Abstract
Human melanoma contains multipotent stem cells that express the neural crest stem cell marker CD271. CD271-expressing melanoma cells in murine xenografts give rise to metastatic tumor. However, a comprehensive clinical investigation of its role in different stages of melanomagenesis has not been well studied. We studied CD271 expression with immunohistochemistry in 11 cases of banal melanocytic nevus, 9 cases of primary cutaneous melanoma, 10 cases of primary mucosal melanoma, 5 cases of metastatic melanoma in regional lymph nodes, and 11 cases of metastatic melanoma in the brain. In addition, 9 cases of metastatic, high-grade adenocarcinomas from breast and lung to the brain were studied as controls. The staining was scored based on the number of positive cells and analyzed by student t-test. All banal melanocytic nevi showed negative to equivocal staining. Primary cutaneous melanomas showed variable patterns, mucosal melanomas were mostly negative, and metastases to lymph nodes ranged from negative to moderate positivity. In contrast, all 11 cases of metastatic melanoma to the brain showed moderate (4 cases) to strong positivity (7 cases). Metastases from lung and breast origin were used as controls and showed negative to weakly positive staining in all but one case. Statistically, CD271 has significantly increased expression in metastatic melanoma to the brain when compared to the other groups studied (P < 0.05). The findings suggest that CD271 expression is specifically increased in metastatic melanoma to the brain. Further prospective study for the role of CD271 in prediction of melanoma brain metastasis as well as prognosis assessment will be of great clinical significance.
Keywords: Melanoma, metastatic melanoma, CD271, tumor stem cells, melanoma prognosis
Introduction
Melanoma arising from skin is the most lethal skin cancer and accounts for approximately seventy-five percent of skin cancer related death [1,2]. The overall survival rate with advanced stage melanoma is still low, with less than fifty percent with regional metastasis and fifteen percent with distant metastasis, in spite of current advancements in therapeutic modalities. Early diagnosis and effective treatment are critical for arresting tumorigenesis and metastasis in melanoma [2,3].
The current understanding of tumorigenesis and metastasis in melanoma is founded on the concept of cancer stem cell theory. This model shows promises in the study of tumorigenesis of several cancers. “Cancer stem cell”, which is a relative misnomer, refers to a small population of relatively slow-cycling cells with continuous self-renewal ability residing in the bulk of a tumor and conferring the major tumor initiation and propagation capacity in the entire tumor. This theory has been well supported by hematological malignancies such as chronic myelogenous leukemia which are largely driven by uncontrolled proliferation and cancer stem cells with phenotypic maturation of the offspring tumor cells [4-6]. The presence of tumor stem cells in solid tumors is still controversial. Numerous studies have explored new or existing surface markers established by previous research for cancer stem cells. The current gold standard to define a cancer stem cell or initiating-cell depends on the ability of trace amount of marker-sorted cells to generate the same type of tumor in xenografted immunodeficient experimental animals, and many types of cancer stem cell have been identified following this methodology [5]. Among these studies, melanoma is one of the most extensively investigated, yet controversial models.
Melanoma has phenotypic features of melanocytes, a derivative of the neural crest stem cells. Several groups have observed that only relatively small populations of melanoma cells are tumor-initiating cells in xenografted immunodeficient nude mice. These cancer stem cells express the stem cell surface marker CD133 (a transmembrane glycoprotein) [7], ABCB5 (a membrane transporter protein) [8], CD166 (activated leukocyte adhesion molecule) [9], Nestin (neuroepithelial stem cells cytoplasmic intermediate filament) [7,10], and Sox2 (transcriptional factor for maintenance of neural crest stem cell pluripotency) [11], and CD271 [12]. CD271, also known as nerve growth factor receptor, p75NTR, TNFRSF16, and Gp80-LNGFR, is a transmembrane signaling receptor involved in negative cell cycle regulation and is specific for neural crest origin [13]. CD271 positive melanoma stem cells have shown correlation with a higher metastatic potential and worse prognosis in immunodeficient mice, and to metastasis but not prognosis in humans [14,15]. However, a comprehensive clinical investigation of CD271’s role in different stages of melanomagenesis has not been well studied.
Materials and methods
Patients
Cases were identified from the files of the Department of Pathology at the University of Oklahoma Medical Center from archived formalin fixed paraffin embedded blocks, including 11 cases of banal melanocytic nevi (control), 9 cases of primary cutaneous melanoma, 10 cases of primary mucosal melanoma, 5 cases of metastatic melanoma in regional lymph nodes, and 11 cases of metastatic melanoma in the brain. In addition, 9 cases of metastatic high-grade adenocarcinomas from breast and lung to the brain were also identified as controls (Table 1). Data regarding patient age and sex was also collected. The study was approved by the institutional review board of the University of Oklahoma Health Sciences Center.
Table 1.
Demographic information of patients by type of lesion
| Lesion Type | n | Sex (M:F) | Age range (median) years |
|---|---|---|---|
| Banal nevus | 11 | 5:6 | 22-75 (56) |
| Primary cutaneous melanoma | 9 | 5:4 | 33-92 (65) |
| Primary mucosal melanoma | 10 | 6:4 | 41-88 (61) |
| Melanoma lymph node metastasis | 5 | 3:2 | 56-91 (70) |
| Melanoma brain metastasis | 11 | 6:5 | 29-74 (57) |
| Breast carcinoma brain metastasis | 4 | 0:4 | 34-67 (53) |
| Lung carcinoma brain metastasis | 5 | 4:1 | 45-72 (61) |
Histology
The sections were analyzed for melanoma staging factors including vertical growth, ulceration and metastasis.
Immunohistochemistry
Thin sections (3 microns thick) were used to minimize formalin fixation generated autofluoresence. CD271 mouse monoclonal antibody (1:500) [16] validated in immunohistochemistry and immunofluorescence in addition to flow cytometry, was tested by immunohistochemistry with routine protocol previously described in detail in other studies [12].
Statistical analysis
The positivity of CD271 staining was scored based on a modification of the scoring system developed by Setia et al. [17]: scattered and/or small cluster, less than 25 positive cells/10 high power fields (hpf), score 0; scattered pattern and/or small cluster, 26-50 cells/10 hpf, score 1/weakly positive; scattered pattern and/or small cluster, 51-75 cells/ 10 hpf, score 2/moderately positive; scattered pattern and/or small cluster and/or large cluster, 76-100 cells/10 hpf, score 3; any pattern, 100-500 cells/10 hpf, score 4; any pattern with > 500 cells/10 hpf, score 5 (score above 3 considered strongly positive).
Statistical analysis was performed with Microsoft Excel. Student t tests were used to compare means of the CD271 staining scores. P-values < 0.05 were considered statistically significant.
Results
Table 1 shows the types of melanocytic lesions studied and associated patient demographics.
The distribution of CD271 positive cells was most commonly scattered single cells, less frequently as small clusters (< 20 cells), occasionally as large clusters (> 100 cells), rarely as very large clusters (> 500 cells), or in a mixed pattern (Figure 1).
Figure 1.
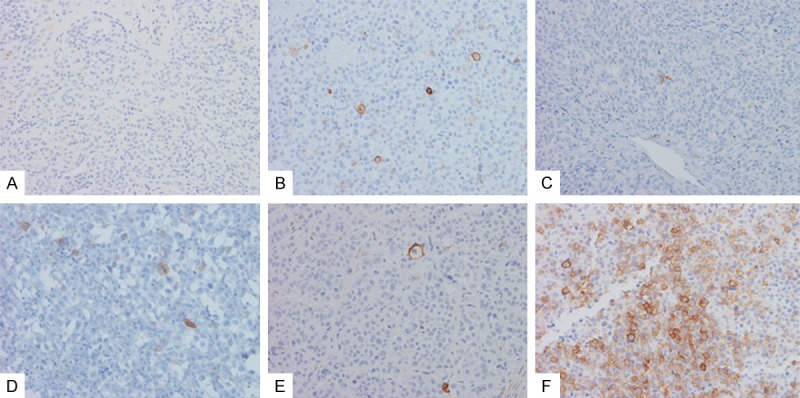
Immunohistochemical staining of CD271 in banal nevus (A), primary cutaneous melanoma (B), primary mucosal melanoma (C), melanoma lymph node metastasis (D), metastatic carcinoma (E) and metastastic melanoma to the brain (F).
In all cases, the CD271 positively stained cells showed a membranous pattern, and there was no significant difference of staining intensity. The highest CD271 scores were observed in the group of melanoma brain metastasis and variably in primary cutaneous melanoma group. Therefore, the CD271 scores of the melanoma brain metastasis group were used as a reference to compare to other groups individually.
The CD271 staining was first examined in 9 cases of primary cutaneous melanoma, including superficial spreading melanoma, nodular melanoma, lentigenous melanoma and one case of acral melanoma (from the toe) (Figure 1B, Table 2). No desmoplastic melanoma was included. CD271 staining was variable with two cases of strong positivity (score > 3), three cases of moderate positivity (score between 2), and 4 cases of negative staining or weak positivity (score 0-1).
Table 2.
Pathologic features and CD271 scores in 9 cases of primary cutaneous melanoma
| Site | Breslow depth | Clark level | Ulceration | Mitosis (per mm2) | CD271 score |
|---|---|---|---|---|---|
| Temple | 4.75 | V | N/P | 2 | 1 |
| Forehead | 2 | IV | N/P | 2 | 2 |
| Scalp | 3 | III | N/P | 18 | 0 |
| Neck | 0.3 | II | P | < 1 | 2 |
| Shoulder | 0.36 | II | N/P | < 1 | 4 |
| Back | 7 | IV | N/P | 4 | 5 |
| Cheek | 1.23 | IV | N/P | 2 | 0 |
| Cheek | 4 | V | N/P | 10 | 0 |
| Toe | 3.6 | IV | P | 7 | 2 |
The investigation of CD271 staining was further expanded to include all lesions, including banal melanocytic nevi, primary mucosal melanoma, melanoma lymph node metastasis, melanoma brain metastasis, breast carcinoma brain metastasis, and lung carcinoma brain metastasis (Figure 1A and 1C-F, Table 3). All cases of banal melanocytic nevi showed negative to focal equivocal staining (Score 0-1). Mucosal melanoma cases were mostly negative (score 0) except 2 cases which were weakly to moderately positive (score 1-2). Cases of lymph node metastasis ranged from negative to moderately positive staining (score 0-2).
Table 3.
Comparison of CD271 scores in melanoma brain metastasis group and/or primary cutaneous melanoma group with other groups
| Banal Nevus | Primary Cutaneous | Primary Mucosal | Melanoma Lymph Node Met | Carcinoma Brain Met | Melanoma Brain Met | |
|---|---|---|---|---|---|---|
| CD271 score (Mean) | 0.09 | 1.78 | 0.3 | 1 | 0.67 | 3.64 |
| P value-1* | < 0.001 | 0.01 | < 0.001 | < 0.001 | < 0.001 | Ref. |
| P value-2* | 0.01 | Ref. | 0.02 | 0.16 |
Indicates the p values by student t test, comparing each individual group with the reference group across the same row.
The CD271 expression was significantly increased in cases of metastatic melanoma to the brain, with a mean score of 3.64 when compared to all other groups (P < 0.01) (Table 3); it is noted that the expression of CD271 in cases of lung and breast carcinoma brain metastasis was also significantly lower than melanoma brain metastasis, indicating the increased CD271 expression is relatively specific in the latter.
The CD271 expression in primary cutaneous melanoma was overall higher than banal nevus and primary mucosal melanoma, in spite of the high variation (P < 0.05) (Table 3); no significant difference of CD271 expression was observed between the primary cutaneous melanoma group and the melanoma lymph node metastasis group (P = 0.16), however it should be noted that the sample size in the latter was much smaller (Table 3).
Discussion
Although the concept of stem cells in melanoma is still under investigation, several markers that are claimed to be melanoma stem cell markers have shown the potential to be effective diagnostic and prognostic markers, including the previously mentioned markers CD133, ABCB5, CD166, Nestin, and Sox2. New drugs and immunotherapy related with cancer stem cell targeting have been studied as well [18]. However, it is noteworthy that few studies concerning the relationship of the novel markers to clinical stages in melanoma have been performed up to now.
Our data suggest expression of melanoma stem cell marker CD271 is specifically increased in metastatic melanoma to the brain. Given the poor outcome after melanoma metastasis to the brain, it would be expected that CD271 expression in melanoma may correlate with patient’s poor prognosis. However, it should be noted that Mohamed et al. demonstrated that CD271 expression did not correlate with patient prognostic factors including Breslow depth, Clark level, sentinal lymph node status, pathologic stage, recurrence, or death, in spite of an observed significant correlation of its expression to melanoma metastasis [15].
Further prospective study for the role of CD271 in prediction of melanoma brain metastasis as well as prognosis assessment will be of great clinical significance. Our findings have proven the clinical usefulness of the melanoma stem cell marker CD271, and support the necessity in development of targeted therapy against the melanoma stem cells.
Acknowledgements
Funding for this project was provided by a research grant from the University Of Oklahoma Health Sciences Center Department Of Pathology. We thank the Peggy and Charles Stephenson Cancer Center at the University of Oklahoma, Oklahoma City, OK and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM103639 for the use of Histology and Immunohistochemistry Core, which provided immunohistochemistry and photographic services.
Disclosure of conflict of interest
None.
References
- 1.Kyrgidis A, Tzellos TG, Triaridis S. Melanoma: Stem cells, sun exposure and hallmarks for carcinogenesis, molecular concepts and future clinical implications. J Carcinog. 2010;9:3. doi: 10.4103/1477-3163.62141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Ko JM, Fisher DE. A new era: melanoma genetics and therapeutics. J Pathol. 2011;223:241–250. doi: 10.1002/path.2804. [DOI] [PubMed] [Google Scholar]
- 4.Gazit R, Weissman IL, Rossi DJ. Hematopoietic stem cells and the aging hematopoietic system. Semin Hematol. 2008;45:218–224. doi: 10.1053/j.seminhematol.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 6.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102–107. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- 8.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Kempen LC, van den Oord JJ, van Muijen GN, Weidle UH, Bloemers HP, Swart GW. Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am J Pathol. 2000;156:769–774. doi: 10.1016/S0002-9440(10)64943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma BK, Manglik V, Elias EG. Immuno-expression of human melanoma stem cell markers in tissues at different stages of the disease. J Surg Res. 2010;163:e11–15. doi: 10.1016/j.jss.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Laga AC, Lai CY, Zhan Q, Huang SJ, Velazquez EF, Yang Q, Hsu MY, Murphy GF. Expression of the embryonic stem cell transcription factor SOX2 in human skin: relevance to melanocyte and merkel cell biology. Am J Pathol. 2010;176:903–913. doi: 10.2353/ajpath.2010.090495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, Longaker MT, Weissman IL. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomellini E, Lagadec C, Polakowska R, Le Bourhis X. Role of p75 neurotrophin receptor in stem cell biology: more than just a marker. Cell Mol Life Sci. 2014;71:2467–2481. doi: 10.1007/s00018-014-1564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, Seifert B, Moch H, Dummer R, van den Broek M, Sommer L. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098–3109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed A, Gonzalez RS, Lawson D, Wang J, Cohen C. Tumor stem cells (CD271, c-kit, SOX10) in Melanomas: prognostic and outcome implications. Appl Immunohistochem Mol Morphol. 2014;22:142–145. doi: 10.1097/PAI.0b013e3182910a3d. [DOI] [PubMed] [Google Scholar]
- 16.Cuthbert RJ, Churchman SM, Tan HB, McGonagle D, Jones E, Giannoudis PV. Induced periosteum a complex cellular scaffold for the treatment of large bone defects. Bone. 2013;57:484–492. doi: 10.1016/j.bone.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Setia N, Abbas O, Sousa Y, Garb JL, Mahalingam M. Profiling of ABC transporters ABCB5, ABCF2 and nestin-positive stem cells in nevi, in situ and invasive melanoma. Mod Pathol. 2012;25:1169–1175. doi: 10.1038/modpathol.2012.71. [DOI] [PubMed] [Google Scholar]
- 18.Pietra G, Manzini C, Vitale M, Balsamo M, Ognio E, Boitano M, Queirolo P, Moretta L, Mingari MC. Natural killer cells kill human melanoma cells with characteristics of cancer stem cells. Int Immunol. 2009;21:793–801. doi: 10.1093/intimm/dxp047. [DOI] [PubMed] [Google Scholar]


