Abstract
Objective: To detect the effects of 17β-estradiol (E2) on the expression of calbindin-D9k (CaBP-9k) in pituitary GH3 cells, and to determine the antagonistic effect of a selective estrogen receptor (ER) antagonist (ICI 182 780) on CaBP-9k expression. Methods: A rat pituitary prolactinoma cell line (GH3 cells) was used in an in vitro model. The localization of CaBP-9k in GH3 cells was observed by immunofluorescence. GH3 cells were cultured with the addition of E2 medium for 24 hours. The levels of CaBP-9k mRNA and protein expression in different groups were analyzed by RT-PCR and Western blot analysis. The ER antagonist, ICI 182 780, was added to GH3 cells before E2 (10-8 M) at a concentration of 10-6 M to investigate the regulation of an ER-mediated pathway on CaBP-9k expression. Results: E2 had a stimulatory effect on CaBP-9k expression of GH3 cells in a dose-dependent manner; the level of CaBP-9k expression was higher when treated with a higher concentration of E2. ICI 182 780 suppressed the stimulatory effect of E2 on CaBP-9k expression in GH3 cells. The level of CaBP-9k expression was significantly reduced by co-administration of E2 with ICI 182 780 in GH3 cells. The immunoprecipitation results confirmed that CaBP-9k interacts directly with ERα, and E2 increases the interaction between CaBP-9k and ERα. Conclusion: Estrogen induces CaBP-9k expression via an ERα-mediated pathway and CaBP-9k directly combines with ERα, suggesting that CaBP-9k is involved in the biological effects mediated by an ER pathway in GH3 cells.
Keywords: GH3 cells, estrogen, estrogen receptor, ICI 182 780, CaBP-9k
Introduction
Calbindin-D9k (CaBP-9k) is a member of the family of calcium-binding proteins with a high affinity for calcium; CaBP-9k is expressed in the intestines, kidneys, lungs, bones, uterus, placenta, and pituitary gland [1,2]. CaBP-9k, which is involved in calcium absorption and transfer, is regulated at the transcriptional and post-transcriptional levels by 1,25-dihydroxy vitamin D3 in the intestines [3], and sex steroid hormones in the uterus and placenta [4]. Calcium is an important element in the process of cell signaling and maintenance of cell function, therefore CaBP-9k may be an important element of cellular signal transduction pathways that regulate cell growth, survival, differentiation, and apoptosis, and participate in several important biological processes [5].
The GH3 cell line, which is a well-established rat pituitary cell line sensitive to estrogen stimulation, is often used as an in vitro research model for pituitary prolactinomas. GH3 cells have been shown to express a high level of estrogen receptor (ER), which plays an important role in GH3 cell proliferation, apoptosis, and prolactin secretion [6]. Recent studies have shown that the level of CaBP-9k expression in GH3 cells is regulated by exogenous estrogen in vitro, and ER may be involved in the regulatory mechanism [6-8]; however, the role of CaBP-9k in pituitary adenoma tumorigenesis and the underlying regulatory mechanism is unclear. In the present study we used the GH3 cell line as an in vitro model of pituitary adenomas to determine the effects of estrogens on the induction of CaBP-9k mRNA and protein, and to determine the possible role of ER in the regulation process. GH3 cells were treated with 17β-estradiol (E2). The effects of E2 on the induction of CaBP-9k mRNA and protein were examined by semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) and Western blot assays. Additionally, an ER antagonist (ICI 182 780) was used as an inhibitor of ER to examine the potential involvement of the ER pathway in the induction of CaBP-9k in GH3 cells. Co-immunoprecipitation was used to determine if CaBP-9k interacts directly with ER and whether or not the expression of CaBP-9k in GH3 cells is related to an ER pathway.
Materials and methods
Cells and reagents
The rat pituitary cell line, GH3, was purchased from the American Type Culture Collection (ATCC; Rockville, MD, USA). Dimethyl sulfoxide (DMSO) and 17β-estradiol (E2) were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). The ER antagonist, ICI 182 780, was obtained from Tocris (Ellisville, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), phenol red-free DMEM, and fetal bovine serum (FBS) were obtained from Gibco BRL (Grand Island, NY, USA). Trizol reagent was purchased from Invitrogen Life Technologies. The cell lysis liquid, RIPA, and a BCA Protein Assay kit were obtained from Thermo (Rockford, IL, USA). Rat monoclonal antibodies against CaBP-9k, ERα, and ERβ, anti-β-actin antibody, horseradish peroxidase-conjugated secondary antibody, and FITC-conjugated secondary antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture and treatment
GH3 cells were maintained in DMEM medium containing 10% FBS (Gibco) and 100 U/ml of penicillin-streptomycin (Gibco) in a 37°C shaker containing 5% CO2 and dispersed in a 6-well plate. When the cells grew to 70%~80% fusion, the culture was changed to a phenol red-free DMEM medium containing 5% activated carbon-FBS glucan without hormone and 100 U/ml penicillin-streptomycin. The cells were continuously cultivated in this culture for 5 days before use.
Experiment 1: GH3 cells were treated with different concentrations of E2 (10-8, 10-9, and 10-10 M). E2 was dissolved in 0.1% (vol/vol) DMSO, and the vehicle control group (DMSO) was set up. Total RNA or protein was extracted 24 h after administration of E2.
Experiment 2: A group of GH3 cells was first given ICI 182 780 (10-6 M) 30 min before E2. The vehicle control group and a separate E2 group (10-8 M) were set up. Cells were collected after 24 h for total RNA or protein extraction. Each group of experiments was repeated 3 times.
Immunofluorescence assay
GH3 cells were cultured in 24-well plates, stained with immunofluorescence when the cells reached 50% fusion by adherent growth, washed 3 times with pre-warmed PBS, fixed with 4% formaldehyde for 30 min at room temperature, washed 3 times with PBS, washed 3 times with PBS after a 5-min permeabilization with 0.2% Triton X-100, and sealed with 5% bovine serum albumin (BSA) at room temperature for 30 min. Goat anti-rat CaBP-9k antibody was added, the plates were placed in a wet box, incubated overnight at 4°C, and washed 3 times with PBS. Donkey anti-goat FITC antibody was added, incubated for 30 min at room temperature without light, washed 3 times with PBS, stained with 1 ml DAPI working liquid mu (1 μg/ml) for 10 min avoiding light, washed 3 times with PBS, and observed under fluorescence microscopy.
RT-PCR
Total RNA of the cells was extracted using the Trizol method. The concentration of RNA was detected using a microplate reader, with purity determined by the ratio of A260/A280. TaKaRa RT-M/MLV kits were used for the reverse transcription reaction. The total reaction volume was 25 μl (total RNA volume, 1 μg; 20 μM oligo [dT; 2 μl]; and DEPC, 3 μl). The mixture was heated at 72°C for 5 min after blending, placed on ice immediately for 5 min, and M-MLV (1 μl), 5X buffer (5 μl), dNTP (2 μl), RNase inhibitor (1 μl), and DEPC (8 μl) was added, and put into the PCR amplifier for reaction after blending at 42°C for 1 h and 72°C for 15 min. The mixture was placed on ice for cooling for 20 min, and the cDNA templates were obtained. The PCR reaction was performed according to the TaKaRa Ex-Taq DNA polymerase instructions. The reaction conditions were as follows: 95°C denaturation for 5 min; 95°C for 30 s; annealing for 30 s; 72°C extension for 45 s; and 72°C extension for 10 min after reaching the number of cycles. Different primers, the annealing temperature, and the cycle numbers are shown in Table 1.
Table 1.
Primer sequences and reaction conditions
| Primer pair (5’-3’, forward and reverse) | Tm (°C) | Cycles | Size (bp) |
|---|---|---|---|
| CaBP-9k | |||
| F: ATG AGC GCT AAG AAA TCT | 55 | 30 | 287 |
| R: TCA TTC TGATAA CTT TTT GA | |||
| GAPDH | |||
| F: AAC GGA TTT GGC CGT ATT | 54 | 25 | 300 |
| R: AGC CTT CTC CAT GGT GAA GA |
Western blot analysis
The cultured cells were collected after 24 h, counted (1 × 106 cells), the RIPA lysate was added for ice bath cracking for 30 min, centrifuged at 12000 r/min (centrifugal radius = 13.5 cm) and 4°C for 30 min, the supernatant was collected, and the protein concentration was determined by the BCA method. Following SDS-PAGE electrophoresis, the proteins were transferred to PVDF membranes, sealed in 5% BSA at room temperature for 2 h, and thrice-washed with TBS-T for 10 min each. Goat anti-rat CaBP-9k antibody (working concentration = 1:200) was added with β-actin as an internal reference, and incubated overnight at 4°C. Donkey anti-goat IgG-HRP antibody (working concentration = 1:2000) was added, gently shaken for 2 h at room temperature, and the membrane was washed. An ECL chemiluminescence detection kit was used to detect hybridization signals. Quantity one software was used to detect the gray degree of the strip. The relative ratio of each group was measured according to the gray degree of the internal reference β-actin. Each experiment was repeated three times and the results were averaged.
Co-immunoprecipitation
Cells in the logarithmic growth phase were selected and RIPA lysis buffer and protease inhibitors were added for ice bath cracking for 30 min, centrifuged at 12000 r/min (centrifugal radius = 13.5 cm) for 30 min at 4°C, the supernatants were obtained, and the protein concentrations of a small amount of supernatant were determined using the BCA method. Twenty μl of Protein G agarose beads (PBS formulated as a 50% concentration) were added to every 400 μg of cell lysate supernatant, incubated for 60 min at 4°C to remove non-specific hybrid protein, centrifuged at 12000 r/min (centrifugal radius 13.5 cm) for 30 s at 4°C, and the supernatant was collected. Tw μg of goat anti-rat Calbindin-D9k antibody (IP antibody) was added to 500 μl of total protein, and the antigen-antibody mixture was gently shaken and incubated overnight at 4°C. The next day, 20 μl of Protein G was added to capture the antigen-antibody complex, gently shaken at 4°C for 3 h, centrifuged at 12000 r/min (centrifugal radius = 13.5 cm) for 30 s at 4°C, the supernatant was discarded, and thrice-washed with cold RIPA buffer (without a protease inhibitor). The supernatant was completely exhausted, the loading buffer was added, boiled for 5 min at 100°C, and the proteins were separated by SDS-PAGE, transferred to a PVDF membrane, sealed with 5% BSA for 1 h at room temperature, the membrane was thrice-washed with PBS-T, rabbit anti-rat ERα antibody was added, and incubated overnight at 4°C. The membrane was thrice-washed with PBS-T, goat anti-rabbit HRP antibody was added, and incubated at 37°C for 1 h. The membrane was thrice-washed with PBS-T, the ECL reagents were added to the PVDF membrane, and placed in the ECL chemiluminescence analyzer. Images were acquired.
Statistical analysis
All experiments were repeated ≥ 3 times. SPSS 18.0 software was used for statistical analysis. The Student’s t-test was used for comparison between the two sets of data, and ANOVA analysis was used for group comparison. A P < 0.05 was considered statistically significant.
Results
Detection of CaBP-9k expression in GH3 cells by immunofluorescence
As shown in Figure 1, green phosphor was detected on GH3 cells, which represented the expression of CaBP-9k. GH3 cells were simultaneously stained with DAPI in the nuclei. The pictures were superimposed. It was shown that CaBP-9k may be expressed in the cytoplasm.
Figure 1.

Detection of CaBP-9k expression in GH3 cells by immunofluorescence.
Analysis of the differential effect of E2 on the level of CaBP-9k expression in GH3 cells based n RT-PCR and Western blot analysis
GH3 cells were treated with different concentrations of E2 (10-8, 10-9, and 10-10 M) for 24 h. The vehicle control group (VE) was set up. Based n RT-PCR and Western blot analysis, the levels of CaBP-9k mRNA and protein expression increased when treated with higher concentrations of E2. Figure 2 shows the results of RT-PCR. Western blot results are shown in Figure 3 (*indicates P < 0.01).
Figure 2.

Detection of the effect of E2 on the level of CaBP-9k mRNA expression in GH3 cells based on RT-PCR.
Figure 3.

Detection of the effect of E2 on the level of CaBP-9k protein expression in GH3 cells based on Western blot analysis.
Investigation of the antagonistic effect of ICI 182 780 on E2-induced CaBP-9k expression in GH3 cells through RT-PCR and Western blot analysis
VE was the vehicle control group. The E2 group was given 10-8 M E2. The E2 + ICI group was given 10-6 M ICI 182 780 30 min before administration of E2; the delivery time was 24 h. The level of CaBP-9k expression in each group of GH3 cells was detected by RT-PCR and Western blot analysis, as shown in Figures 4 and 5. The results showed that ICI 182 780 can inhibit E2-induced CaBP-9k expression at the genetic and protein levels. The E2 group was compared to the VE group (*represents P < 0.01; the E2 + ICI group was compared to the E2 group, **means P < 0.01).
Figure 4.

Detection of the antagonistic effect of ICI 182 780 on E2-induced mRNA expression of CaBP-9k in GH3 cells by RT-PCR.
Figure 5.
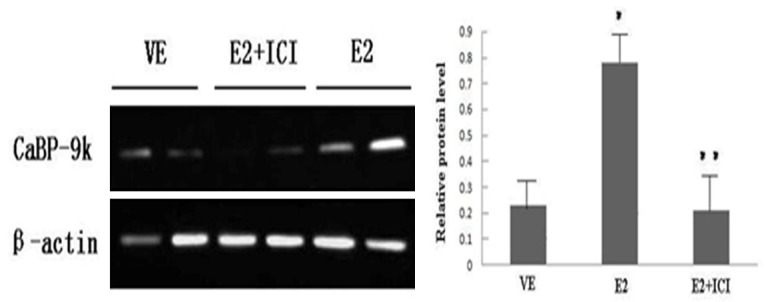
Detection of the antagonistic effect of ICI 182 780 on E2-induced protein expression of CaBP-9k in GH3 cells by Western blot analysis.
Analysis of interactions between CaBP-9k and ERα by co-immunoprecipitation
It has been shown that estrogen plays an important role in the occurrence and development of prolactinomas [6], which is closely related to the activation of ER signal transduction pathways. Although numerous studies have revealed that ER signaling pathways play a key role in the occurrence, development, and drug resistance of prolactinomas, the exact mechanism remains unclear. Our previous in vitro experiments involving GH3 cell lines showed that E2 can promote the proliferation of GH3 cells and PRL release through ER signal transduction pathways (mainly ERα), whereas the ER antagonist, ICI 182 780, can inhibit E2-induced cell proliferation and PRL release [10].
The present study showed that the level of CaBP-9k expression is closely related to E2. As the selective antagonist of ERα, ICI 182 780 can antagonize the effect of E2-induced CaBP-9k expression of GH3 cells. Therefore, we analyzed the interaction between CaBP-9k and ERα by immunoprecipitation. The protein of interest gained by the immunoprecipitation method was treated with the CaBP-9k antibody and purified through combination with Protein G, denatured, separated by electrophoresis, and transferred to a membrane. Then, the ERα antibody was added to create a hybridoma. The interaction between the two was analyzed. The results are shown in Figure 6. In the GH3 cells, lysis buffer of the E2 and vehicle control groups, protein bands were hybridized in both immune coprecipitation complexes consistent with the target protein, suggesting that CaBP-9k and ERα can combine with each other directly in GH3 cells, and after the administration of E2, the interaction between the two may increase.
Figure 6.

Analysis of interactions between CaBP-9k and ERα by co-immunoprecipitation.
Discussion
Prolactinomas are the most common pituitary adenoma, accounting for 45% of pituitary adenomas and 40%-60% of hormone-secreting tumors. Currently, the pathogenesis of prolactinomas is unclear; however, the present study confirmed that estrogen plays an important role in the occurrence and development of prolactinomas, which may due to the activation of ER pathways [6,10]. In our previous research, we studied the effect of estrogen and ER on the proliferation of prolactinoma cells, the secretion of prolactin, and the function of ER antagonist in the treatment of prolactinomas. Based on the in vitro experiments of the GH3 cell line, we found that estrogen promoted the proliferation of prolactinoma cells and pituitary prolactin release significantly through ER signal transduction pathways (mainly ERα), while the selective ER antagonist, ICI, 182 780, inhibited estrogen-induced GH3 cell proliferation and prolactin release. A study abroad also confirmed that estrogen and ER play an important role in the proliferation and apoptosis of a prolactinoma cell line, and prolactin secretion [6].
Based on the current study we found that the level of CaBP-9k mRNA and protein expression in GH3 cells was increased by estrogen, while antagonized by the ER antagonist, ICI 182 780, suggesting that ER plays a decisive role in the regulation of CaBP-9k expression. We further analyzed the interaction between CaBP-9k and ERα by co-precipitation and showed that CaBP-9k and ERα combines and interacts directly in GH3 cells, and the interaction between CaBP-9k and ERα may increase via E2. This suggests that the expression of CaBP-9k in pituitary adenoma is related to the effect of estrogen, which is most likely mediated by an ERα pathway.
Why CaBP-9k was highly expressed in prolactin cells with active prolactin secretion and proliferation? What role does CaBP-9k play in cell proliferation, secretion of prolactin, and tumor invasive growth, and how does CaBP-9k function? From a comprehensive analysis of the relationship between estrogen, ER, and CaBP-9k in GH3 cells, and the fact that the level f CaBP-9k expression in GH3 cells is increased significantly while inducing GH3 cell proliferation and prolactin secretion by estrogen in vitro, we can determine the relationship between CaBP-9k expression in GH3 cells and estrogen. Therefore, we suggest that CaBP-9k may be involved in the downstream biological effects brought out by ER-mediated signaling pathways in GH3 cells, such as cell proliferation, apoptosis, and prolactin secretion.
The regulation of prolactinomas by estrogen depends on the expression of ER, which is mainly expressed in the nucleus, with a small part expressed in the cell membrane. ER in the nucleus and membrane are interrelated. Estrogen can directly enter the nucleus to combine with the nuclear estrogen receptor (nER), act on the specific binding sites of the target gene-estrogen response element (ERE), and initiate gene transcription. Pathways which act through nER are referred to as classical or genomic pathways, while estrogen in combination with the estrogen receptor membrane (mER) and mER activates the kinase cascade with the G protein-coupled signal directly or indirectly act through mER and are referred to as non-classical or non-genomic pathways.
In prolactin cells, estrogen has been shown to activate prolactin mRNA transcription through ERE, promote prolactin cell proliferation, and regulate the storage and release of prolactin, whereas the ERE is located upstream of the 5’-regulatory region [11,12]. According to the literature, the CaBP-9k gene contains an incomplete palindromic sequence with 15 base-pairs, which is highly homologous with ERE. This sequence is positioned between exon 1 and intron A nucleotides +51 to +65 in the CaBP-9k gene. The interaction between this region and ERα may be the target where estrogen regulates CaBP-9k mRNA promoter activity and its level of expression, i.e., mRNA expression of CaBP-9k is regulated by ERα-ERE pathway. It suggests that CaBP-9k may regulate cell proliferation and secretion of prolactin cells through the action mode of genomic estrogen. What signal transduction pathway estrogen has taken to affect the occurrence and development of prolactinomas and what role CaBP-9k has played will be the subjects of future studies.
Acknowledgements
This study is supported by National Natural Science Foundation of China, Grant No. 81472343 and Natural Science Foundation of Jilin Province, Grant No. 20111509.
Disclosure of conflict of interest
None.
References
- 1.Dang VH, Nguyen TH, Choi KC, Jeung EB. A calcium-binding protein, Calbindin-D9k, is regulated through an estrogen-receptor-mediated mechanism following xenoestrogen exposure in the GH3 cell line. Toxicol Sci. 2007;98:408–415. doi: 10.1093/toxsci/kfm120. [DOI] [PubMed] [Google Scholar]
- 2.Hong EJ, Ji YK, Choi KC, Manabe N, Jeung EB. Conflict of estrogenic activity by various phthalates between in vitro and in vivo models related to the expression of Calbindin-D9k. J Reprod Dev. 2005;51:253–263. doi: 10.1262/jrd.16075. [DOI] [PubMed] [Google Scholar]
- 3.Jung EM, An BS, Yang H, Choi KC, Jeung EB. Biomarker genes for detecting estrogenic activity of endocrine disrupts via estrogen receptors. Int J Environ Res Public Health. 2012;9:698–711. doi: 10.3390/ijerph9030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vo TT, Jeung EB. An evaluation of estrogenic activity of parabens using uterine calbindin-D9k gene in an immature rat model. Toxicol Sci. 2009;112:68–77. doi: 10.1093/toxsci/kfp176. [DOI] [PubMed] [Google Scholar]
- 5.Lee GS, Kim HJ, Jung YW, Choi KC, Jeung EB. Estrogen receptor alpha pathway is involved in the regulation of Calbindin-D9k in the uterus of immature rats. Toxicol Sci. 2005;84:270–277. doi: 10.1093/toxsci/kfi072. [DOI] [PubMed] [Google Scholar]
- 6.Kansra S, Yamagata S, Sneade L, Foster L, Ben-Jonathan N. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Mol Cell Endocrinol. 2005;239:27–36. doi: 10.1016/j.mce.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Yoo YM, Jeung EB. Melatonin-induced estrogen receptor alpha-mediated calbindin-D9k expression plays a role in H2O2-mediated cell death in rat pituitary GH3 cells. J Pineal Res. 2009;47:301–307. doi: 10.1111/j.1600-079X.2009.00714.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim SM, Jung EM, An BS, Hwang I, Vo TT, Kim SR, Lee SM, Choi KC, Jeung EB. Additional effects of bisphenol A and paraben on the induction of calbindin-D9k and progesterone receptor via an estrogen receptor pathway in rat pituitary GH3 cells. J Physiol Pharmacol. 2012;63:445–455. [PubMed] [Google Scholar]
- 9.Watson CS, Jeng YJ, Hu GZ, Wozniak A, Bulayeva N, Guptarak J. Estrogen- and xenoestrogen-induced ERK signaling in pituitary tumor cells involves estrogen receptor-α interactions with G protein-αi and caveolin I. Steroids. 2012;77:424–432. doi: 10.1016/j.steroids.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo YC, Liu J, Wang W. Differential effects of estrogen receptor antagonists on pituitary GH3 cell proliferation, apoptosis, PRL secretion, and ER protein expression. Chinese Journal of Neurosurgery. 2012;28:285–290. [Google Scholar]
- 11.Yoo YM, Jeung EB. Melatonin-induced calbindin-D9k expression reduces hydrogen peroxide-mediated cell death in rat pituitary GH3 cells. J Pineal Res. 2010;48:83–93. doi: 10.1111/j.1600-079X.2009.00730.x. [DOI] [PubMed] [Google Scholar]
- 12.Vo TT, An BS, Yang H, Jung EM, Hwang I, Jeung EB. Calbindin-D9k as a sensitive molecular biomarker for evaluating the synergistic impact of estrogenic chemicals on GH3 rat pituitary cells. Int J Mol Med. 2012;30:1233–1240. doi: 10.3892/ijmm.2012.1122. [DOI] [PubMed] [Google Scholar]


