Abstract
A malignant gastrointestinal neuroectodermal tumor (GNET), a distinctive entity covering the characteristics of clear cell sarcoma (CCS) of gastrointestinal tract described recently, arising primarily in the ileum of a 33-year-old woman is reported. Histologically, the neoplasm involved the full thickness of the intestinal wall. Tumor cells, mainly displayed epithelioid or polygonal appearance with oval or round nuclei, arranged in strand, nested, and solid pattern with prominent pseudopapillary architecture instead of the familiar histological image with multinucleated osteoclast-like giant cells. They were positive for vimentin, S-100, synaptophysin, CD56 and CD99 protein, but negative for AE1/AE3, EMA, CEA, LCA, Desmin, CK7, CK20, Villin, CgA, CD117, Dog-1, GFAP, Melan-A, HMB-45, CD34, CR, WT1, D2-40. Fluorescence in situ hybridization (FISH) showed the presence of chromosomal translocation involving EWSR. The patients lived through a calm period after a tumor resection and 4 cycles of chemotherapy combining ifosfamide and epirubicin. This case demonstrates that GNET is a rare tumor in gastrointestinal tract, and furthermore, various misleading histological characteristics should been taken into consideration in the diagnosis.
Keywords: Malignant gastrointestinal neuroectodermal tumor, clear cell sarcoma, ileum, pseudopapillary, immunochemistry, FISH
Introduction
Primary CCS of the gastrointestinal tract is extraordinarily rare, which frequently presents infiltrative growth rich in osteoclast-type giant cells with uniform expression of S-100 protein but lacks melanocytic differentiation compared to its soft counterpart. However, the term of “GNET” was proposed by Stockman et al [1] owing to the tumor showing characteristics of neural differentiation with lacking melanocytic features. Histologically, multinucleated osteoclast-like giant cells, as a useful diagnostic clue, often present but not always. In this study, we added a case of GNET with uncommon pseudopapillary architecture to investigate the clinicopathologic, immunohistochemical, and molecular features and differential diagnosis.
Clinical history
A 33-year-old female was admitted for abdominal abscess of right lower quadrant with recurrent fever administrated by anti-inflammatory drugs after appendicectomy. A computed tomography scan showed a lump of 61 × 40 mm in maximum cross-section with mixed density, relative demarcation (Figure 1). In the laparotomy, the mass was located in the contralateral jejunum mesenteric side and adhesive with omentum majus, and a segmental resection of the ileum were performed, with regional mesenteric lymph nodes removed. No other remnant or metastatic lesions were found. The patients lived through a calm period after a tumor resection and 4 cycles of chemotherapy combining ifosfamide and epirubicin.
Figure 1.
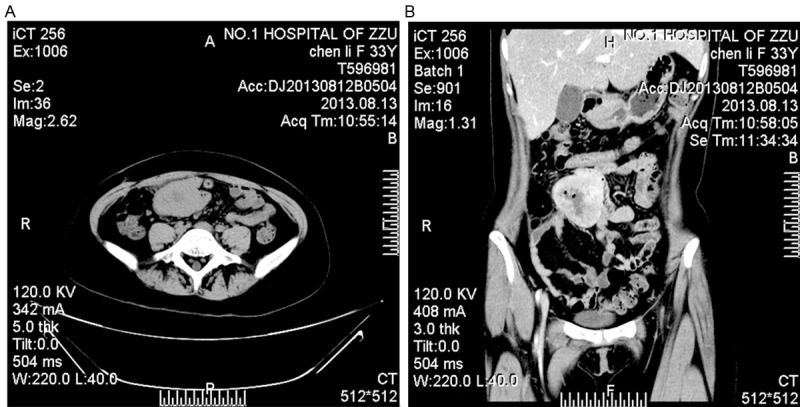
A, B. Axial and enhanced coronal CT revealed a lump with relative demarcation, communicating with the intestinal lumen.
Materials and methods
The surgical specimen were fixed in 4% buffered formalin, embedded routinely in paraffin and then stained with hematoxylin and eosin. Immunohistochemical studies were performed by En Vision technique using commercially antibodies in the Ventana BenchMark XT instrument (Ventana Systems, Tucson AZ). The antibodies included CD56, EMA, LCA, CEA, CK7, CK20, Villin, CgA, Dog-1, CD34, CR, D2-40, WT-1 (all above from Ventana, prediluted) and vimentin (1:200; Dako, Carpinteria, CA), AE1/AE3 (1:200; Dako) S-100 protein (1:2000; Dako), HMB45 (1:200; Dako), Melan-A (1:100; Dako), GFAP (1:500; Dako), synaptophysin, CD117 (1:400; Dako), Desmin (1:100; Dako), CD99 (1:50; Dako).
Fluorescence in situ hybridization evaluation for EWS rearrangement was performed on the 4-μm thick paraffin sections with the LSI EWSR1 (22q12) dual-color, break-apart probe (Abbott/Viysis, Downers Grove, IL), based on the manufacturer’s instruction.
Results
Grossly, the tumor revealed a firm and white-tan cut surface without distinct demarcation, involving the entire thickness of the intestinal wall. The average tumor size was 5.5 cm, ranging from 3.5 to 6 cm. Histologically, the GNET showed a strand, nested, and solid pattern with prominent pseudopapillary architecture (Figures 2, 3). The tumor cells mainly displayed epithelioid or polygonal appearance with eosinophilic cytoplasm and oval or round nuclei (Figure 3). Mitosis figures were uniformly scanty (< 1/10 HPFs). Necrosis and the multinucleated tumor cells, the hallmark we observed in most cases, were absent. Metastasis in the mesenteric lymph nodes was not found.
Figure 2.
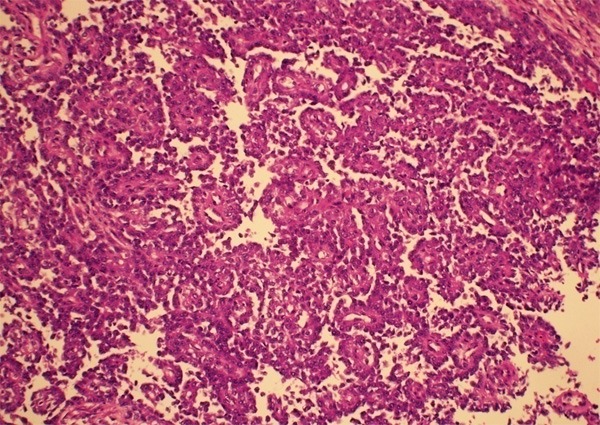
GNET with distinct pseudopapillary architecture.
Figure 3.
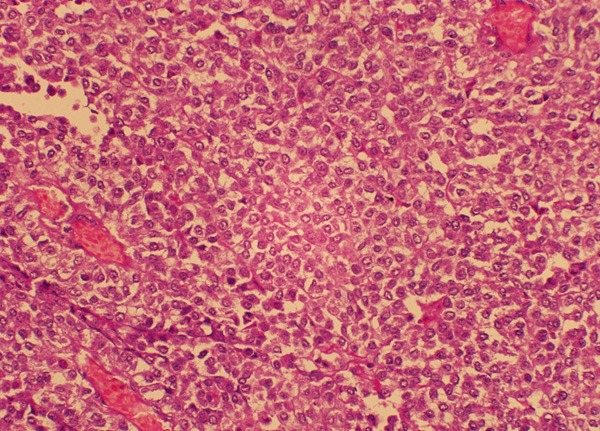
Relatively solid area of GNET composed of the relatively monomorphic epithelioid or polygonal cells with eosinophilic cytoplasm and vesicular nuclei.
The tumor cells were positive for S100 protein (Figure 4), CD56 (Figure 5), CD99, whereas negative for AE1/AE3, CK7, CEA, LCA, CgA, synaptophysin, Melan-A, HMB45, CD117, Dog-1, CD34, CR, D2-40, WT-1, Desmin. Proliferative index Ki-67 was approximately 20%.
Figure 4.
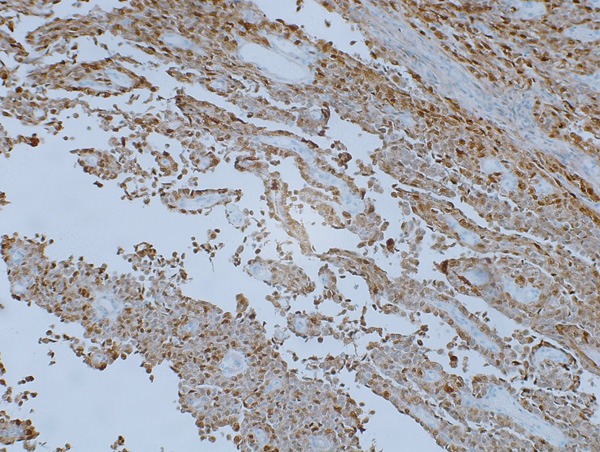
S-100 was strongly and diffusely positive in both cytoplasmic and nuclear pattern.
Figure 5.

Tumor cells were positive for CD56.
FISH analysis demonstrated the translocation signals in 80 out of 100 nuclei, which, typically, presented as one juxtaposed green and red signal and a significant separated, signal green and red signal per nucleus, indicating the presence of the EWS gene rearrangement (Figure 6).
Figure 6.
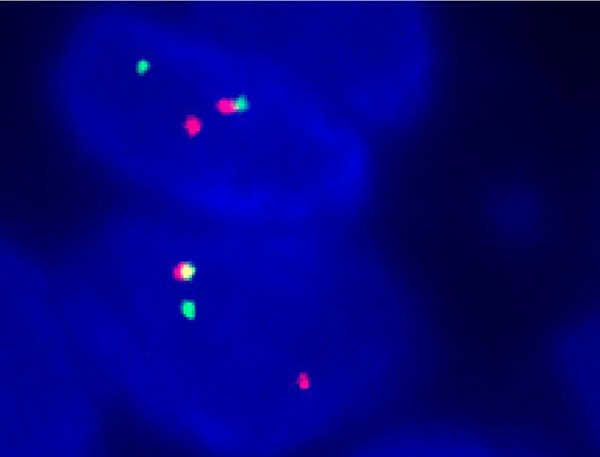
Representative image of EWSR gene rearrangement. The two cells showed 2 intact normal fused signals, and a separate red and green signal indicating the translocation of one copy of the EWSR per nucleus.
Discussion
Firstly described by Enzinger [2], clear cell sarcoma in tendons and aponeuroses is a distinct entity adopting evidence of melanocytic differentiation [3]. The term “GNET” proposed by Stockman et al [4] recently, could more readily tell the features of CCS in gastrointestinal tract, albeit, in some regards, resembling CCS of soft parts in morphology, immunohistochemitry, and genetics. The biological behavior seems to be aggressive, with local recurrence, lymph node or visceral metastases, particularly involving in liver, but more indolent compared to the classical CCS of soft tissue [4]. However, the process in our case appeared to escape a sinister prognosis, for the patient had been in a significant palliation without recurrence for 12 months.
Clinically, the tumor has a slight female predominance, and mainly affects the young aged to middle-aged adults [5]. Similar to their soft tissue counterparts, GNETs are relatively small with the median size 5 cm [5]. And the most common feature of these tumors is the significant transmural involvement of the gastrointestinal wall, with, sometimes, mucosal ulceration [6]. Histologically, the tumor cells arranged in nested, fascicular growth pattern and punctuated by fibrous septa, infiltrate in full-thickness of the gastrointestinal tract. The cells are polygonal, oval, or, sometimes, spindle with variable eosinophilic or clear cytoplasm [1,5]. The nuclei are round or oval and significantly vesicular with small nucleoli, occasionally prominent. Multinucleated neoplastic giant cells can be observed in the majority of the cases, which is a useful clue for the diagnosis of GNET [4,7,8]. As to our case, without osteoclast-like giant cells was pseudopapillary architecture significantly conspicuous except for the nested-and-solid area. Pseudopapillary structure with superimposed relatively uniform epithelioid or oval tumor cells stemmed from the fact that the stratified cells far away from capillaries failed in adherence and hence separated and fell off. This prominent pseduopapillary appearance is extremely rare, and has barely described by two reports up to now [1,4]. Immunohistochemically, the vast majority of the tumor cells show a consistent immunophenotype, with strong expression of S100 protein and absent expression of HMB-45, Melan-A and tyrosinase, indicating a deficiency in melanocytic differentiation. The positive expression of other neuroectodermal markers, such as CD56, NSE, synaptophysin, can be demonstrated in some cases [5]. Although GNET shares a compelling genetic feature with CCS in soft part, with a presence of EWS-ATF1 or EWS-CREB1 fusion, that would not be conclusive proof of a link between these two tumors [4]. Because EWS, a “promiscuous” gene, seems to fuse with many different partner genes and the translocation has been identified in diverse sarcoma as well as some non-mesenchymal tumors. Take the former for example, they include CCS of soft tissue, GENT, myxoid liposarcoma, extraskeletal myxoid chondrosarcoma, angiomatoid fibrous histocytoma, Ewing sarcoma, sclerosing epithelioid fibrosarcoma, low-grade fibromyxoid sarcoma and so on [9]. Consistently, there existed EWS rearrangement by FISH in our case. But the probe we used didn’t reveal the partner gene juxtaposed with EWS. However, an unverified speculation needs a further investigation that the cases with a significant pseudopapillary architecture in morphology tend to be EWS-CREB fusion rather than EWS-ATF1 according to the limited reported literature [1,10]. And furthermore, at the ultrastructural level, melanosomes were not observed in any reported cases, and a distinctive neural differentiation was confirmed by Stockman et al, which possessing multiple interdigitating cell process containing dense core granules and synaptic bulbs-like vesicles suggesting tumor’s origination from autonomic nervous system-related primitive cell of neural crest derivation [1,6]. So it is reasonable to substitute the designation “malignant gastrointenstinal neuroectodermal tumor” (GNET) for the term “clear cell sarcoma” in the gastrointestinal tract.
The first evaluation about the differential diagnosis of GNET should rule out gastrointestinal stromal tumors (GIST), the most frequent mesenchymal tumors involved in digestive tract, which are usually positive for CD117, Dog-1, and CD34 but negative for GNET [11]. Other differential diagnosis includes carcinoma, metastatic malignant melanoma, perivascular epithelioid cell neoplasm (PEComas), epithelioid malignant peripheral nerve sheath tumor (MPNST), and the CCS of soft tissue involving the gastrointestinal tract. Although GNET usually is composed of nests of relatively monomorphic epithelioid cells, it is not difficult to eliminate the diagnosis of carcinoma by means of a panel of epithelial marker, such as AE1/AE3, CAM5.2, CK7, CK20, CEA. Metastatic malignant melanoma and Perivascular epithelioid cell tumor (PEComas) are often rather tough to distinguish from GNET on histological ground. Generally, there exists cutaneous or mucosal involvement and relatively rare scatter significantly numerous osteoclast-like gaint cells in conventional malignant melanoma [4]. PEComas are usually composed of epithelioid, but, as a biphasic tumor, occasionally spindled, elongated cells with granular eosinophilic to clear cytoplasm and focal perivascular accentuation [12]. The above two commonly demonstrate melanocytic makers as HMB45, Melan-A, and TFE3, which, however, are scarcely appreciated in GNET. In addition, PEComas are characterized by immunopositivity with myoid makers like desmin, smooth muscle actin, calponin [12]. Epithelioid MPNSTs most often display multilobulated appearance and nested or corded growth pattern. The eosinophilic or amphophilic epithelioid tumor cells show vacuolated nuclei and prominent nucleoli embedded in a mucus-abundant stroma. Presence of areas, at least focally, of typical spindle cells with an alternating hypocellularity and hypercellularity transformation and negative immunoreactivity to melanoma antigens and scattered S-100 protein expression, combined with electron microscopy, if necessary, can facilitate the diagnosis. To distinguish GENT from CCS of soft tissues involving gastrointestinal tract is also challenging. Generally, deficiency of original lesions in soft tissues and presence of osteoclast-like gaint cells, and absent expression of specific melanocytic-associated markers, such as HMB45 and Melan-A, aid in correct diagnosis [4]. Under the electron microscopy, GNET also demonstrates the differential diagnostic features of total lack of melanoctyic differentiation evidence and showing neural differentiation [1].
In conclusion, GNET is a rare tumor with distinctive morphologic features and lack of melanocytic makers and of evidence of neural differentiation. The pseudopapillary architecture in histology most rarely comes cross. Although it shares some features with CCS in the soft tissues, a collection of ways can separate them and another similar neoplasms by the distinctly morphologic, immunohistochemical, genetic, and ultrastructural characteristics.
Acknowledgements
This work was financially supported by the first affiliated hospital of Zhenzhou University.
Disclosure of conflict of interest
None.
References
- 1.Stockman DL, Miettnen M, Suster S, Spagnolo D, Dominguez-Malagon H, Hornick JL, Asday V, Chou PM, Amanuel B, VanTuinen P, Zambrano EV. Malignant gastrointenstinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol. 2013;36:857–868. doi: 10.1097/PAS.0b013e31824644ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enzinger FM. Clear cell sarcoma of tendons and aponeuroses. An analysis of 21 cases. Cancer. 1965;18:1163–1174. doi: 10.1002/1097-0142(196509)18:9<1163::aid-cncr2820180916>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Deenik W, Mooi WJ, Rutgers EJ, Peterse JL, Hart AA, Kroon BB. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 30 cases. Cancer. 1999;86:969–975. [PubMed] [Google Scholar]
- 4.Kosemehmetoglu K, Folpe AL. Clear cell sarcoma of tendons and aponeuroses, and osteoclast-rich tumour of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: a review and update. J Clin Pathol. 2010;63:416–423. doi: 10.1136/jcp.2008.057471. [DOI] [PubMed] [Google Scholar]
- 5.Comin CE, Novelli L, Tornaboni D, Messerini L. Clear cell sacrcoma of the ileum: report of a case and review of literature. Virchows Arch. 2007;451:839–845. doi: 10.1007/s00428-007-0454-z. [DOI] [PubMed] [Google Scholar]
- 6.Antonescu CR, Nafa K, Segal NH, Dal Cin P, Ladanyi M. EWS-CREB1: a recurrent variant fusion in clear cell sarcoma-association with gastrointestinal location and absence of melanocytic differentiation. Clin Cancer Res. 2006;12:5356–62. doi: 10.1158/1078-0432.CCR-05-2811. [DOI] [PubMed] [Google Scholar]
- 7.Joo M, Chang SH, Kim H, Gardnar JM, Ro JY. Primary gastrointestinal clear cell sarcoma: report of 2 cases, one case associated with IG4-related sclerosing disease, and a review of literature. Ann Diagn Pathol. 2009;13:30–35. doi: 10.1016/j.anndiagpath.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Huang W, Zhang X, Li D, Chen J, Meng K, Wang Y, Lu Z, Zhou X. Osteoclast-rich tumor of the gastrointestinal tract with features resembling those of clear cell sarcoma of soft parts. Virchows Arch. 2009;448:200–203. doi: 10.1007/s00428-005-0051-y. [DOI] [PubMed] [Google Scholar]
- 9.Fisher C. The diversity of soft tissue tumors with EWSR1 gene rearrangements: a review. Histopathology. 2014;64:134–50. doi: 10.1111/his.12269. [DOI] [PubMed] [Google Scholar]
- 10.Shenjere P, Salman WD, Singh M, Mangham DC, Williams A, Eyden BP, Howard N, Knight B, Benerjee SS. Intra-abdominal clear-cell sarcoma: a report of 3 cases, including 1 case with unusual morphological features, and review of the literature. Int J Surg Pathol. 2011;20:378–385. doi: 10.1177/1066896911425485. [DOI] [PubMed] [Google Scholar]
- 11.Abdulkader I, Cameselle-Teijeiro J, de Alava E, Ruiz-Ponte C, Used-Aznar MM, Forteza J. Intestinal clear cell sarcoma with melanocytic differentiation and EWS [corrected] rearrangement: report of a case. Int J Surg Pathol. 2008;16:189–193. doi: 10.1177/1066896907306841. [DOI] [PubMed] [Google Scholar]
- 12.Armah HB, Parwani AV. Perivascular epithelioid cell tumor. Arch Pathol Lab Med. 2009;133:648–654. doi: 10.5858/133.4.648. [DOI] [PubMed] [Google Scholar]


