Abstract
To summarize the clinicopathological features, therapeutic regimens and outcomes for the patients with undifferentiated embryonal sarcoma of the liver (UESL), 9 cases of UESL were retrospectively reviewed. Complete clinical history, lab studies, imaging examinations and pathological findings were collected for analysis. Overall survival and progression free survival were assessed by Kaplan-Meier Survival Analysis. The patients were 6 to 37 years old, and included 6 males and 3 females. The tumor size ranged from 5 to 26 cm. Pathologically, the tumors consisted of proliferations of medium sized spindle, oval or stellate shaped pleomorphic cells loosely or compactly arranged in an edematous or myxoid matrix, with scattered bizarre multinucleated giant cells. All of the 9 cases were treated with radical resections, 6 of 9 cases received chemothrepy for postoperative treatment. All follow-up data were available, 4 of 9 cases had recurrence, and 2 patients were died. Time to recurrence in these cases was 19, 4, 29, 14 months. The mean overall survival (OS) was 58.25 ± 9.1 months and the mean progression-free survival (PFS) was 39.55 ± 11.6 months. UESL is a potential treatable malignance when treated with combined multiagent chemotherapy after resection.
Keywords: Liver, undifferentiated embryonal sarcoma, hepatectomy, prognosis
Introduction
Undifferentiated (embryonal) sarcoma of the liver (UESL), first documented in 1978, is a rare and highly malignant hepatic neoplasm of mesenchymal origin and shows a divergent differentiation. It occurs predominantly in children, with a peak incidence in the age range of 6-10 years [1,2]. The diagnosis of UESL may be difficult because it may show overlap features with several other tumors. The standard treatment for UESL has not been defined, likely due to the rarity of the disease and a paucity of prospective clinical trials. Moreover, the usual postoperative course of UES is relatively unknown. Therefore, collecting more clinicopathologic and therapeutic data and summarizing the previously reported cases are important.
In this retrospective multi-institutional study, we present our experience in diagnosis, treatment and outcome of 9 cases of UESL and reviewed the literature with the aim of expanding our understanding of this rare and deadly malignancy.
Patients and methods
Patients
From Jan, 2005 to Dec, 2013, 9 cases of UESL were retrieved from the pathology files of The First Affiliated Hospital of Sun Yat-Sen University and Nanfang Hospital of Southern Medical University, Guangzhou, China. Data including clinical and histological features, management methods, and outcomes of the patients were obtained from patient medical records. The patients gave informed consent for the use of their medical records for research, and approval for the retrospective review was obtained from the Ethics Committee of The First Affiliated Hospital of Sun Yat-Sen University and Nanfang Hospital of Southern Medical University.
Clinical and pathological evaluation
The information of clinical presentation, imaging studies and lab examination were collected from the medical records. Haematoxylin and eosin (HE) slides and all special stains were reviewed and all pathologic diagnoses were made according to the WHO classification of tumors of the digestive system (2010) [3]. Immunohistochemistry was performed on representative blocks using a panel of antibodies and the EnVision + system. Appropriate positive and negative controls were used throughout the experiment. Staining was considered positive when > 5% of cells showed staining with the appropriate pattern.
Outcome evaluation
The overall survival (OS) duration was calculated from the time of diagnosis to the time of death or the last follow-up. Progression-free survival (PFS) was measured from the time of diagnosis to the time of treatment failure, recurrence, or death from UESL.
Statistical analysis
Survival analysis was performed with the Kaplan-Meier method. Statistical analyses were per-formed using the SPSS Statistics 19.0 software package (SPSS Inc., Chicago, IL, USA).
Results
Clinical findings
The patients, including 6 males and 3 females, aged from 6-37 years (mean age is 15.3 years). They all were primary lesions. Lesions involved the right lobe in 4 cases, right and caudal lobe in 1 case and the left lobe in 4 cases. The main presenting complains were abdominal pain and mass. Fever and anorexia also presented in Case No. 9.
Imaging studies
Ultrasonography scan showed either hetero-echoic mass containing some echoless areas (in Case No. 2, 3, 4, 6, 7, 8, 9) or hyper-echoic (in Case No. 1). Computerized tomography (CT) or Magnetic resonance imaging (MRI) showed a hypodense mass with a multicystic appearance with an enhanced peripheral rim. The hypodense area of the mass did not enhance in the enhanced scan (Figure 1A, 1B). Most of the cases were misdiagnosed as malignant mesenchymal tumor or angiosarcoma, malignant fibrous histiocytoma (MFH) in imaging study (in Case No. 2, 4, 6, 7, 8, 9). Two cases were misdiagnosed as hepatocellular carcinoma (HCC), and one case was considered as teratoma or echinococcosis. The clinical data are summarized in Table 1.
Figure 1.
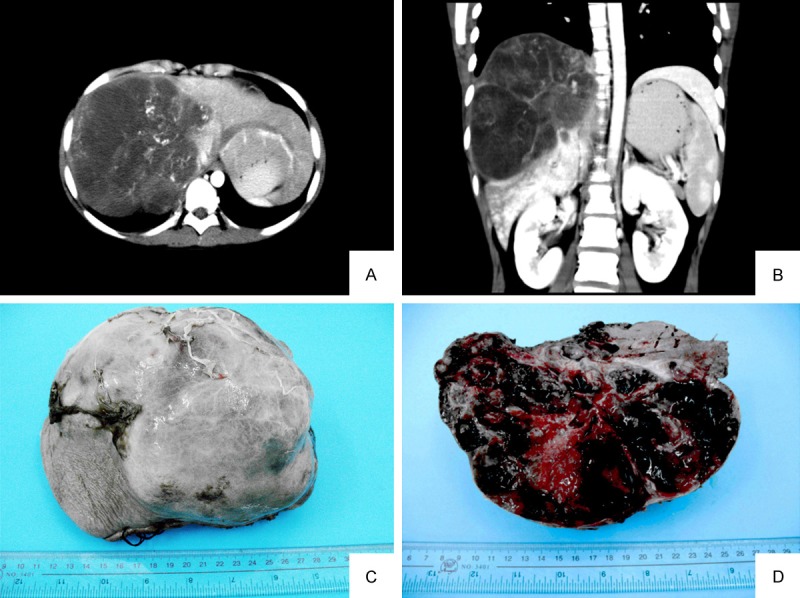
A, B. CT scan showed a hypodense mass with a multicystic appearance in the right lobe liver. Peripheral rim of the mass was enhanced but not the hypodense area in enhanced scan (Case No. 8). C. The tumor was globular and well demarcated with complete encapsulation (Case No. 9). D. The cut-surface is grey-white, with prominent haemorrhagic and necrotic areas.
Table 1.
Clinical features of 9 cases
| Case | Gender/Age | Chief Complain | Location | BUS | CT/MRI | Imaging diagnosis |
|---|---|---|---|---|---|---|
| 1 | Male/37 | Upper abdominalPain | Left | Hyper-echoic | Hypodense | Hepatocellular carcinoma? |
| 2 | Male/13 | Right upper abdominal pain suddenly | Right | Hetero-echoic | Hypodense | Mesenchymal Tumor |
| 3 | Male/7 | Upper abdominal mass | Left | Hetero-echoic | N/A | Teratoma or Echinococcosis |
| 4 | Female/24 | Upper abdominal Pain | Right | Hetero-echoic | N/A | Angiosarcoma? |
| 5 | Male/14 | Upper abdominal Pain | Left | N/A | Hypodense | Hepatocellular carcinoma? |
| 6 | Female/14 | Right upper abdominal pain | Right | Hetero-echoic | Hypodense | Malignant fibrous histiocytoma |
| 7 | Male/6 | Upper abdominal mass | Right and caudal lobe | Hetero-echoic | Hypodense | Malignant mesenchymal tumor |
| 8 | Female/9 | Upper abdominal mass | Left | Hetero-echoic | Hypodense | Malignant mesenchymal tumor |
| 9 | Male/14 | Right upper abdominal pain, fever, anorexia | Right | Hetero-echoic | Hypodense | Angiosarcoma |
BUS, B Ultrasonography Scan; CT, Computerized tomography; MRI, Magnetic resonance imaging; N/A, Not Available.
Laboratory examination
The 9 cases had no relation to hepatitis B and C virus infection. The serum alkaline phosphatase (ALP) activity and lactate dehydrogenase (LDH) were increased in most of the cases, the mean level was 180.6 IU/L and 336.3 IU/L (normal level: ALP < 110, LDH (114-240)). Serum α-fetoprotein (AFP), cancer antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA) levels were within the normal range. Cancer antigen 125 (CA125) was increased in Case No. 5, 8. In addition, γ-glutamyltransferase (GGT), leucine aminopeptidase (LAP) was slightly increased in some cases.
Gross features
The largest dimensions of the tumors in the cases were ranged from 5 to 26 cm. Most of cases showed a large, solitary mass. The cut surfaces were variegated, mainly solid and grey-white, with some cysitc gelatious areas. Haemorrhagic and necrotic areas were common (Figure 1C, 1D).
Microscopically and immunohistochemistry
The tumors were separated from the adjacent compacted hepatic parenchyma by a fibrous pseudocapsule. The tumors consisted of proliferations of medium sized spindle, oval or stellate shaped pleomorphic cells loosely or compactly arranged in an edematous or myxoid matrix, with scattered bizarre multinucleated giant cells (Figure 2A). Variable sized eosinophilic globules were present in the cytoplasm of large tumor cells or in extracellular matrix (Figure 2B). Haemorrhage and necrosis were frequently seen. In all cases, satellite lesions and tumor embolus were not found in sections. Foci of haemangiocytoma-like compact areas with collagenization were present in case No. 2 (Figure 2C). Fibroblast-like fascicles and bundles were seen in compact areas in Case No. 8 (Figure 2D). With regard to immunohistochemistry, eosinophilic gobules were typical positive for PAS and diastase resistant. Most tumor cells consistently were positive for markers a1-antitrypsin (a1-AT) and vimentin (Figure 2E, 2F). While makers CK, CK19, actin, desmin, CD56 showed positive staining occasionally in some cases. Makers MyoD1, HHF35, S-100, CD31, CD34, Hepatocyte and AFP were all immunonegative in our cases.
Figure 2.
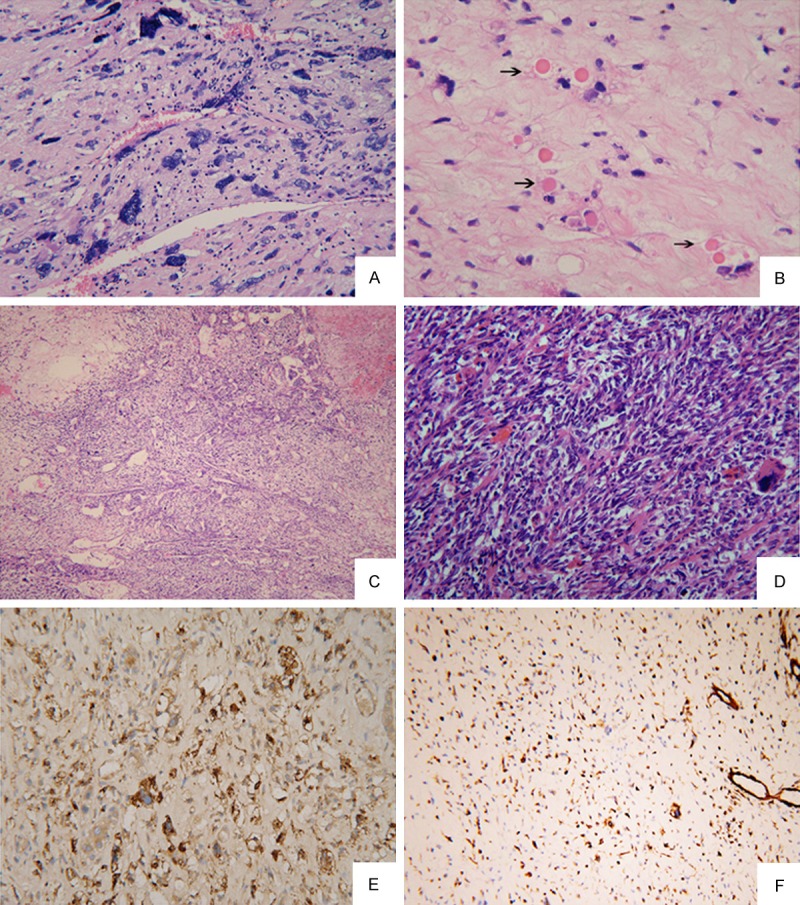
(A) There were foci of uniform small round cells admixed with a small number of pleomorphic cells in an edematous background (HE × 200) (Case No. 4); (B) Variable sized eosinophilic globules were present in the cytoplasm of large tumor cells or in extracellular matrix (arrow) (HE × 200); (C) Haemangiocytoma-like compact areas with collagenization (HE × 100) (Case No. 2); (D) Fibroblast-like fascicles and bundles were seen in compact areas (HE × 200) (Case No. 8); (E, F) Tumor cells were strongly positive for AAT (E) and Vimentin (F) (Case No. 4) (Envision Immunohistochemistry, × 100).
In addition to those features, interestingly, some zones containing UESL and mesenchymal hamartomas of the liver (MHL) coud be found in Case No. 3, 4 (Figure 3A). Part of areas corresponded to a typical UESL composed of pleomorphic malignant stromal spindle to stellate-shaped elements with numerous mitoses (Figure 3B). Close to UESL area, there were some peripheric MHL-like areas consisted of a loose myxoid stroma populated by spindle or stellateshaped cells without cytonuclear atypia or mitoses, and dilated bile duct (Figure 3C).
Figure 3.
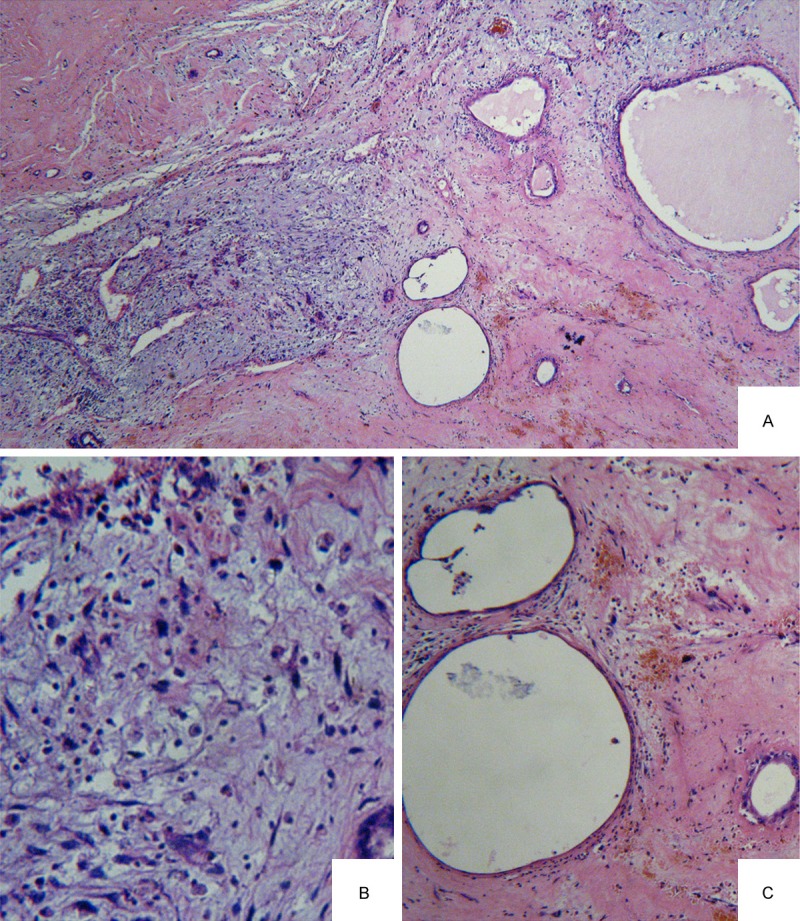
A. Transitional zones containing a histological mixture of the UESL and MHL (HE × 100) (Case No. 4); B. UESL areas showed pleomorphic malignant stromal spindle to stellate-shaped elements with numerous mitoses (HE × 200); C. MHL-like areas consisted of a loose myxoid stroma without cytonuclear atypia or mitoses and dilated bile duct (HE × 200).
Treatment
Radical resections (lobectomies) were performed in all cases. In Case No. 1, the patient received transcatheter arterial chemoembolization (TACE: lipiodol, epirubicin, and hydroxy camptothecin) untill intrahepatic recurrence in the 19 month. No preoperative treatments but postoperative chemotherapy were performed in Case No. 2, 3, 4, 5, 6 with a standard regimens for sarcoma (MAID: mesna + adriamycin + iphosphamide + dacarbazine). In Case No. 6, because the tumor invaded diaphragma, partial diaphragma resection and neoplasty were performed simultaneously. In Case No. 7, he received chemotherapy 4 times (pirarubicin-adriamycin + cis-diaminedichloroplatinum) and interventional therapy 2 times but had no significant effect before surgery. Therefore, lobectomy and postoperative chemotherapy was performed.
Outcomes and survival analysis
The follow-up information of all cases was available (Table 2). In Case No. 1, TACE was performed when intrahepatic recurrence occurred in the 19 month, unfortunately, distant metastasis emerged in the right lower lung and lymph node of mediastinum in the 24 month. He died of multi-organ tumor metastasis in the 34 month. In Case No. 6, who died in the 47 months while intrahepatic recurrence appeared in the 14 months. The other cases were alive (range from 5 to 76 months). In additional, the time to recurrence was 19, 4, 29, 14 months in Case No. 1, 4, 5, 6. No recurrent lesions were detected in the other cases by routine B-mode ultrasonography examination every 3 months. The OS and PFS analysis was performed using the Kaplan-Meier method. The mean OS was 58.25 ± 9.1 months and the mean PFS was 39.55 ± 11.6 months (Figure 4A, 4B).
Table 2.
Treatment and prognosis data of 9 cases
| Case | Treatment | Chemotherapy regimens | Follow-up |
|---|---|---|---|
| 1 | Lobectomies | No | DOD, 34 mo. intrahepatic recurrence in the 19 mo, treated with TACE. Tumor metastasis in the 24 mo. |
| 2 | Lobectomies + Chemotherapy | MAID | FOD, 76 mo No recurrence |
| 3 | Lobectomies + Chemotherapy | MAID | FOD, 18 mo No recurrence |
| 4 | Lobectomies + Chemotherapy | MAID | FOD, 24 mo (recurrence in the 4mo ) |
| 5 | Lobectomies + Chemotherapy | MAID | FOD, 50 mo. (intrahepatic recurrence in the 29 mo. Treated with segmentectomy |
| 6 | Lobectomies + Chemotherapy | MAID | DOD, 47 mo. Intrahepatic recurrence in the 14 mo |
| 7 | Chemotherapy + TACE + Lobectomies | THP-ADM + CDDP | FOD, 29 mo. No recurrence |
| 8 | Lobectomies + Chemotherapy | MAID | FOD, 5 mo |
| 9 | Lobectomies + Chemotherapy | MAID | FOD, 5 mo |
DOD, dead of disease; FOD, free of disease; TACE, transcatheter arterial chemoembolization (lipiodol, epirubicin, and hydroxy camptothecin); MAID, mesna + adriamycin + iphosphamide + dacarbazine; THP-ADM, pirarubicin-adriamycin; CDDP, cis-diaminedichloroplatinum.
Figure 4.
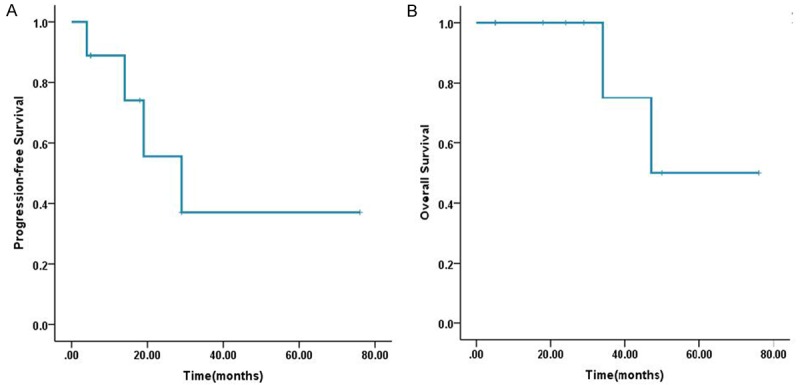
(A, B) Kaplan-Meier Overall survival (A) and Progression-free survival (B) curves.
Discussion
Undifferentiated embryonal sarcoma is the third most common malignant tumor of the liver in children, accounting for 13% of hepatic malignancies in this age group. There were over 150 cases were reported untill 2012 [4-6]. In this report, we have presented a descriptive study of 9 cases. The majority of the patients are children, the mean age is 15.3 years. The primary presenting complaints of UESL is always upper abdominal pain or mass that may be correlated with the relatively large size of the tumors. Other complaints, such as nausea, anorexia, fever, or headache, may also be presented [7,8]. Laboratory studies, such as CA125, CEA, CA199 are nonspecific for this entity, while ALP and LDH elevate generally in most of cases. In imaging study, typical ultrasonography scan (US) showed hepatomegaly and a heterogeneous mass containing some echoless areas, and CT scan showed a lesion occupying a large space with cystic areas, hypodense areas that do not enhance in an enhanced scan, compression or displacement around the tissue [9,10]. However, it is hard to make an accurate diagnosis through imaging study while it should be considered along with the age of the patient and the size of the tumor.
Grossly, the tumor is usually a large, solitary mass, predominantly solid but often with variable areas of hemorrhage, necrosis and cystic degeneration. Tumors with and without capsules have been reported. The tumor size often exceeds 10 cm and can be as large as 30 cm. The right lobe of the liver is more commonly involved [6,7].
Histologically, the tumor has a variable but distinctive sarcomatous appearance. It is composed of loosely arranged, medium-large spindles, oval and stellate pleomorphic cells with poorly defined cell borders, and giant cells with severe atypia [11-14]. In addition, as in our report, there are prominent eosinophilic, PAS+, and diastase-resistant globules in the tumor cell cytoplasm and extracellular matrix. Although its pathological origin remains unclear, ultrastructural and immunohistochemical studies have shown its fibroblastic, histiocytic, myoblastic, myofibroblastic, rhabdomyoblastic and leiomyoblastic differentiation [15]. Most UESL are diffusely positive for vimentin, a1-AT, and focally positive for cytokeratin, desmin, muscle-specific actin, CD68, myoglobin, non-specific enolase, S100, and CD34, suggesting that embryonal sarcoma is an “undifferentiated” sarcoma, since it may display partial differentiation [16,17].
The pathogenesis of UESL remains unclear. Some authors assumed that UESL can arise within mesenchymal hamartomas of the liver (MHL), which is the second commonest benign liver tumor in children. Karyotypic analysis of UESL arising in MHL has demonstrated rearrangements of region 19q13.4, including t(11;19)(q11;q13.3-13.4) [18,19]. In present study, some transitional zones including both histological features supporting the assumptions that MHL and UESL may belong to the same family of mesenchymal hepatic tumors, the latter representing the malignant entity of the spectrum. However, further study about the association between them need to be performed in future.
A standard treatment for UESL has not been defined. Uchiyama et al [20] proposed that surgical resection combined with chemotherapy is the most effective therapy for UESL patients with tumor rupture. Additionally, the authors suggested that chemotherapy containing 3-4 types of drugs, such as adriamycin, cisplatin and others, demonstrated beneficial therapeutic effects and significantly improved survival rates. Yu et al [21] suggested that the best method for improving survival is total resection, regardless of whether the tumor is ruptured. Li et al [5] demonstrated that a UESL patient who underwent interventional therapy and surgical resection exhibited a prolonged survival compared with patients who underwent surgical resection only. In addition to that traditional therapy, hepatic and stem cells transplantation were performed to this malignance. Kelly et al. [22] have demonstrated that hepatic transplantation is an effective therapeutic method for UESL patients whose tumor is not able to be resected or who have postoperative recurrence of the tumor. And recently, Plant et al. [6] also showed that in cases of unresectable primary tumor or recurrent and refractory disease isolated to the liver, orthotopic liver transplantation (OLT) is a potential therapeutic option. Noguchi K [23] reported a long-term survival case of UESL who was treated with a high dose of etoposide and radiation therapy, then followed with peripheral blood stem cell transplantation.
In previously reported literatures, the prognosis of UESL is poor with historically overall survival of < 37.5% at 5 years and the majority of patients die of tumor recurrence in the upper abdomen or metastasis to lung, pleura, and peritoneum within 2 years of the initial operation. However, more and more literatures reported that the tumor is potentially treatable [6,24,25]. In our study, the Case No. 2 is alive and free of disease for more than 6 years without recurrence. And the overall survival (OS) of our case series is close to 5 years (mean OS is 58.25 ± 9.1 months), the progression-free survival (PFS) is also more than 3 years (mean PFS is 39.55 ± 11.6 months). These data also support the idea that UESL is treatable. In light of recent literatures and our retrospective study, we hold the idea that multiagent chemotherapy after resection can significantly improve the survival and some other novel treatments like hepatic or stem cell transplantation provide the potential opportunity to cure this tumor.
Disclosure of conflict of interest
None.
References
- 1.Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978;42:336–348. doi: 10.1002/1097-0142(197807)42:1<336::aid-cncr2820420151>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Lack EE, Schloo BL, Azumi N, Travis WD, Grier HE, Kozakewich HP. Undifferentiated (embryonal) sarcoma of the liver. Clinical and pathologic study of 16 cases with emphasis on immunohistochemical features. Am J Surg Pathol. 1991;15:1–16. [PubMed] [Google Scholar]
- 3.Bosman FT, Carneiro F, Hruban RH. WHO classification of tumours of the digestive system. 4th edition. Lyon: IARC Press; 2010. pp. 327–330. [Google Scholar]
- 4.Pachera S, Nishio H, Takahashi Y, Yokoyama Y, Oda K, Ebata T, Igami T, Nagino M. Undifferentiated embryonal sarcoma of the liver: case report and literature survey. J Hepatobiliary Pancreat Surg. 2008;15:536–44. doi: 10.1007/s00534-007-1265-y. [DOI] [PubMed] [Google Scholar]
- 5.Li XW, Gong SJ, Song WH, Zhu JJ, Pan CH, Wu MC, Xu AM. Undifferentiated liver embryonal sarcoma in adults: a report of four cases and literature review. World J Gastroenterol. 2010;16:4725–32. doi: 10.3748/wjg.v16.i37.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plant AS, Busuttil RW, Rana A, Nelson SD, Auerbach M, Federman NC. A single-institution retrospective cases series of childhood undifferentiated embryonal liver sarcoma (uels): success of combined therapy and the use of orthotopic liver transplant. J Pediatr Hematol Oncol. 2013;35:451–5. doi: 10.1097/MPH.0b013e318271c948. [DOI] [PubMed] [Google Scholar]
- 7.Baron PW, Majlessipour F, Bedros AA, Zuppan CW, Ben-Youssef R, Yanni G, Ojogho ON, Concepcion W. Undifferentiated embryonal sarcoma of the liver successfully treated with chemotherapy and liver resection. J Gastrointest Surg. 2007;11:73–75. doi: 10.1007/s11605-006-0044-4. [DOI] [PubMed] [Google Scholar]
- 8.Buetow PC, Buck JL, Pantongrag-Brown L, Marshall WH, Ros PR, Levine MS, Goodman ZD. Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology. 1997;203:779–783. doi: 10.1148/radiology.203.3.9169704. [DOI] [PubMed] [Google Scholar]
- 9.Lashkari HP, Khan SU, Ali K, Sashikumar P, Mukherjee S. Diagnosis of undifferentiated embryonal sarcoma of the liver-importance of combined studies of ultrasound and CT scan. J Pediatr Hematol Oncol. 2009;31:797–8. doi: 10.1097/MPH.0b013e3181b534e6. [DOI] [PubMed] [Google Scholar]
- 10.Massani M, Caratozzolo E, Baldessin M, Bonariol L, Bassi N. Hepatic cystic lesion in adult: a challenging diagnosis of undifferentiated primary embryonal sarcoma. G Chir. 2010;31:225–8. [PubMed] [Google Scholar]
- 11.Ma L, Liu YP, Geng CZ, Tian ZH, Wu GX, Wang XL. Undifferentiated embryonal sarcoma of liver in an old female: case report and review of the literature. World J Gastroenterol. 2008;14:7267–70. doi: 10.3748/wjg.14.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Cai JQ, Bi XY, Zhao JJ, Huang Z, Lu HZ, Zhou HT. [Diagnosis,treatment, and prognostic of liver sarcoma: analysis of 16 cases] . Zhonghua Yi Xue Za Zhi. 2008;88:1537–9. [PubMed] [Google Scholar]
- 13.Kaur J, Dey P, Das A. Fine needle aspiration cytology of undifferentiated (embryonal) sarcoma of liver in an adult male. Diagn Cytopathol. 2010;38:547–8. doi: 10.1002/dc.21254. [DOI] [PubMed] [Google Scholar]
- 14.Gupta C, Iyer VK, Kaushal S, Agarwala S, Mathur SR. Fine needle aspiration cytology of undifferentiated embryonal sarcoma of the liver. Cytopathology. 2010;21:414–6. doi: 10.1111/j.1365-2303.2009.00732.x. [DOI] [PubMed] [Google Scholar]
- 15.Wei ZG, Tang LF, Chen ZM, Tang HF, Li MJ. Childhood undifferentiated embryonal liver sarcoma: clinical features and immunohistochemistry analysis. J Pediatr Surg. 2008;43:1912–9. doi: 10.1016/j.jpedsurg.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Kiani B, Ferrell LD, Qualman S, Frankel WL. Immunohistochemical analysis of embryonal sarcoma of the liver. Appl Immunohistochem Mol Morphol. 2006;14:193–7. doi: 10.1097/01.pai.0000173052.37673.95. [DOI] [PubMed] [Google Scholar]
- 17.Zheng JM, Tao X, Xu AM, Chen XF, Wu MC, Zhang SH. Primary and recurrent embryonal sarcoma of the liver: clinicopathological and immunohistochemical analysis. Histopathology. 2007;51:195–203. doi: 10.1111/j.1365-2559.2007.02746.x. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan MJ, Swanson PE, Knoll J, Taboada EM, Dehner LP. Undifferentiated embryonal sarcoma with unusual features arising within mesenchymal hamartoma of the liver: report of a case and review of the literature. Pediatr Dev Pathol. 2001;4:482–9. doi: 10.1007/s10024001-0047-9. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Chen H, Jin M, Wang X, Lee J, Xu W, Zhang R, Li S, Niu J. Molecular cytogenetic characterization of undifferentiated embryonal sarcoma of the liver: a case report and literature review. Mol Cytogenet. 2012;5:26. doi: 10.1186/1755-8166-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchiyama M, Iwafuchi M, Yagi M, Iinuma Y, Kanada S, Yamazaki S, Ohtaki M, Shirai Y. Treatment of ruptured undifferentiated sarcoma of the liver in children: a report of two cases and review of the literature. J Hepatobiliary Pancreat Surg. 2001;8:87–91. doi: 10.1007/s005340170055. [DOI] [PubMed] [Google Scholar]
- 21.Yu DC, Tandon R, Bohlke AK, Steiner RB, Haque S, Florman SS. Resection of a large, ruptured, undifferentiated (embryonal) sarcoma of the liver in a child: a case report and review of the literature. J La State Med Soc. 2009;161:41–44. [PubMed] [Google Scholar]
- 22.Kelly MJ, Martin L, Alonso M, Altura RA. Liver transplant for relapsed undifferentiated embryonal sarcoma in a young child. J Pediatr Surg. 2009;44:e1–3. doi: 10.1016/j.jpedsurg.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi K, Yokoo H, Nakanishi K, Kakisaka T, Tsuruga Y, Kamachi H, Matsushita M, Kamiyama T. A long-term survival case of adult undifferentiated embryonal sarcoma of liver. World J Surg Oncol. 2012;10:65. doi: 10.1186/1477-7819-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisogno G, Pilz T, Perilongo G, Ferrari A, Harms D, Ninfo V, Treuner J, Carli M. Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer. 2002;94:252–7. doi: 10.1002/cncr.10191. [DOI] [PubMed] [Google Scholar]
- 25.Kim DY, Kim KH, Jung SE, Lee SC, Park KW, Kim WK. Undifferentiated (embryonal) sarcoma of the liver: combination treatment by surgery and chemotherapy. J Pediatr Surg. 2002;37:1419–23. doi: 10.1053/jpsu.2002.35404. [DOI] [PubMed] [Google Scholar]


