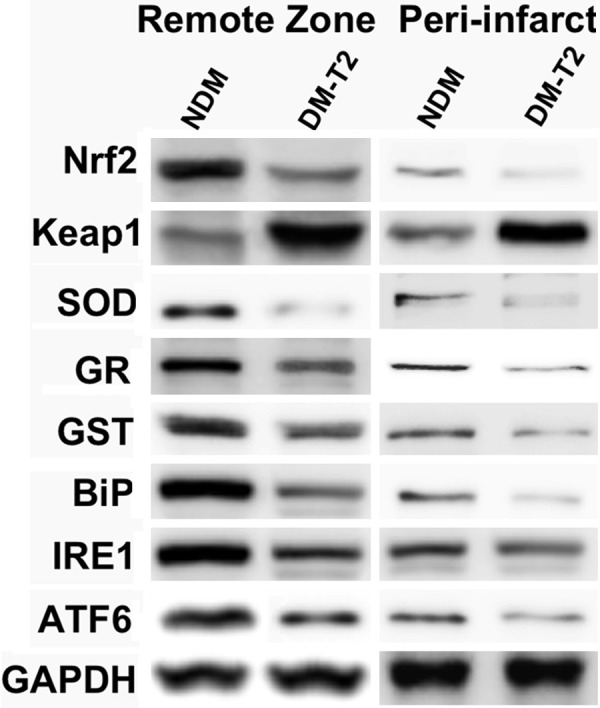
Represents the western blot analysis of Nrf2-mediated antioxidant system proteins. The biopsies samples were taken from remote, peri-infarct zones in myocardium of diabetic Type 2 (DM-T2) and non-diabetic (NDM) patients.
RT-PCR analysis of Keap1/Nrf2 gene
We had investigated the mRNA levels of Keap1 and Nrf2 gene using real-time PCR. The mRNA levels of Keap1 gene in diabetic cardiomyopathy showed 3 fold increase in remote zone and 5 fold increase in peri-infarct zone than that of non-diabetic patients. However, the mRNA level of Nrf2 was found decreased from below normal level (Figure 2). These results suggest and speculate that epigenetic modification in promoter DNA of Keap1 gene might have occurred.
Figure 2.

Represents the real-time PCR analysis of Nrf2 and Keap1 mRNA levels. The biopsies samples were taken from remote, peri-infarct zones in myocardium of diabetic Type 2 (DM-T2) and non-diabetic (NDM) patients.
Bisulphite DNA sequencing analysis of keap1 promoter gene
As per our speculation, we an increased DNA demethylation in the promoter region (between -430 and -110) of Keap1 gene in peri-infarct zones in myocardium of type-2 diabetic (DM-T2). However, a very minimal or no demethylation was observed in non-diabetic samples. Moreover, the peri-infarct zone showed increased demethylation than the remote zone in both diabetic and non-diabetic samples (Figure 3A). The percentage of demethylation in peri-infarct and remote zone of both diabetic and non-diabetic samples was calculated from the bisulphate DNA sequencing (Figure 3B).
Figure 3.
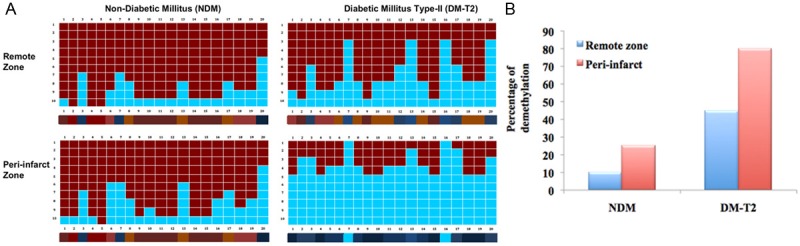
The bisulphate DNA sequencing analysis of Keap1 promoter DNA in remote, peri-infarct zones in myocardium of non-diabetic (NDM) and type-2 diabetic (DM-T2). A. Represent the methylation status of CpG dinucleotides in Keap1 promoter region (between -430 and -110); B. Represent the percentage of demethylation calculated from the bisulphate DNA sequencing.
Discussion
Oxidative stress plays an important role in the development and progression of chronic diabetic complications. Diabetes causes mitochondrial superoxide overproduction in endothelial cells of both large and small vessels. This increased superoxide production causes the activation of several signal pathways involved in the pathogenesis of chronic complications [17] such as diabetic cardiomyopathy. Extra generation of reactive oxygen species (ROS), induced by hyperglycemia, is considered as the main reason for the development of these diabetic complications. Transcription factor, NFE2-related factor 2 (Nrf2), is a master regulator of cellular detoxification response and redox status, and also provides a protective action from various oxidative stresses and damages. Under basal conditions, Nrf2 binds to Keap1, which is a substrate adaptor protein for a Cul 3-dependent E3 ubiquitin ligase complex, directing nrf2 for proteasomal degradation [18]. Oxidative stress, electrophiles, and sulforaphane-like inducers disrupt the Keap1/Nrf2 complex: Nrf2 translocates to the nucleus and, combining with small maf protein [19], induces ARE linked gene expression [20]. Sulforaphane releases nrf2 from Keap1 by modification of critical cysteine thiol residues [21]. Keap1 has concurrent increased susceptibility to degradation but also has ARE-linked gene expression and may be induced by Nrf2 activation, providing an auto-regulatory feedback loop [22]. Nrf2 also undergoes nuclear export, establishing cytoplasmic/nuclear dynamic shuttling [23].
In the present study, the status of Nrf2 mediated antioxidant system in biopsies of diabetic and non-diabetic patients were investigated. The antioxidant proteins levels in diabetic patients were found significantly decreased when compared to the non-diabetic patients. Similarly, the high impact of decreased antioxidant levels was found in peri-infarct zone when compared to remote zone. These results were consistent with the report that failure of active Nrf2/Keap1 system in vascular endothelial cells [24] of diabetic cardiomyopathy. There found a drastic increase in level of Keap1 protein in diabetic cardiomyopathy suggesting that the keap1 actively participated in directing nrf2 for proteasomal degradation. Thereby preventing the expression of Nrf2 downstream antioxidant genes. Since several studies have indicated that the induction of ROS by high glucose in the cultured cardiovascular cells and renal cells, whether high glucose could elevate the Nrf2 expression and activation and its downstream gene expression in these cells has been investigated [11,25,26]. However, our study suggests that prolonged exposure or chronic diabetic condition will lead to the failure of this protective antioxidant system.
To reveal the mechanism behind the drastic Keap1 increase in diseased samples, we have quantified the mRNA level of Keap1 and Nrf2 genes using real-time PCR. Interestingly, 5 fold increase in mRNA levels of Keap1 gene was found in diabetic samples. However, the Nrf2 levels were below normal. These results suggested that methylated CpGs suppresses and demethylated CpGs up-regulates the Keap1 mRNA levels in diabetic samples. The half-life of the mRNAs of the house keeping genes is generally short and they do not survive for a long time [27]. We suggest that the 5-fold increase in the mRNA of Keap1 might be due to an activation of the transcription due to CpG demethylation of the Keap1 promoter. To further confirm this, the DNA methylation statuses of the Keap1 gene in diabetic and non-diabetic samples were examined. The Bisulphite se-quencing of Keap1 promoter DNA in diabetic samples reveals high level of demethylation, the samples from peri-infarct zones were more pronounced than that of remote zones. Recent evidence suggests methylation of histones and DNA are dynamic processes, which coordinate gene expression with environmental stimuli. Unlike histone acetylation which is activating, histone methylation can be transcriptionally activating or repressing depending on the residue that is methylated such as, trimethylation on histone subunit H3 residue lysine-4 (H3K4me3) is typically found on promoters that are actively transcribed, while trimethylation on histone subunit H3 residue lysine-9 (H3K9me3) is typically found on promoters that are repressed [28]. One possible explanation is that methylation changes the conformation of the histone, either making it more or less accessible to transcription machinery. Conversely, DNA methylation is typically repressing and often occurs in CpG islands, regions of DNA typically found in promoters which signal for silencing when methylated [29]. The study of methylation in diabetes is just beginning. Only a select few methyltransferases (eg. Set7) and demethylases (eg. LSD1) have been studied so far but in vitro data suggests that methylation may turn out to be an important mechanism in glucose-induced oxidative stress [30].
Altogether, our present study had demonstrated the failure of Nrf2 mediated antioxidant system in diabetic cardiomyopathy by measuring the protein, mRNA levels of antioxidants. The drastic change in Keap1 level had direct us to focus on DNA methylation analysis, which in fact revealed the actual mechanism behind the failure of Nrf2 mediated antioxidant system in the diseased samples. The study on promoter demethylation had become a novel concept to reveal the mechanistic role in the progression of diseases.
Disclosure of conflict of interest
None.
References
- 1.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: A summary of a Congress Series sponsored by UNESCO MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 2.Haskins K, Bradley B, Powers K. Oxidative stress in type 1 diabetes. Ann N Y Acad Sci. 2003;1005:43–54. doi: 10.1196/annals.1288.006. [DOI] [PubMed] [Google Scholar]
- 3.Folli F, Corradi D, Fanti P, Davalli A, Paez A, Giaccari A, Perego C, Muscogiuri G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev. 2011;7:313–24. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 4.Miki T, Yuda S, Kouzu H, Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev. 2013;18:149–66. doi: 10.1007/s10741-012-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–45. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 6.Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–40. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Sykiotis GP, Bohmann D. Stress-activated cap‘n’collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 10.de Haan JB. Nrf2 activators as attractive therapeutics for diabetic nephropathy. Diabetes. 2011;60:2683–4. doi: 10.2337/db11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57:2809–17. doi: 10.2337/db06-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negi G, Kumar A, Joshi RP, Sharma SS. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem Biophys Res Commun. 2011;408:1–5. doi: 10.1016/j.bbrc.2011.03.087. [DOI] [PubMed] [Google Scholar]
- 13.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasso FC, Torella D, Carbonara O, Ellison GM, Torella M, Scardone M, Marra C, Nasti R, Marfella R, Cozzolino D, Indolfi C, Cotrufo M, Torella R, Salvatore T. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol. 2005;46:827–34. doi: 10.1016/j.jacc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 16.Rohde C, Zhang Y, Reinhardt R, Jeltsch A. BISMA-fast and accurate bisulfite sequencing data analysis of individual clones from unique and repetitive sequences. BMC Bioinformatics. 2010;11:230. doi: 10.1186/1471-2105-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng B, Ruiz MA, Chakrabarti S. Oxidative-stress-induced epigenetic changes in chronic diabetic complications. Can J Physiol Pharmacol. 2013;91:213–20. doi: 10.1139/cjpp-2012-0251. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–9. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motohashi H, Shavit JA, Igarashi K, Yamamoto M, Engel JD. The world according to Maf. Nucleic Acids Res. 1997;25:2953–59. doi: 10.1093/nar/25.15.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang DD, Lo SC, Sun Z, Habib GM, Lieberman MW, Hannink M. Ubiquitination of Keap1, a BTB-kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathway. J Biol Chem. 2005;280:30091–9. doi: 10.1074/jbc.M501279200. [DOI] [PubMed] [Google Scholar]
- 21.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–13. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee OH, Jain AK, Papusha V, Jaiswal AK. An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. J Biol Chem. 2007;282:36412–20. doi: 10.1074/jbc.M706517200. [DOI] [PubMed] [Google Scholar]
- 23.Jain AK, Bloom DA, Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Biol Chem. 2005;280:29158–68. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- 24.Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. J Biol Chem. 2003;278:703–11. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 25.He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol. 2009;46:47–58. doi: 10.1016/j.yjmcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:850–60. doi: 10.2337/db09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paschoud S, Dogar AM, Kuntz C, Grisoni-Neupert B, Richman L, Kuhn LC. Destabilization of interleukin-6 mRNA requires a putative RNA stem-loop structure, an AU-rich element, and the RNA-binding protein AUF1. Mol Cell Biol. 2006;26:8228–41. doi: 10.1128/MCB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper ME, El-Osta A. Epigenetics: mechanisms and implications for diabetic complications. Circ Res. 2010;107:1403–13. doi: 10.1161/CIRCRESAHA.110.223552. [DOI] [PubMed] [Google Scholar]
- 29.Miranda TB, Jones PA. DNA methylation: the nuts and bolts of repression. J Cell Physiol. 2007;213:384–90. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- 30.Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, Calkin AC, Brownlee M, Cooper ME, El-Osta A. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes. 2009;58:1229–36. doi: 10.2337/db08-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]


