Abstract
Spinal cord is an important target of volatile anesthetics in particular for the effect of immobility. Intrathecal injection of volatile anesthetics has been found to produce subarachnoid anesthesia. The present study was designed to compare spinal anesthetic effects of emulsified volatile anesthetics, and to investigate the correlation between their spinal effects and general effect of immobility. In this study, halothane, isoflurane, enflurane and sevoflurane were emulsified by 30% Intralipid. These emulsified volatile anesthetics were intravenously and intrathecally injected, respectively. ED50 of general anesthesia and EC50 of spinal anesthesia were determined. The durations of general and spinal anesthesia were recorded. Correlation analysis was applied to evaluate the anesthetic potency of volatile anesthetics between their spinal and general effects. ED50 of general anesthesia induced by emulsified halothane, isoflurane, enflurane and sevoflurane were 0.41 ± 0.07, 0.54 ± 0.07, 0.74 ± 0.11 and 0.78 ± 0.08 mmol/kg, respectively, with significant correlation to their inhaled MAC (R2 = 0.8620, P = 0.047). For intrathecal injection, EC50 of spinal anesthesia induced by emulsified halothane, isoflurane, enflurane and sevoflurane were 0.35, 0.27, 0.33 and 0.26 mol/L, respectively, which could be predicted by the product of inhaled MAC and olive oil/gas partition coefficients (R2 = 0.9627, P = 0.013). In conclusion, potency and efficacy of the four emulsified volatile anesthetics in spinal anesthesia were similar and could be predicted by the product of inhaled MAC and olive oil/gas partition coefficients (MAC × olive oil/gas partition coefficients).
Keywords: Emulsified volatile anesthetics, spinal anesthesia, general anesthesia
Introduction
The application of diethyl ether in clinic is regarded as the beginning of modern anesthesia, which greatly improved surgical procedures. Although volatile anesthetics are commonly used, the exact mechanism of volatile anesthetics to induce general anesthesia is still unclear.
Many studies have indicated that spinal cord is an important target of volatile anesthetics for immobility [1,2]. However, the exact spinal mechanism of volatile anesthetics is still unknown. By previous studies, emulsified isoflurane was found to produce regional anesthesia [3-7] and typical subarachnoid anesthesia was achieved by intrathecal administration of volatile anesthetics [6,7]. The endpoint of subarachnoid anesthesia of volatile anesthetics (inhibition of nociceptive reflex, e.g. tail-clamping) is extremely similar to the endpoint of immobility induced by systemic inhalation of volatile anesthetics. In addition, intrathecal injection of pharmacological agents has been widely applied to determine spinal molecular targets of immobility induced by volatile anesthetics [8-10]. However, it is elusive whether spinal anesthesia of volatile anesthetics and their effect of immobility with systemic inhalation share common mechanisms.
MAC (minimum alveolar concentration) of volatile anesthetics indicates their general potency for inhibition of nociceptive reflex and significantly correlates with oil/gas partition coefficients (anesthetic with higher oil/gas partition coefficients has lower MAC). For two volatile anesthetics, the anesthetic with higher oil/gas partition coefficient would be distributed to brain or spinal cord more easily, thus, the exact concentrations of the two volatile anesthetics in spinal cord might be similar. By classic anesthetic theory of volatile anesthetics (e.g. Meyer-Overton Rule), product of inhaled MAC and oil/gas partition coefficients (MAC × oil/gas partition coefficients) might be a constant. Therefore, although MAC of volatile anesthetics are various, their spinal potency of immobility might be similar and correlate with the product of inhaled MAC and olive oil/gas partition coefficients (MAC × olive oil/gas partition coefficients). For the present study, we hypothesized that anesthetic potency of emulsified volatile anesthetics in spinal anesthesia would be predicted by the product of inhaled MAC and olive oil/gas partition coefficients if subarachnoid anesthesia of volatile anesthetics and their effect of immobility with systemic inhalation share similar mechanism. This study was designed to evaluate this hypothesis.
Material and methods
With the approval from the Institutional Animal Experimental Ethics Committee of Sichuan University (Chengdu, Sichuan, China), Sprague-Dawley rats weighing 200 to 300 g, evenly composed of males and females, were used in the present study. Rats were housed in cages with free access to food and water, and were kept on a 12-hour light-dark cycle.
Emulsified volatile anesthetics were prepared in our laboratory according to a well-established protocol [5]. For example, 100 ml 8% (v/v) emulsified isoflurane contained 8 ml pure liquid isoflurane. E-halothane, E-isoflurane, E-enflurane and E-sevoflurane were the abbreviations for emulsified halothane, emulsified isoflurane, emulsified enflurane and emulsified sevoflurane, respectively. Halothane was purchased from Halocarbon Laboratories (River Edge, NJ, US). Isoflurane and enflurane was supplied by Abbott Pharmaceutical Co., Ltd. of Shanghai (Shanghai, China). Sevoflurane was the product from Maruishi Pharmaceutical Co., Ltd. (Tokyo, Japan). Thirty percent Intralipid (30% soybean oil, 1.2% lecithin, 1.7% glycerin, water, and sodium hydroxide; pH = 8), the solvent of emulsified volatile anesthetics, was purchased from the Sino-Swed Pharmaceutical Co., Ltd. (Wuxi, Jiangsu, China). One percent lidocaine was prepared by diluting 2% lidocaine (Shanghai Fortune Zhaohui Pharmaceutical Co., Ltd., Shanghai, China) with normal saline.
Determination of general anesthetic ED50 of emulsified volatile anesthetics
To ensure anesthetic potency of volatile anesthetics would be unaffected by the preparation of lipid emulsion, general anesthetic potency of emulsified volatile anesthetics was determined. An up-and-down method [11] was applied to determine general anesthetic ED50 (for loss of righting reflex) of the four emulsified volatile anesthetics. Venipuncture was performed on the middle of rat tail with a 24-gauge intravenous cannula (Terumo Medical Corp., Tokyo, Japan). MAC (minimum alveolar concentration) of the four volatile anesthetics in rats was 1.24%, 1.45%, 2.20%, and 2.80% [12] respectively for halothane, isoflurane, enflurane and sevoflurane. According to inhaled MAC of the four volatile anesthetics and preliminary experiments, the initial doses received by the first rats in up-and-down method were 0.379, 0.462, 0.809, 0.858 mmol/kg, respectively for halothane, isoflurane, enflurane and sevoflurane. Loss of righting reflex on forelimbs of rats was considered as successful general anesthesia. After each successful general anesthesia, the doses of emulsified volatile anesthetics were multiplied by 0.85 fold; after each failure, the doses were divided by 0.85 fold. The up-and-down procedure was repeated until six successive crossover points (successful to failure) recorded. Durations of general anesthesia (from loss to revival of righting reflex) were recorded on the rats that developed successful general anesthesia.
Determination of spinal anesthetic EC50 of emulsified volatile anesthetics
The method of intrathecal administration in rats was used in this study. Briefly, the subarachnoid catheter (PE-10, Scientific Commodities INC. Lake Havasu City, Arizona, US) was inserted between L5-L6 of the rat and about 1 cm advanced the cephalic direction to place the distal end of the catheter at about L2-L3. One day before formal experiment, 1% lidocaine at volume of 20 μL was intrathecally injected to confirm the place of the catheter. For 1% lidocaine at volume of 20 μL, typical subarachnoid anesthesia in rats was developed: no nociceptive reflex to tail-clamping stimulus and lose of ability to support body weight for hind limbs.
The injection volume of emulsified volatile anesthetics was 150 μL/kg. The Bliss method [13] was applied to measure spinal anesthetic EC50 of the four emulsified volatile anesthetics. Loss of ability to support body weight on hind limbs was regarded as successful motor blockade and sensory blockade was determined by tail-clamping test at middle part of the tail. No aversive response to tail-clamping stimulus was considered as successful sensory blockade. The rats with both successful motor and sensory blockades were regarded as successful spinal anesthesia. The experimental groups for Bliss experiment was listed below (Table 1).
Table 1.
The experimental design and results for Bliss experiment to determinespinal anesthetic EC50 of emulsified volatile anesthetics
| N in each group | E-halothane** | E-isoflurane§ | E-enflurane† | E-sevoflurane‡ | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Groups | Anesthetized|| | Groups | Anesthetized | Groups | Anesthetized | Groups | Anesthetized | |
| 8 | 6.4% (0.59 M*) | 8 | 6.4% (0.53 M) | 8 | 6.0% (0.50 M) | 7 | 4.0% (0.29 M) | 5 |
| 8 | 5.1% (0.47 M) | 7 | 5.1% (0.42 M) | 7 | 4.8% (0.38 M) | 5 | 3.2% (0.24 M) | 3 |
| 8 | 4.1% (0.38 M) | 5 | 4.1% (0.34 M) | 6 | 3.8% (0.31 M) | 4 | 2.6% (0.19 M) | 2 |
| 8 | 3.3% (0.31 M) | 3 | 3.3% (0.27 M) | 4 | 3.1% (0.26 M) | 3 | 2.1% (0.15 M) | 1 |
| 8 | 2.6% (0.24 M) | 1 | 2.6% (0.21 M) | 3 | 2.5% (0.21 M) | 2 | 1.6% (0.12 M) | 0 |
| 8 | 2.1% (0.19 M) | 1 | 2.1% (0.17 M) | 2 | 2.0% (0.17 M) | 1 | 1.3% (0.10 M) | 0 |
| 8 | 1.7% (0.16 M) | 0 | 1.7% (0.14 M) | 0 | 1.6% (0.13 M) | 0 | 1.1% (0.08 M) | 0 |
| Spinal EC50 | 3.73% (3.24, 4.34) | 3.29% (2.73, 3.90) | 3.99% (3.32, 4.89) | 3.53% (3.06, 4.56) | ||||
Indicated the number of rats that developed successful spinal anesthesia for each concentration group.
Spinal EC50 of emulsified volatile anesthetics were analyzed by probit analysis and expressed as mean (95% confidence limits).
Indicated emulsified halothane;
Indicated emulsified isoflurane;
Indicated emulsified enflurane;
Indicated emulsified sevoflurane;
Indicated mol/L.
Comparison of spinal anesthetic effects of emulsified volatile anesthetics
To compare spinal anesthetic effects of the four emulsified volatile anesthetics, 0.30 mol/L (about their spinal EC50) of E-halothane, E-isoflurane, E-enflurane and E-sevoflurane were intrathecally administrated in rats (n = 12). Every rat received all the four emulsified volatile anesthetics at an interval of two days and the experimental protocol was listed below (Table 2). For every emulsified volatile anesthetic, percentage of rats that developed successful spinal anesthesia, onset time of spinal anesthesia, and duration of motor and sensory blockades were recorded after intrathecal administration.
Table 2.
The experimental protocol to compare spinal anesthetic effects of the four emulsified volatile anesthetics
| n = 12 | The order for intrathecal administration | |||
|---|---|---|---|---|
| A (n = 3) | E-halothane* | E-isoflurane** | E-enflurane§ | E-sevoflurane† |
| B (n = 3) | E-sevoflurane | E-halothane | E-isoflurane | E-enflurane |
| C (n = 3) | E-enflurane | E-sevoflurane | E-halothane | E-isoflurane |
| D (n = 3) | E-isoflurane | E-enflurane | E-sevoflurane | E-halothane |
Twelve rats were divided into four groups, as group A to D. Every rat received all the four emulsified volatile anesthetics at interval of two days according to the experimental order revealed in this table. For example, in group A, there were 3 rats received agents in an order from emulsified halothane, to emulsified isoflurane, to emulsified enflurane and to emulsified sevoflurane.
Indicated emulsified halothane;
Indicated emulsified isoflurane;
Indicated emulsified enflurane;
Indicated emulsified sevoflurane.
Statistical analysis
The data were analyzed by SPSS 16.0 (SPSS Inc., Chicago, IL). For up-and-down experiment, general anesthetic ED50 of emulsified volatile anesthetics was calculated by averaging the doses of the six crossovers and expressed as mean ± SD. One-way analysis of variance (ANOVA) with S-N-K post hoc test was applied to compare the ED50 of general anesthesia as well as the duration of general anesthesia. For Bliss experiment, probit analysis was applied to determine spinal anesthetic EC50 of emulsified volatile anesthetics. One-way analysis of variance (ANOVA) with S-N-K post hoc test was applied to compare the onset time and the duration of motor and sensory blockade among the four emulsified volatile anesthetics. In all cases, P < 0.05 was considered as statistically significant.
To analyze general and spinal effects of emulsified volatile anesthetics, correlation analysis was applied between inhaled MAC and intravenous general ED50; olive oil/gas partition and duration of general anesthesia; as well as the correlation between spinal anesthetic EC50 and the product of inhaled MAC × olive oil/gas partition coefficient of the four volatile anesthetics.
Results
By intravenous administration, general anesthetic ED50 (for loss of righting reflex) of emulsified halothane, emulsified isoflurane, emulsified enflurane and emulsified sevoflurane were 0.41 ± 0.07, 0.54 ± 0.07, 0.74 ± 0.11 and 0.76 ± 0.08 mmol/kg and the durations of general anesthesia were 116 ± 18, 39 ± 9, 58 ± 13 and 23 ± 7 seconds, respectively. Significant differences were found in ED50 and anesthetic durations between each two emulsified volatile anesthetics (P < 0.01, Table 3). By correlation analysis, general anesthetic ED50 of the four emulsified volatile anesthetics and their inhaled MAC were in significant correlation (R2 = 0.8620, P = 0.047, Figure 1A). Durations of general anesthesia of the four emulsified volatile anesthetics were perfectly predicted by their olive oil/gas partition coefficients (Figure 1B, R2 = 0.9810, P = 0.006). These results indicated that the potency and efficacy of emulsified volatile anesthetics were unaffected by the preparation of lipid emulsion.
Table 3.
Correlation between spinal anesthetic EC50 of the four emulsified volatile anesthetics and their MAC × olive oil/gas partition coefficients
| λ | MAC in rats (% ) | MAC × λ | Spinal EC50 (mol/L) | |
|---|---|---|---|---|
| Halothane | 224.0 ± 9.0 | 1.24 | 277.76 | 0.35 |
| Isoflurane | 88.2 ± 1.3 | 1.45 | 127.89 | 0.27 |
| Enflurane | 103.0 ± 4.0 | 2.20 | 226.6 | 0.33 |
| Sevoflurane | 47.5 ± 0.8 | 2.80 | 133.0 | 0.26 |
λ: olive oil/gas partition coefficient; All the data of inhaled MAC and olive oil/gas partition coefficients in this table were from the study by Taheri S et al. [12].
Figure 1.
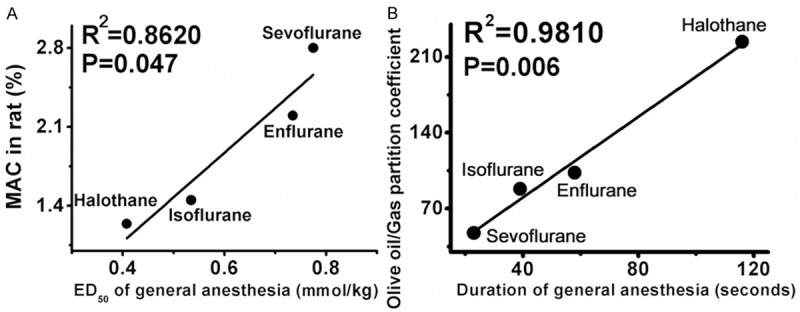
The correlation between inhaled MAC and intravenous general anesthetic ED50 of the four emulsified volatile anesthetics. The intravenous general anesthetic ED50 of the four volatile anesthetics could be predicted by their inhaled MAC (R2 = 0.8620, P = 0.047). This significant correlation indicated that the preparation of lipid emulsion did not affect potency and efficacy of emulsified volatile anesthetics.
For intrathecal administration, spinal anesthetic EC50 (v/v) of emulsified halothane, emulsified isoflurane, emulsified enflurane and emulsified sevoflurane were 3.73% (3.24%, 4.34%), 3.29% (2.73%, 3.90%), 3.99% (3.32%, 4.89%) and 3.53% (3.06%, 4.56%), respectively (Figure 2). Data were expressed as mean (95% confidence limits). If calculated in molar concentrations, spinal anesthetic EC50 of emulsified halothane, emulsified isoflurane, emulsified enflurane and emulsified sevoflurane were 0.35, 0.27, 0.33 and 0.26 mol/L, respectively. Spinal anesthetic EC50 of the four emulsified volatile anesthetics were similar (P > 0.05) and could not be predicted by neither their inhaled MAC nor olive oil/gas partition coefficients. Inhaled MAC and olive oil/gas partition coefficients of the four volatile anesthetics were according to the study by Taheri et al [12]. The spinal anesthetic EC50 (potency) of the four emulsified volatile anesthetics could be predicted by the product of MAC and their olive oil/gas partition coefficients (R2 = 0.9627, P = 0.013, Table 3 and Figure 3).
Figure 2.
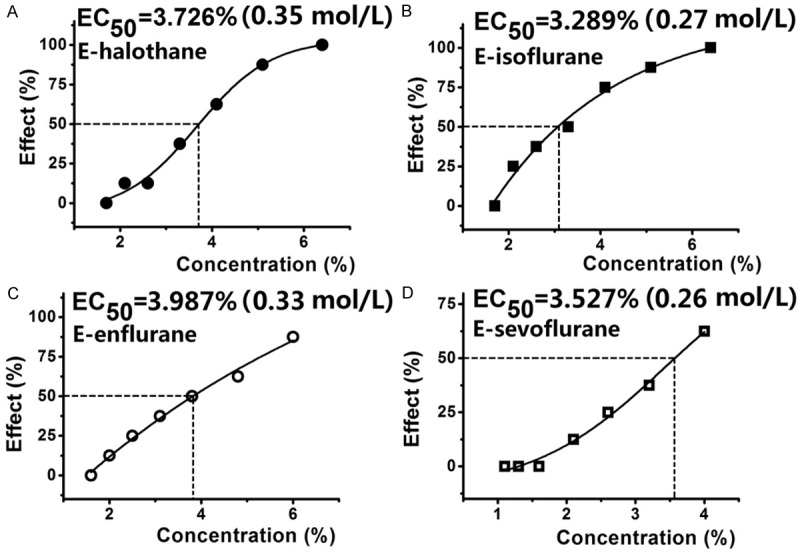
The spinal anesthetic effect-concentration curves for the four emulsified volatile anesthetics. All the four emulsified volatile anesthetics produced typical subarachnoid anesthesia in rats in a concentration-dependent manner and no significant difference was found among the four emulsified volatile anesthetics when they were intrathecally administrated in rats.
Figure 3.
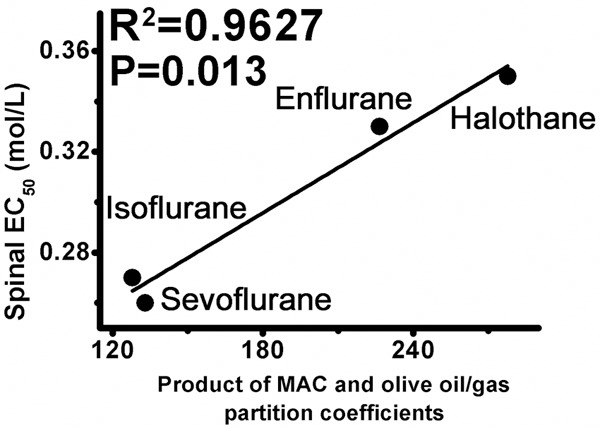
The correlation between spinal anesthetic EC50 and product of inhaled MAC and olive oil/gas partition coefficients (MAC × olive oil/gas partition coefficients). Spinal anesthetic EC50 of the four emulsified volatile anesthetics could be predicted by their product of inhaled MAC and their olive oil/gas partition coefficients (R2 = 0.9627, P = 0.013). Spinal anesthesia of volatile anesthetics and their effect of immobility might share common mechanisms.
At concentration of 0.30 mol/L, durations of motor blockade were 10.1 ± 1.8, 11.0 ± 2.6, 10.0 ± 1.4 and 11.4 ± 2.8 min for emulsified halothane, emulsified isoflurane, emulsified enflurane and emulsified sevoflurane, respectively and durations of sensory blockade were 6.9 ± 1.6, 7.6 ± 1.6, 6.2 ± 2.0 and 6.7 ± 2.1 min, respectively, without significant difference between each two emulsified volatile anesthetics (P > 0.05, Table 4).
Table 4.
General and spinal anesthetic effects of the four emulsified volatile anesthetics
| General anesthesia | Spinal anesthesia | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| ED50 (mmol/kg) | Duration (s) | Ratio (n = 12) | Onset (s) | Durations of blockade (min) | ||
|
|
||||||
| Motor | Sensory | |||||
| E-halothane* | 0.41 ± 0.07 | 116 ± 18 | 6 | 39 ± 9 | 10.1 ± 1.8 | 6.9 ± 1.6 |
| E-isoflurane** | 0.54 ± 0.07 | 39 ± 9 | 7 | 41 ± 7 | 11.0 ± 2.6 | 7.6 ± 1.6 |
| E-enflurane§ | 0.74 ± 0.11 | 58 ± 13 | 6 | 42 ± 8 | 10.0 ± 1.4 | 6.2 ± 2.0 |
| E-sevoflurane† | 0.78 ± 0.08 | 23 ± 7 | 7 | 46 ± 5 | 11.4 ± 2.8 | 6.7 ± 2.1 |
“Ratio” indicated the number of rats that developed successful spinal anesthesia for each emulsified volatile anesthetic (n = 12). The onset time of general anesthesia was not observed because general anesthesia induced by emulsified volatile anesthetics was occurred immediately after injection.
Indicated emulsified halothane;
Indicated emulsified isoflurane;
Indicated emulsified enflurane;
Indicated emulsified sevoflurane.
Discussion
In the present study, we compared general and spinal anesthetic effects of the four emulsified volatile anesthetics. By intravenous injection, the general anesthetic ED50 of the four emulsified volatile anesthetics were correlated to their inhaled MAC and durations of general anesthesia were perfectly predicted by their olive oil/gas partition coefficients. Therefore, the potency and efficacy of volatile anesthetics were unaffected by the preparation of lipid emulsion, excluding the possibility that emulsified preparation could affect effects of volatile anesthetics. Thus, similar potency of the four emulsified volatile anesthetics in spinal anesthesia resulted from their pharmacological properties. Of note, for this study, endpoint of general anesthesia was loss of righting reflex and was different to that of spinal anesthesia (inhibition of nociceptive reflex), thus, we did not directly compare general and spinal anesthetic effects of emulsified volatile anesthetics.
The present study demonstrated that all the study emulsified volatile anesthetics could produce typical subarachnoid anesthesia with similar potency and durations. Spinal anesthetic EC50 of the four emulsified volatile anesthetics could not be predicted by their general anesthetic ED50. While the product of inhaled MAC and olive oil/gas partition coefficients (MAC × olive oil/gas partition coefficients) could perfectly predict spinal potency of volatile anesthetics. The deviation of spinal anesthetic EC50 between halothane (0.35 mol/L) and sevoflurane (0.26 mol/L) is small, although their MAC (1.24% vs. 2.80%) is significantly different. This result indicates that all volatile anesthetics might induce spinal anesthesia with relatively similar potency. And the small deviation in regional anesthetic potency of volatile anesthetics is consistent with previous studies that effects of volatile anesthetics on underlying molecular targets of general anesthesia were similar [14-20]. The concentration of halothane in brain and/or spinal cord would be significantly higher than the concentration of sevoflurane when they are inhaled at the same concentration and liposolubility of volatile anesthetics is the main factor to affect their absorption and distribution in vivo. For usual application, volatile anesthetics were inhaled and distributed to targets through various equilibriums including alveoli-blood, blood-brain and blood-spinal cord equilibriums. Thus, at their MAC respectively, the exact concentrations of halothane and sevoflurane in spinal cord might be similar. Therefore, although MAC of volatile anesthetics are various, their spinal potency of immobility might be similar and correlate with the product of inhaled MAC and olive oil/gas partition coefficients (MAC × olive oil/gas partition coefficients).
Spinal cord has been identified as an important anesthetic target of volatile anesthetics for immobility [1,2]. Previous studies suggested that volatile anesthetics could produce typical subarachnoid anesthesia with intrathecal administration [6,7]. In the present study, the potency of emulsified volatile anesthetics in spinal cord could be perfectly predicted by the product of MAC and olive oil/gas partition coefficients, indicating that subarachnoid anesthetic effect of volatile anesthetics and their effect of immobility might share common mechanisms. Based on the results of this study, intrathecal administration of volatile anesthetics could be an appropriate method to determine spinal mechanism of volatile anesthetics.
For experimental design, we used up-and-down method to determine general anesthetic ED50 whereas Bliss method was chosen for spinal anesthetic EC50. The up-and-down method is an efficient way to measure ED50 and/or EC50 with small sample size. However, we chose Bliss method to determine EC50 of spinal anesthesia of the four emulsified volatile anesthetics because we could not make sure to induce 50% successful spinal anesthesia under saturated concentration of volatile anesthetics in 30% Intralipid, in particular for sevoflurane (saturated concentration of sevoflurane in 30% Intralipid is only about 4%). If the EC50 of emulsified sevoflurane to induce spinal anesthesia was larger than 4%, up-and-method could not be available.
There were still some limitations in the present study. Firstly, it would be better evidence if exact concentrations of volatile anesthetics in spinal cord were similar between inhalation and intrathecal injection. However, for technological obstacles and volatile properties of volatile anesthetics, we could not determine their exact concentrations in spinal cord after administration. Secondly, we did not test spinal anesthetic potency of all volatile anesthetics.
In summary, emulsified volatile anesthetics produced typical spinal anesthesia with similar potency. The product of inhaled MAC and olive oil/gas partition coefficients (MAC × olive oil/gas partition coefficients) could perfectly predict spinal potency of emulsified volatile anesthetics. Thus, subarachnoid anesthetic effect of volatile anesthetics and their effect of immobility might share common mechanisms.
Acknowledgements
This study was supported by the grant 2014M552361 (to Dr. Cheng Zhou) from the National Postdoctoral Foundation of China; and the grant 81401139 (to Dr. Cheng Zhou) from the National Natural Science Foundation of China. The authors thank Dr. Jing Yang (Department of Anesthesiology, West China Hospital, Sichuan University, Chengdu, China), for instructive advice and useful suggestions on article preparation.
Disclosure of conflict of interest
None.
References
- 1.Eger EI 2nd, Raines DE, Shafer SL, Hemmings HC Jr, Sonner JM. Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth Analg. 2008;107:832–848. doi: 10.1213/ane.0b013e318182aedb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J, Chai YF, Gong CY, Li GH, Luo N, Luo NF, Liu J. Further proof that the spinal cord, and not the brain, mediates the immobility produced by inhaled anesthetics. Anesthesiology. 2009;110:591–5. doi: 10.1097/ALN.0b013e3181974bfd. [DOI] [PubMed] [Google Scholar]
- 3.Chai YF, Yang J, Liu J, Song HB, Yang JW, Liu SL, Zhang WS, Wang QS. Epidural anaesthetic effect of the 8% emulsified isoflurane: a study in rabbits. Br J Anaesth. 2008;100:109–115. doi: 10.1093/bja/aem298. [DOI] [PubMed] [Google Scholar]
- 4.Zhou C, Gan J, Liu J, Luo WJ, Zhang WS, Chai YF. The interaction between emulsified isoflurane and lidocaine is synergism in intravenous regional anesthesia in rats. Anesth Analg. 2011;113:245–250. doi: 10.1213/ANE.0b013e31821e9797. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Yang J, Liu J, Gong CY, Gan J, Zhang X, Luo WJ, Li GH. Reversible conduction block in isolated toad sciatic nerve by emulsified isoflurane. Anesth Analg. 2010;110:1024–1029. doi: 10.1213/ANE.0b013e3181d2732f. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Wu W, Liu J, Liao DQ, Kang Y, Chen XD. Inhibition of voltage-gated sodium channels by emulsified isoflurane may contribute to its subarachnoid anesthetic effect in beagle dogs. Reg Anesth Pain Med. 2011;36:553–9. doi: 10.1097/AAP.0b013e3182324d18. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Fernandez J, Parodi E, Garcia P, Matute E, A-Gomez-de-Segura I, Cediel R, Gilsanz F. Clinical actions of subarachnoid sevoflurane administration in vivo: a study in dogs. Br J Anaesth. 2005;95:530–4. doi: 10.1093/bja/aei205. [DOI] [PubMed] [Google Scholar]
- 8.Ishizaki K, Yoon DM, Yoshida N, Yamazaki M, Arai K, Fujita T. Intrathecal administration of N-methyl-D-aspartate receptor antagonist reduces the minimum alveolar anaesthetic concentration of isoflurane in rats. Br J Anaesth. 1995;75:636–8. doi: 10.1093/bja/75.5.636. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Sharma M, Eger EI, Laster MJ, Hemmings HC Jr, Harris RA. Intrathecal veratridine administration increases minimum alveolar concentration in rats. Anesth Analg. 2008;107:875–8. doi: 10.1213/ane.0b013e3181815fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowley TJ, Daniel D, Flood P. The role of adrenergic and cholinergic transmission in volatile anesthetic-induced pain enhancement. Anesth Analg. 2005;100:991–5. doi: 10.1213/01.ANE.0000147708.73945.B3. [DOI] [PubMed] [Google Scholar]
- 11.Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15:47–50. doi: 10.1016/s0149-7634(05)80090-9. [DOI] [PubMed] [Google Scholar]
- 12.Taheri S, Halsey MJ, Liu J, Eger EI 2nd, Koblin DD, Laster MJ. What solvent best represents the site of action of inhaled anesthetics in humans, rats, and dogs? Anesth Analg. 1991;72:627–34. doi: 10.1213/00000539-199105000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Carmines EL, Carchman RA, Borzelleca JF. A method for the evaluation of dose-effect data utilizing a programmable calculator. J Environ Pathol Toxicol. 1980;4:23–30. [PubMed] [Google Scholar]
- 14.Ouyang W, Herold KF, Hemmings HC Jr. Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function. Anesthesiology. 2009;110:582–90. doi: 10.1097/ALN.0b013e318197941e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso RA, Yarnakura T, Susan J, Chavez-Noriega LE, Harris RA. Human neuronal nicotinic acetylcholine receptors expressed in xenopus oocytes predict emcacy of halogenated compounds mat disobey the Meyer-Ouerton rule. Anesthesiology. 1999;91:1370–7. doi: 10.1097/00000542-199911000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Johnson CB, Taylor PM. Comparison of the effects of halothane, isoflurane and methoxyflurane on the electroencephalogram of the horse. Br J Anaesth. 1998;81:748–753. doi: 10.1093/bja/81.5.748. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa SK, Tanaka E, Shin MC, Kotani N, Akaike N. Volatile anesthetic effects on isolated GABA synapses and extrasynaptic receptors. Neuropharmacology. 2011;60:701–10. doi: 10.1016/j.neuropharm.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Grasshoff C, Antkowiak B. Effects of isoflurane and enflurane on GABAA and glycine receptors contribute equally to depressant actions on spinal ventral horn neurones in rats. Br J Anaesth. 2006;97:687–94. doi: 10.1093/bja/ael239. [DOI] [PubMed] [Google Scholar]
- 19.Olsen RW, Li GD. GABAA receptors as molecular targets of general anesthetics: identification of binding sites provides clues to allosteric modulation. Can J Anesth. 2011;58:206–15. doi: 10.1007/s12630-010-9429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horishita T, Eger EI 2nd, Harris RA. Effects of volatile aromatic anesthetics on voltage-gated Na+ channels expressed in xenopus oocytes. Anesth Analg. 2008;107:1579–1586. doi: 10.1213/ane.0b013e318184b966. [DOI] [PMC free article] [PubMed] [Google Scholar]


